Abstract
Introduction
Information about how physical exercise affects patients with amnestic mild cognitive impairment (aMCI) due to Alzheimer's disease (AD) is still missing. This study evaluated the impact of multicomponent exercise training on cognition and brain structure in aMCI subjects with cerebral spinal fluid positive AD biomarkers.
Methods
Forty aMCI subjects were divided in training (multicomponent exercise thrice a week for 6 months) and nontraining groups. Assessments included cardiorespiratory fitness, neurocognitive tests, and a structural magnetic resonance imaging using 3.0 T scanner. FreeSurfer software analyzed hippocampal volume and cortical thickness.
Results
The training group showed increased volume in both hippocampi and better performance in episodic memory test after 6 months. In contrast, the nontraining group declined in functional activities, recognition, and cardiorespiratory fitness for the same period.
Discussion
Multicomponent exercise seems to improve hippocampal volume and episodic memory, and maintains VO2max in aMCI due to AD.
Keywords: aMCI due to AD, Multicomponent exercise, Cognition, Hippocampus, Cortical thickness
1. Introduction
Over the past few decades, much effort has been made to develop a treatment that could bring some cognitive improvement in patients with Alzheimer's disease (AD), because the current pharmacologic treatment available has limited effects. Identifying and targeting subjects at risk for developing dementia (i.e., amnestic mild cognitive impairment [aMCI] subjects), as well as developing nonpharmacologic treatments are research priorities. In this context, physical exercise has been demonstrated to be an effective nonpharmacologic strategy for combating cognitive decline and hippocampal degeneration in the cognitively healthy elderly [1], [2], [3], [4], [5] and in those with genetic risk for AD [6]. Similarly, converging evidence demonstrated the benefits of practicing aerobic, resistance, or balance training on cognition [7], [8], [9] and hippocampal volume increase [10] in aMCI subjects.
Another type of exercise, which has been gaining prominence, is the multicomponent exercise program (MEP). By definition, the MEP is a program that contains aerobic and resistance exercises, balance, coordination practicing, and flexibility stimulation in its core. Interestingly, previous studies have suggested that the combination of exercise regimens has better wide-ranging effects on old adults than just one type of exercise [11], possibly having a larger effect on cognition than either aerobic or strength training programs alone. Similar to aerobic and resistance interventions, the MEP training is also associated with improvement in general global cognitive status in aMCI subjects [12] and in patients with AD [13]. Furthermore, besides improving logical memory and maintaining general cognitive function, MEP training was associated with less whole brain cortical atrophy in older adults with aMCI [14].
Although physical exercise clearly shows some benefits in cognition and even in brain structure in the elderly, it is not well established if MEP training can have these effects in aMCI subjects with pathophysiological evidence of AD (i.e., amyloidosis and neurodegeneration shown by altered p-tau or hippocampal atrophy). Including only aMCI subjects due to AD in our sample diminishes the heterogeneity of these subjects and aid in selecting the appropriate population for preclinical therapeutic intervention. Because the diagnostic criteria for MCI in previous studies emphasize the presence of memory complaints and the absence of dementia, the diagnostic accuracy can be lower, especially in this early phase when the symptoms are vague. To answer this question, we assessed the influence of a 6-month MEP training on episodic memory and functional activities, as well as on the brain structure (cortical thickness and hippocampal volume) in aMCI with pathophysiological evidence of AD.
2. Methods
2.1. Study design
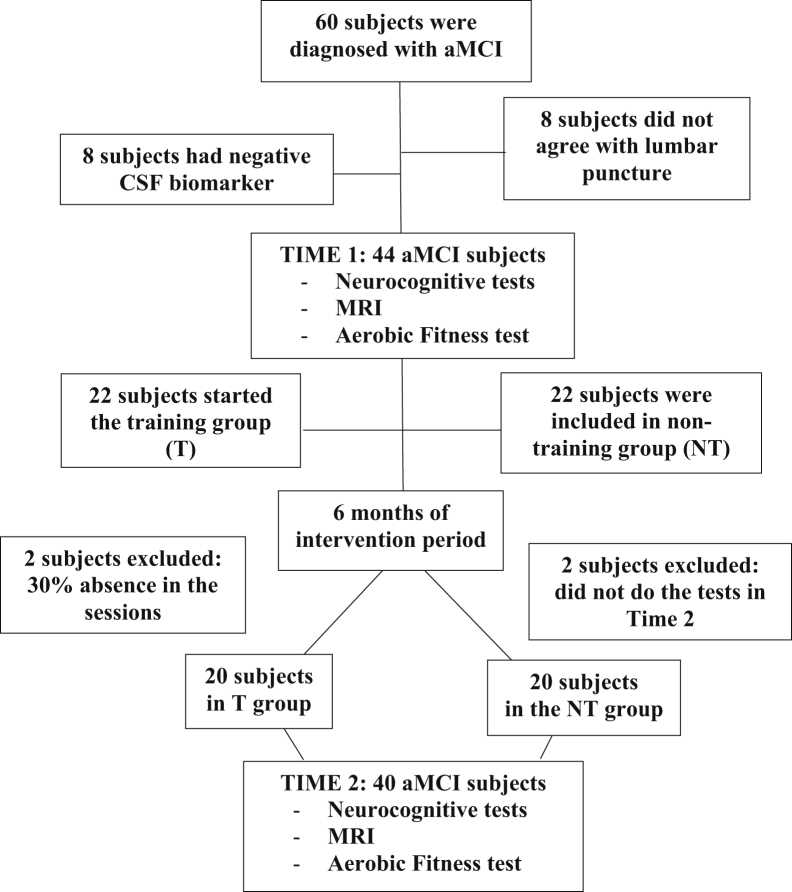
We conducted a 26-week, single-blinded, MEP intervention trial with sedentary older participants diagnosed with aMCI (mean age 68.3 ± 4.8, 56.5% female). The study was approved by the ethics committee of University of Campinas and all participants signed a written informed consent before any procedure. The study had a total of 40 participants, who were divided into two groups: Training (T, n = 20) and nontraining (NT, n = 20), according to their own will of participation (nonrandomized). All participants underwent neuropsychological assessment, magnetic resonance imaging (MRI) scanning, and aerobic fitness examination twice: 1 week before the intervention period (time 1) and 1 week after the 26-week intervention period (time 2) (Fig. 1).
Fig. 1.
The flow chart of recruitment and exclusion of participants. Abbreviations: aMCI, amnestic mild cognitive impairment; MRI, magnetic resonance imaging.
2.2. Participants
Participants were diagnosed as aMCI by using the core criteria of the National Institute on Aging/Alzheimer's Association for MCI [15], [16] and had a Clinical Dementia Rate [17] score of 0.5 (with an obligatory memory score of 0.5), estimated on the basis of a semistructured interview. All participants had pathophysiological evidence of AD in cerebral spinal fluid (CSF; amyloid beta [Aβ]1–42 < 416.5 pg/mL and Aβ1–42/p-tau < 9.5 pg/mL) [18], memory cognitive complaint confirmed by poor performance on episodic memory test (Rey Auditory Verbal Learning Test [RAVLT]) [19], and the absence of dementia. We excluded individuals with any cardiologic disease that could compromise the exercise performance, other neurologic or psychiatric diseases, Hachinski ischemic score [20] >4, Fazekas scale >1 [21], head injury with loss of consciousness, use of sedative drugs 24 h before neuropsychological testing, history of drug or alcohol addiction, and prior chronic exposure to neurotoxic substances. T and NT groups did not differ in age, gender, body mass index, activity level (self-reported mean endurance per week), or medicine intake (anticholinesterase, beta-adrenergic blocking agents, metformin, diuretics, antihyperlipidemic statins, antidepressive, anxiolytic, thyroid medication) (see “baseline group comparison”).
2.3. Aerobic fitness assessment
After obtaining the physician's approval to engage in cardiorespiratory fitness testing, the participants performed a graded maximal exercise test, the protocols of which consisted of start walking at a speed of 3 mph without slope, and then increase 2% every 2-minute intervals. Measures of oxygen uptake, heart rate, and blood pressure were continuously monitored by a cardiologist. The test was finished when the subject reached volitional exhaustion and/or symptom limitation.
Measures of oxygen utilization during a treadmill test provide a direct index of cardiorespiratory fitness (considered the gold standard). Oxygen uptake peak (VO2 peak) or VO2max indicates the functional capacity of cardiorespiratory function and is often considered as the benchmark indicator of cardiorespiratory fitness [22]. VO2max was defined as the highest recorded VO2 value after two of the three criteria were met [1]: a plateau in peak VO2 between two or more workloads [2]; a respiratory exchange ratio >1.00; and [3] a heart rate equivalent to their age-predicted maximum.
In this test, we also calculated the anaerobic threshold and the respiratory compensation point. The first one is defined as the level of exercise intensity in which lactic acid builds up in the body faster than it can be cleared away, whereas the second one is the metabolic rate for a maximal incremental test, from which the control of the acid-base balance is lost [23]. In addition, metabolic equivalent of task (MET) scores (1 MET = 1 kcal/kg/h or 3.5 mL/kg/min) were calculated to assess the self-reported physical activity level through International Physical Activity Questionnaire [24].
2.4. Neuropsychological evaluation
Neuropsychologists administered to all participants a cognitive, functional, and neuropsychiatric battery without knowledge of clinical diagnosis. Global cognitive status was measured using the Mini-Mental State Examination [25], and episodic memory was evaluated by the RAVLT (subitems encoding—RAVLT, delayed recall-A7, and recognition-Rec) [19]. To evaluate the presence of confounding depressive symptoms, we used Beck Depression Inventory [26]. We used the Pfeffer Functional Scale [27] to evaluate functionality. The test assesses 10 common activities, focusing on complex cognitive/social functioning. The informant report's total score (range 0–10, meaning 0 independency in functional activities and 10 great dependency on them) was obtained.
2.5. MRI acquisition
All patients underwent MRI using a 3 T Achieva-Intera Philips Scanner. Routine T1- and T2-weighted sequences were performed for all subjects to exclude unrelated abnormalities. We used volumetric T1-weighted images of the brain acquired using a standard eight-channel head coil: sagittal orientation; voxel matrix, 240 × 240 × 180; voxel size, 1 × 1 × 1 mm³; repetition time/echo time, 7/3.201 ms; and flip angle 8°.
2.6. CSF sample
CSF was obtained from all participants once (time 1), by lumbar puncture of the L3/L4 or L4/L5 intervertebral space, using a 25-gauge needle, and collected via a syringe in 12-mL polypropylene tubes (Sarstedt, Nümbrecht, Germany). A small amount of CSF was used for routine analysis, including total cells (leucocytes and erythrocytes), total protein, and glucose. Within 2 hours, CSF samples were centrifuged at 800g for 10 minutes to remove cells and stored at −80°C until AD biomarker analysis for protein analysis. CSF Aβ1–42, total tau, and p-tau proteins were measured using Inno-Bia Alzbio3 kit (Innogenetics, Gent, Belgium).
2.7. Intervention protocol
2.7.1. T group
The goal of the MEP sessions was to accumulate 30 minutes of different types of exercises in the intensity prescribed by the treadmill test (see “aerobic fitness assessment” session). To achieve the target intensity, participants were wearing a heart rate monitor during the training sessions. We defined the target intensity being the minimal heart rate value from the anaerobic threshold and the maximum value from the respiratory compensation point. Although both thresholds were individualized in the test, we could estimate an intensity between 70% and 90% of the maximum heart rate of each participant.
In each session, 10 minutes were dedicated for warming up and cooling down (5 minutes at the beginning of the session and 5 minutes at the end). During the first 4 weeks of the intervention, the sessions were shorter (between 20 and 30 minutes/session), so the participants could start feeling more confident doing the proposed exercise, using the monitor, and trying to achieve the target heart rate. From the fifth week onward, the participants were stimulated to achieve and maintain the target heart rate as much as possible during the 30 minutes of core content. A range of aerobic exercises was offered including outdoor walking and jogging, circuit training using rubber bands, exercises using different kinds of balls, and dancing. Once a week, the exercise was walking or jogging, depending on the participant condition. The others two sessions of the week included circuit training, in which the participants had to do some resistance exercises using rubber bands and between the exercises had to walk or run; sports like basketball, volleyball, and tennis adapted to the elderly; or dancing.
2.7.2. NT group
Participants in the NT group did not take part in any intervention session. They were advised to continue with their normal routine, but without initiating any new activity, including physical exercise. We kept track of it by calling them weekly and asking about their activities. In addition to that, the treadmill test showed their unchanged aerobic fitness at the end of the intervention period.
2.8. MRI preprocessing
To calculate cortical thickness and hippocampal volume, structural T1-weighted images were analyzed using FreeSurfer software (V. 5.3.) (http://surfer.nmr.mgh.harvard.edu/) [28]. Images were corrected for inhomogeneity from magnetic field, ranged to Talairach and Tournoux atlas [29], and skull-stripped. Voxels were labeled as white and gray matter, and CSF. Cortical thickness was calculated as the shortest distance between the pial and white surface at each vertex across the cortical mantle. We used a Gaussian filter with 10-mm full width half maximum for smoothing. We estimated total intracranial volume, and the volume for both hippocampus was calculated. We considered 34 bilateral cortical regions defined by parcellation according to the anatomic atlas of Desikan et al. [30]. All the metrics were analyzed later on, using IBM Statistical Package for the Social Sciences version 22 (SPSS Inc, Chicago, IL).
2.9. Statistical analysis
Using SPSS, we first tested the normal distribution of the data using the Kolmogorov-Smirnov test. To verify if there were differences between groups at baseline (time 1), we did a t test for independent samples and χ2 test was used for comparison of categorical variables, such as sex. Groups were considered similar when P > .05. The intervention effect was first analyzed using analysis of variance (ANOVA) for repeated measures with “group” (T and NT) as between-groups factor and “time” (time 1 and time 2) as within-groups factor. We aimed to verify the intervention effect on the following dependent variables: cognition, functional dependency, aerobic fitness, cortical thickness, and hippocampal volume. As our data satisfied the sphericity condition (Mauchly's test, P > .05), SPSS already corrects for multiple comparisons [31].
3. Results
3.1. Baseline group comparison
Comparison between T and NT at time 1 showed that there was no significant difference between groups in any baseline characteristics (Table 1).
Table1.
Comparison of baseline characteristics between training (T) and nontraining (NT) groups
| Characteristics | T | NT | P value |
|---|---|---|---|
| Gender (% female) | 60% | 61% | .94 |
| Age (y) | 69.4 (5.2) | 68.9 (5.5) | .86 |
| Education (y) | 8.3 (5.7) | 6.9 (4.2) | .25 |
| Body mass index | 26.3 (4.2) | 27.5 (3.9) | .44 |
| Medicine for, n (%) | |||
| Hypertension | 6 (30) | 6 (30) | .82 |
| Diabetes | 3 (15) | 2 (10) | .72 |
| Dyslipidemia | 4 (20) | 1 (5) | .34 |
| Depressive symptoms | 7 (35) | 6 (30) | .91 |
| Anticholinesterase | 2 (10) | 3 (3) | .72 |
| Cognitive performance | |||
| PFS score | 1.9 (3.1) | 1.8 (3.6) | .95 |
| MMSE score | 26.3 (2.1) | 25.5 (2.2) | 1.0 |
| RAVLT score | 31.0 (8.0) | 35.7 (15.6) | .10 |
| RAVLT A7 | 4.0 (2.7) | 4.9 (3.5) | .15 |
| RAVLT Rec | 5.5 (6.1) | 9.3 (4.6) | .34 |
| BDI | 6.2 (6.4) | 7.2 (9.6) | .14 |
| CSF biomarkers | |||
| Aβ1–42 (pg/mL) | 370.4 (195.6) | 355.16 (118.8) | .78 |
| Total tau (pg/mL) | 86.3 (52.3) | 74.7 (37.7) | .64 |
| p-tau (pg/mL) | 38.3 (20.3) | 43.5 (24) | .22 |
| Physical performance | |||
| IPAQ (METs/wk) | 18.6 (18.5) | 18.2 (20.8) | .72 |
| VO2max (mL/kg/min) | 19.7 (5.4) | 18.9 (3.6) | .08 |
Abbreviations: Aβ1–42, amyloid beta; BDI, Beck Depressive Inventory; CSF, cerebral spinal fluid; IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent of task; MMSE, Mini-Mental State Examination; PFS, Pfeffer Functional Scale; p-tau, tau phosphorylated; RAVLT encoding, encoding of Rey Auditory Verbal Learning Test; RAVLT A7, delayed recall of Rey Auditory Verbal Learning Test; RAVLT Rec, Rey Auditory Verbal Learning Test true recognition (i.e., recognition minus false positives); Total tau, total tau protein; VO2max, maximum oxygen intake.
NOTE. Data are presented as average (standard deviation).
3.2. Aerobic fitness change
Repeated measures ANOVA yielded a significant between-groups interaction for VO2max, indicating a difference between the two groups in maximum oxygen consumption after the intervention (higher in the T group). Paired t test showed a significant decrease in VO2max for the NT group after 6 months, whereas the T group had a stable VO2max (Table 2, Fig. 2).
Table 2.
Effects of intervention in aerobic fitness, neuropsychological tests, and functional activity in training (T) and nontraining (NT) groups
| T |
NT |
Between-group (T time 2 vs. NT time 2) |
T paired t test (time 2 − time 1) |
NT paired t test T (time 2 − time 1) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Time 1 | Time 2 | Time 1 | Time 2 | F | P value | t | P value | t | P value |
| VO2max | 19.7 (5.4) | 21.8 (6.5) | 18.9 (3.6) | 15.9 (3.9) | 15.7 | .004 | −1.7 | .09 | 7.0 | .00002 |
| PFS | 1.9 (3.1) | 1.5 (1.7) | 1.8 (3.6) | 2.6 (2.9) | 5.7 | .034 | 0.98 | .35 | −3.19 | .009 |
| MMSE | 26.3 (2.1) | 24.53 (6.2) | 25.5 (2.2) | 25.95 (2.5) | 1.3 | .26 | 1.6 | .13 | 0.32 | .75 |
| RAVLT | 31.0 (8.0) | 34.6 (11.3) | 35.3 (15.0) | 34.6 (10.0) | 0.16 | .68 | −0.52 | .61 | 0.54 | .60 |
| RAVLT A7 | 4.0 (2.7) | 5.9 (5.1) | 4.9 (3.5) | 5.2 (3.6) | 4.6 | .045 | −2.4 | .04 | 0.35 | .73 |
| RAVLT Rec | 5.5 (6.1) | 8.6 (9.6) | 9.3 (4.6) | 5.1 (8.8) | 7.8 | .018 | −1.51 | .15 | 2.8 | .02 |
Abbreviations: PFS, Pfeffer Functional Scale; MMSE, Mini-Mental State Examination; RAVLT encoding, encoding of Rey Auditory Verbal Learning Test; RAVLT A7, delayed recall of Rey Auditory Verbal Learning Test; RAVLT Rec, Rey Auditory Verbal Learning Test true recognition (i.e., recognition minus false positives); VO2max, maximum oxygen intake.
NOTE. Data are presented as average ± standard deviation. P values are in bold with statistical significant (P < .05 or P < .01).
Fig. 2.
Differences in VO2max after 6 months in training and nontraining groups. ∗Significant interaction between-groups; †significant interaction within-groups.
3.3. Cognition and functional activities changes
ANOVA for repeated measures indicated a significant time × group (between-group) interaction in Pfeffer Functional Scale. RAVLT A7, and RAVLT Rec, indicating a difference between T and NT in functional and memory scales after the intervention. Paired t test demonstrated an improvement in RAVLT A7 for the T group, and a significant worse result in Pfeffer and RAVLT Rec in the NT group after the training period (Table 2).
3.4. Cortical thickness change
T group showed increased values in various cortical areas after the intervention period, whereas the NT group showed significant atrophy in those areas. As shown in Table 3, ANOVA displayed a significant time × group interaction for several areas in the left and right hemispheres. However, such results did not survive after false discovery rate correction for multiplicity.
Table 3.
Effects of intervention in cortical thickness in training (T) and nontraining (NT) groups
| T |
NT |
Between-group (T time 2 vs. NT time 2) uncorrected |
Between-group (T time 2 vs. NT time 2) false discovery rate corrected |
||||
|---|---|---|---|---|---|---|---|
| Cortical areas | Time 1 | Time 2 | Time 1 | Time 2 | F | P value | P value |
| Bank of superior temporal sulcus | 2.22 (0.2) | 2.26 (0.1) | 2.21 (0.1) | 2.15 (0.1) | 6.48 | .016 | .07 |
| Caudal anterior cingulate gyrus | 2.54 (0.2) | 2.64 (0.3) | 2.64 (0.3) | 2.60 (0.3) | 4.58 | .041 | .13 |
| Entorhinal cortex | 3.14 (0.4) | 3.24 (0.3) | 3.20 (0.5) | 3.10 (0.5) | 8.92 | .006 | .06 |
| Fusiform gyrus | 2.43 (0.2) | 2.55 (0.2) | 2.52 (0.2) | 2.52 (0.2) | 7.52 | .01 | .06 |
| LH | |||||||
| Lingual gyrus | 1.78 (0.1) | 1.84 (0.1) | 1.82 (0.1) | 1.82 (0.1) | 7.25 | .011 | .31 |
| Inferior temporal gyrus | 2.48 (0.2) | 2.55 (0.2) | 2.57 (0.2) | 2.55 (0.2) | 8.14 | .008 | .39 |
| Middle temporal gyrus | 2.53 (0.2) | 2.60 (0.2) | 2.53 (0.1) | 2.53 (0.2) | 8.12 | .008 | .30 |
| Parahippocampal gyrus | 2.55 (0.3) | 2.60 (0.3) | 2.70 (0.3) | 2.66 (0.2) | 4.16 | .049 | .39 |
| Supra marginal gyrus | 2.21 (0.2) | 2.28 (0.1) | 2.24 (0.1) | 2.23 (0.1) | 8.87 | .006 | .39 |
| Temporal pole | 3.56 (0.3) | 3.67 (0.3) | 3.42 (0.3) | 3.41 (0.3) | 6.2 | .019 | .07 |
| Insula gyrus | 2.75 (0.1) | 2.8 (0.2) | 2.70 (0.2) | 2.63 (0.2) | 6.64 | .015 | .27 |
| RH | |||||||
| Isthmus cingulate gyrus | 2.31 (0.3) | 2.22 (0.2) | 2.22 (0.2) | 2.23 (0.2) | 5.11 | .031 | .35 |
| Pars orbitalis | 2.44 (0.2) | 2.48 (0.2) | 2.44 (0.2) | 2.40 (0.2) | 4.36 | .045 | .38 |
| Pars triangularis gyrus | 2.20 (0.1) | 2.23 (0.2) | 2.19 (0.1) | 2.15 (0.1) | 8.79 | .006 | .19 |
Abbreviations: CC, corpus callosum; FL, frontal lobe; FTPL, frontotemporoparietal area; LH, left hemisphere; OL, occipital lobe; PL, parietal lobe; RH, right hemisphere; TL,temporal lobe; TOL, temporal-occipital lobe.
NOTE. Data are presented as average (standard deviation).
3.5. Hippocampal volume change
As our group of interest was aMCI, we wanted to focus on hippocampal volume. Regarding this important area, ANOVA demonstrated a significant interaction in time × group for both right and left hippocampi (Table 4). Analysis within-groups confirmed a significant change only for the T group in both hippocampi, whereas NT did not present any significant difference.
Table 4.
Changes in hippocampal volume after the intervention period and comparison between groups
| T |
NT |
Between-group (T time 2 vs. NT time 2) |
Within-group T (time 2 − time 1) |
Within-group NT (time 2 − time 1) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | F | P | t | P | t | P | |
| Left hippocampal volume (mm³) | 3258.5 (687.8) | 3538.8 (416.0) | 3486.3 (635.8) | 3423.6 (665.5) | 13.7 | .00003 | −2.9 | .007 | 1.04 | .31 |
| Right hippocampal volume (mm³) | 3369.9 (714.6) | 3663.6 (488.1) | 3584.8 (606.9) | 3544.8 (678.8) | 8.0 | .001 | −2.4 | .03 | 0.56 | .58 |
NOTE. Data are presented as average (standard deviation). P values are in bold with statistical significant (P < .05 or P < .01).
4. Discussion
In the present article, 6 months of MEP training was effective in maintaining cardiorespiratory fitness and increasing significantly right and left hippocampal volume in a sample with aMCI due to AD. Furthermore, the group that participated in the intervention showed better episodic memory and maintained functional activities, whereas the group that did not participate decreased in those variables. To our knowledge, this is the first study that examines the effect of an MEP training on cerebral and clinical characteristics of aMCI with pathophysiological evidence for AD.
The aMCI group who undertook the MEP training showed a significant increase in volume in left, right, and total hippocampus of 9.64%, 8.72%, and 9.18%, respectively, after the 6-month period, whereas the group that did not engage in such training presented a volume loss of 2.82%, 2.19%, and 2.51% in the same regions. The increased hippocampal volume found here corroborate with a recent investigation, in which the participants with probable MCI had higher hippocampal volume by 5.6%, 2.5%, and 4% for left, right, and total hippocampus after 6 months of aerobic training [10]. The decrease in hippocampal volume found in the NT group also aligns with the atrophy expected for MCI subjects previously published (i.e., 2.53% per year) [32]. Our primary finding about the increase in hippocampal volume after the intervention period for the group that engaged in physical training concurs and extends previous observations [3], [10], [33].
The positive impact of training on VO2 peak should also be mentioned. Our study showed that MEP training using an intensity based on aerobic threshold was effective in maintaining VO2 peak in elderly with aMCI due to AD. VO2max decline rate is around 20% per decade at the age of 70 and older, demonstrating a much higher rate when compared with 3% to 6% rate in the 20s and 30s [34]. Although for most healthy adults VO2max has little bearing on everyday life (and is perhaps of lesser practical concern), in the aging population the decline in VO2max is of such importance that the ability of elderly individuals to perform everyday tasks become greatly dependent on VO2max [35]. Maintaining aerobic fitness means, to the aging population, preventing or delaying everyday tasks' dependency, reducing mobility, and mortality.
Besides increase in hippocampal volume and aerobic fitness, the T group showed better episodic memory (recall and recognition) after the intervention, whereas the group that did not undertake the intervention performed worse in episodic memory (recognition) and daily functional activities tests. Although previous studies have already found a positive impact of aerobic exercise on cognition of MCI [36], [37], [38], [39], the present work differs from the others by its inclusion criteria. For instance, even mild levels of depression can produce significant functional impairment [40], and exercise is known to affect mood/apathy [41]. Because our aMCI participants had pathophysiological evidence of AD and the absence of depressive symptoms, we can say with relative certainty that the cognitive changes observed after the training period were at least partly because of the effects of intervention.
Under the biological underpinnings, physical exercise affects some biological mechanisms that contribute to improve neurocognitive function, such as enhanced cerebral blood flow, synthesis of neurotransmitters, vascular brain neurogenesis, and regulation of neurotrophic factors [42], [43], [44]. A recent study demonstrated, with a 3-month aerobic training in healthy older adults, improvement in memory and recognition and hippocampal volume are related to increased hippocampal perfusion because of better fitness [5]. Aerobic training may increase hippocampal volume by increasing levels of a brain-derived neurotrophic factor, highly concentrated in hippocampus and cortex [45], which stimulate neurogenesis and increase the complexity of dendritic network [3], [46]. Moreover, exercise is known as a powerful “tool” against metabolic and cerebrovascular disease [47] and neuroinflammation, which are known to increase the risk of AD [48]. Indeed, literature has shown the protective role of physical exercise against proinflammatory cytokines such as tumor necrosis factor-alpha and interleukin 6 [39], [49], which seem to be higher in MCI subjects [50], [51], [52].
Our study has one major limitation that must be acknowledged: the individuals were not randomly allocated to the two different interventions, instead, the subjects were trained or not based on their own interest and willingness. Among its statistic disadvantages, nonprobability sampling does not allow us to know how well we are representing the population, and we face difficulties in estimating sampling variability and identifying possible bias. Therefore, we cannot certainly claim that the alterations observed after the follow-up period were exclusive because of the MEP training or consequence of other factors related to the willingness of someone in engaging in such trainings. However, we tried to control and balance as much as possible other possible confounding factors between the two groups, such as incidence of hypertension, diabetes, dyslipidemia, depressive symptoms, use of medication, pathophysiological level of tau and Aβ1–42, and physical and cognitive performance.
One of the National Institutes of Health Consensus and State-of-the Science Statements concluded that although there is no conclusive evidence that modifiable factor is associated with reduction in the risk for AD, exercise interventions seem to present promising results [53]. The long preclinical phase of AD provides a critical opportunity for potential interventions, trying to change the trajectory of cognitive decline and loss of independency known to occur in patients with AD. Furthermore, therapeutic interventions applied earlier would be more likely to modify the course of disease. Most studies in the literature, however, present a great variability in MCI characterization [54], in which a substantial proportion of them will never convert to dementia and will remain stable over years in their cognitive functioning. Including only aMCI with pathophysiological evidence of AD increases the chance that our patients are really at a prodromal stage of AD.
Taken together, we found that supervised MEP training not only contributes to the improvement in cardiorespiratory fitness, memory performance, and functionality, but also increases hippocampal volume in aMCI subjects due to AD. Highlighting the many benefits of physical exercise for aMCI subjects, future research should look into different intensities of exercise, longer durations, and also follow-ups, focusing on the mechanisms that explore fully the direction and nature of the association among physical exercise, cognition, and brain.
Research in Context.
-
1.
Systematic review: Increasing evidence demonstrates that physical exercise is an important modifiable factor not only for cardiovascular fitness but also for brain health and dementia prevention. However, most of the studies, so far, have showed the results on amnestic mild cognitive impairment (MCI) clinically diagnosed without separating them in amnestic MCI with CSF positive or negative Alzheimer's disease biomarkers. This is of great relevance given the fact that there are MCI converters and nonconverters based on the pathophysiological changes. Thus, we found it important to verify the effect of physical exercise on this population.
-
2.
Interpretation: Our findings led to a conclusion that, even with the pathophysiology, the practice of physical exercise can still be beneficial to this population.
-
3.
Future directions: The study shows how powerful physical exercise can be, but there is still need to observe and control factors such as previous brain atrophy, genetic risk, different exercise intensities, and follow-up after the intervention period.
Acknowledgments
The authors would like to thank BRAINN—Brazilian Institute of Neuroscience and Neurotechnology.
Funding sources by Fundação da Amparo à Pesquisa do Estado de São Paulo—Fapesp n.2014/02359-9, n.2018/05546-5.
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Teixeira C.V., Gobbi S., Pereira J.R., Vital T.M., Hernandez S.S., Shigematsu R. Effects of square-stepping exercise on cognitive functions of older people. Psychogeriatrics. 2013;13:148–156. doi: 10.1111/psyg.12017. [DOI] [PubMed] [Google Scholar]
- 2.Liu-Ambrose T., Nagamatsu L.S., Voss M.W., Khan K.M., Handy T.C. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33:1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maass A., Duzel S., Brigadski T., Goerke M., Becke A., Sobieray U. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2015;131:142–154. doi: 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 5.Maass A., Duzel S., Goerke M., Becke A., Sobieray U., Neumann K. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2014;20:585–593. doi: 10.1038/mp.2014.114. [DOI] [PubMed] [Google Scholar]
- 6.Smith J.C., Nielson K.A., Woodard J.L., Seidenberg M., Durgerian S., Hazlett K.E. Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease. Front Aging Neurosci. 2014;6:61. doi: 10.3389/fnagi.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greblo J.Z., Krizanic V., Sarabon N., Markovic G. Effects of feedback-based balance and core resistance training vs. Pilates training on cognitive functions in older women with mild cognitive impairment: a pilot randomized controlled trial. Aging Clin Exp Res. 2017;29:1295–1298. doi: 10.1007/s40520-017-0740-9. [DOI] [PubMed] [Google Scholar]
- 8.Nagamatsu L.S., Chan A., Davis J.C., Beattie B.L., Graf P., Voss M.W. Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. J Aging Res. 2013;2013:861–893. doi: 10.1155/2013/861893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagamatsu L.S., Handy T.C., Hsu C.L., Voss M., Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ten Brinke L.F., Bolandzadeh N., Nagamatsu L.S., Hsu C.L., Davis J.C., Miran-Khan K. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br J Sports Med. 2015;49:248–254. doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirk-Sanchez N.J., McGough E.L. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. 2012;12:128. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampaio A., Marques E.A., Mota J., Carvalho J. Effects of a multicomponent exercise program in institutionalized elders with Alzheimer's disease. Dementia (London) 2016 doi: 10.1177/1471301216674558. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Ito K. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One. 2013;8:e61483. doi: 10.1371/journal.pone.0061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. United States: 2011 The Alzheimer's Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj A., Kuceyeski A., Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73:1204–1215. doi: 10.1016/j.neuron.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Forlenza O.V., Radanovic M., Talib L.L., Aprahamian I., Diniz B.S., Zetterberg H. Cerebrospinal fluid biomarkers in Alzheimer's disease: diagnostic accuracy and prediction of dementia. Alzheimers Dement (Amst) 2015;1:455–463. doi: 10.1016/j.dadm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malloy-Diniz L.F., Lasmar V.A., Gazinelli Lde S., Fuentes D., Salgado J.V. The Rey Auditory-Verbal Learning Test: applicability for the Brazilian elderly. Rev Bras Psiquiatr. 2007;29:324–329. doi: 10.1590/s1516-44462006005000053. [DOI] [PubMed] [Google Scholar]
- 20.Hachinski V., Iadecola C., Petersen R.C., Breteler M.M., Nyenhuis D.L., Black S.E. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 21.Fazekas F., Chawluk J., Alavi A., Hurtig H., Zimmerman R. MRI signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJNR Am J Neuroradiol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 22.McArdle W.D., Katch F.I., Katch V.L. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA: 2010. Exercise physiology energy, nutrition, and human performance. 7th ed. [Google Scholar]
- 23.Ghosh A.K. Anaerobic threshold: its concept and role in endurance sport. Malays J Med Sci. 2004;11:24–36. [PMC free article] [PubMed] [Google Scholar]
- 24.Craig C.L., Marshall A.L., Sjostrom M., Bauman A.E., Booth M.L., Ainsworth B.E. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 25.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. England. [DOI] [PubMed] [Google Scholar]
- 26.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer R.I., Kurosaki T.T., Harrah C.H., Jr., Chance J.M., Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talairach J.T.P. Thieme; New York: 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 30.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Park E., Cho M., Ki C.S. Correct use of repeated measures analysis of variance. Korean J Lab Med. 2009;29:1–9. doi: 10.3343/kjlm.2009.29.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Tabatabaei-Jafari H., Shaw M.E., Cherbuin N. Cerebral atrophy in mild cognitive impairment: a systematic review with meta-analysis. Alzheimers Dement (Amst) 2015;1:487–504. doi: 10.1016/j.dadm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aribisala B.S., Gow A.J., Bastin M.E., del Carmen Valdes Hernandez M., Murray C., Royle N.A. Associations between level and change in physical function and brain volumes. PLoS One. 2013;8:e80386. doi: 10.1371/journal.pone.0080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleg J.L., Morrell C.H., Bos A.G., Brant L.J., Talbot L.A., Wright J.G. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 35.Paterson D.H., Govindasamy D., Vidmar M., Cunningham D.A., Koval J.J. Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc. 2004;52:1632–1638. doi: 10.1111/j.1532-5415.2004.52454.x. [DOI] [PubMed] [Google Scholar]
- 36.Porto F.H., Coutinho A.M., Pinto A.L., Gualano B., Duran F.L., Prando S. Effects of aerobic training on cognition and brain glucose metabolism in subjects with mild cognitive impairment. J Alzheimers Dis. 2015;46:747–760. doi: 10.3233/JAD-150033. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira C.V., Gobbi L.T., Corazza D.I., Stella F., Costa J.L., Gobbi S. Non-pharmacological interventions on cognitive functions in older people with mild cognitive impairment (MCI) Arch Gerontol Geriatr. 2012;54:175–180. doi: 10.1016/j.archger.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Strohle A., Schmidt D.K., Schultz F., Fricke N., Staden T., Hellweg R. Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry. 2015;23:1234–1249. doi: 10.1016/j.jagp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Nascimento C.M., Pereira J.R., de Andrade L.P., Garuffi M., Talib L.L., Forlenza O.V. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res. 2014;11:799–805. doi: 10.2174/156720501108140910122849. [DOI] [PubMed] [Google Scholar]
- 40.Defrancesco M., Marksteiner J., Kemmler G., Fleischhacker W.W., Blasko I., Deisenhammer E.A. Severity of depression impacts imminent conversion from mild cognitive impairment to Alzheimer's disease. J Alzheimers Dis. 2017;59:1439–1448. doi: 10.3233/JAD-161135. [DOI] [PubMed] [Google Scholar]
- 41.Deslandes H.S., Helena M., Natacha O., Evandro Silva Freire C., Jerson L., Andrea Patients: a systematic review and meta-analysis. Neuropsychobiology. 2017;67:61–68. [Google Scholar]
- 42.Zhang H.Y., Wang S.J., Xing J., Liu B., Ma Z.L., Yang M. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav Brain Res. 2009;197:103–108. doi: 10.1016/j.bbr.2008.08.012. Netherlands. [DOI] [PubMed] [Google Scholar]
- 43.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker J.A., Hedden T., Carmasin J., Maye J., Rentz D.M., Putcha D. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burock M.A., Dale A.M. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassilhas R.C., Lee K.S., Fernandes J., Oliveira M.G., Tufik S., Meeusen R. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Middleton L.E., Corbett D., Brooks D., Sage M.D., Macintosh B.J., McIlroy W.E. Physical activity in the prevention of ischemic stroke and improvement of outcomes: a narrative review. Neurosci Biobehav Rev. 2012;37:133–137. doi: 10.1016/j.neubiorev.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Honjo K., Black S.E., Verhoeff N.P. Alzheimer's disease, cerebrovascular disease, and the beta-amyloid cascade. Can J Neurol Sci. 2012;39:712–728. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- 49.Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., McTiernan A. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magaki S., Mueller C., Dickson C., Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trollor J.N., Smith E., Agars E., Kuan S.A., Baune B.T., Campbell L. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 2012;34:1295–1308. doi: 10.1007/s11357-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman S.H., Kandel R., Cruz L., Rozkalne A., Newell K., Frosch M.P. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:1205–1212. doi: 10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daviglus M.L., Bell C.C., Berrettini W., Bowen P.E., Connolly E.S., Jr., Cox N.J. NIH state-of-the-science conference statement: preventing Alzheimer's disease and cognitive decline. NIH consens State Sci Statements. 2010;27:1–30. [PubMed] [Google Scholar]
- 54.Roberts R., Knopman D.S. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]