Abstract
Introduction
To investigate whether baseline subjective cognitive complaints (SCCs) predict longitudinal decline on neuropsychological testing and whether SCC increases longitudinally, in the setting of high levels of amyloid burden.
Methods
Two hundred seventy-nine clinically normal older participants (mean age = 73.7 ± 6.1 years) from the Harvard Aging Brain Study, a cohort of community-dwelling individuals, were followed longitudinally (4.27 ± 1.35 years) with annual subjective memory questionnaires and neuropsychological assessment. 11C Pittsburgh compound-B positron emission tomography was used to measure cortical amyloid and to classify status (Aβ+/Aβ−) at baseline.
Results
Higher baseline SCC predicted more rapid cognitive decline on neuropsychological measures among those with elevated amyloid (t = −2.18, P < .0001). In addition, longitudinal report of SCC significantly increased over time, with SCC progression most pronounced among Aβ+ individuals (t = 2.24, P = .0005).
Discussion
SCC may inform risk for future cognitive decline and track progression of self-perceived decline, particularly in those along the AD trajectory, providing potentially important indicators of clinical meaningfulness in AD prevention trials.
Keywords: Preclinical Alzheimer's disease, Subjective cognitive decline, Amyloid, PET imaging
1. Introduction
Experiencing persistent subjective cognitive complaints (SCCs) in the absence of clinical impairment may represent one of the earliest manifestations of Alzheimer's disease (AD) [1], [2]. SCCs offer complementary information to standard neuropsychological assessment in that they reflect the person's own perspective, an important component in tracking early disease progression. Several cross-sectional studies have shown that greater SCCs, in older individuals who are otherwise clinically normal, are associated with AD biomarkers, including increased beta-amyloid (Aβ) and neurodegeneration [3], [4], [5], [6], [7] and greater entorhinal cortical tau burden [8]. Longitudinal studies using baseline SCC to predict cognitive and clinical outcomes have been mixed [9], [10], [11], [12], [13], [14], but a few recent studies have suggested that SCC in the context of elevated AD biomarkers predict worse cognitive and clinical outcomes than SCC alone [15], [16], [17].
The longitudinal trajectory of SCC, particularly in individuals with elevated AD biomarkers, is not known. While it has been shown that cognitive performance on neuropsychological assessment declines more steeply in individuals with preclinical AD [18], [19], [20], it has yet to be determined whether individuals with preclinical AD show an increase in SCC longitudinally. If a longitudinal increase in SCC was observed in individuals on the AD continuum, this would provide further support for the concept of SCC as valuable marker of AD, in that individuals perceive increasing cognitive difficulties as the disease progresses.
In the present study, we sought to examine the role of SCC in predicting and tracking disease progression in clinically normal individuals with abnormal Aβ levels measured by Pittsburgh compound B positron emission tomography (PIB-PET). First, we hypothesized that greater baseline SCC would predict steeper decline on objective neuropsychological measures particularly in the context of elevated Aβ compared to those with lower levels. Second, we predicted that SCC would increase more rapidly in individuals with abnormal Aβ compared to those with lower levels.
2. Methods
2.1. Participants
To be enrolled in HABS at baseline, participants were clinically normal, defined as a global score of 0 on the Clinical Dementia Rating Scale [21], greater than 25 on the Mini–Mental State Examination [22], less than 11 on the 30-item Geriatric Depression Scale [23], and normal performance within validated education-adjusted norms on Logical Memory II delayed recall [24]. A detailed review of medical history and functional performance as well as physical and neurologic examinations confirmed their status as clinically normal. None of the participants had a history of alcoholism, drug abuse, head trauma, or current serious medical or psychiatric illness. All study staff who assessed subjects clinically were blinded to the biomarker status of the subjects. This study included 279 HABS participants at baseline.
Participants were followed for an average of 4.27 ± 1.35 years (range: 2–6 years) with annual cognitive testing. Participants underwent APOE genotyping. Demographics at baseline can be found in Table 1. Study protocols were approved by the Partners Institutional Review Board, and all participants provided informed consent before undergoing any study procedures.
Table 1.
Demographics table at baseline
| All | Aβ+ | Aβ− | |
|---|---|---|---|
| N | 279 | 70 | 209 |
| Age (years) | 73.4 (6.1) | 75.0 (5.7) | 72.9 (6.0) |
| Sex (% female) | 59 | 61 | 59 |
| Years of education | 15.8 (3.0) | 16.3 (3.0) | 15.7 (3.1) |
| APOE ε4 status (% carriers) | 29 | 61 | 18 |
| Logical Memory delayed recall | 13.7 (3.3) | 14.0 (2.1) | 13.6 (3.3) |
| MMSE | 29.0 (1.1) | 28.8 (1.0) | 29.1 (1.1) |
| Free and Cued Selective Reminding Test (/96) | 80.9 (5.9) | 80.7 (6.0) | 81.0 (5.9) |
| Digit Symbol Coding Test | 47.23 (10.7) | 46.9 (10.0) | 47.3 (11) |
| Sum of STIDA (/7) | 1.14 (1.2) | 1.4 (1.3) | 1.03 (1.2) |
Abbreviations: MMSE, Mini–Mental State Examination; STIDA, Structured Telephone Interview of Dementia Assessment.
NOTE. Values represent mean (standard deviation) except for sex and amyloid status in which values represent percentages.
2.2. Test battery and timeline
HABS participants were administered a set of seven yes or no questions annually, adapted from the Structured Telephone Interview for Dementia Assessment (STIDA) used in a large epidemiological study of nurses for assessing cognitive change in older individuals [25], [26]. Answers on these questions (0 = no, 1 = yes) were added together to create a summary score that was used in statistical analyses. Subsequently, a z-score, using the baseline average, was calculated for each year of the study that was used in analyses. Higher scores on the STIDA z-score indicate greater subjective cognitive concerns.
Objective cognitive performance was examined using the Preclinical Alzheimer Cognitive Composite (PACC) [20], [27], a cognitive composite that has previously been shown to be sensitive to amyloid-related decline. The PACC comprised the following neuropsychological tests: (1) Logical Memory delayed recall [24] (2) Free and Cued Selective Reminding Test free and total cued recall score [28], (3) number completed on the digit-symbol test [29], and (4) Mini–Mental State Examination total score [22]. Measures were z-transformed based on the mean and standard deviation from the larger HABS baseline sample and averaged. Lower scores on the PACC z-score indicate lower cognitive performance. Participants were administered measures from the PACC annually.
2.3. PiB-PET imaging
11C Pittsburgh compound-B PET data were collected at baseline, as previously been described in detail [30]. PiB-PET cerebellar gray matter was used as the reference region from the Freesurfer aseg atlas as previously described [31], [32], and a summary distribution volume ratio was used. A composite PiB distribution volume ratio measure of cortical amyloid burden that comprised frontal, lateral, and retrosplenial regions [33] was calculated for each participant. Baseline amyloid status (Aβ+/Aβ−) was classified using a previously reported Gaussian mixture modeling approach with a cutoff of 1.19 [34].
2.4. Statistical analyses
Two sets of longitudinal analyses were conducted. First, linear mixed models in which baseline STIDA score was used to predict longitudinal PACC performance were assessed with covariates that included age, education, and sex. Second, linear mixed models in which baseline Aβ status was used to predict longitudinal STIDA scores were assessed with covariates that included age, education, and sex. We also ran secondary models with GDS and APOE ε4 carrier status in separate models.
In the first series of models, baseline STIDA score and covariates were used to predict PACC score (PACC ∼ STIDA*time + age*time + sex*time + education*time). Subsequent models included an interaction term between baseline Aβ status and STIDA score to predict PACC decline with covariates (PACC ∼ Aβstatus*STIDA*time + age*time + sex*time + education*time).
In addition, we examined the STIDA as a longitudinal outcome to determine whether there was an increase in report of symptoms over time, controlling for covariates (STIDA ∼ time + age*time + sex*time + education*time) and whether differences could be observed by Aβ group (STIDA ∼ Aβstatus*time + age*time + sex*time + education*time). All analyses using Aβ status were repeated using amyloid as continuous variable. We used the statistical package R version 3.3.2 for all statistical analyses.
3. Results
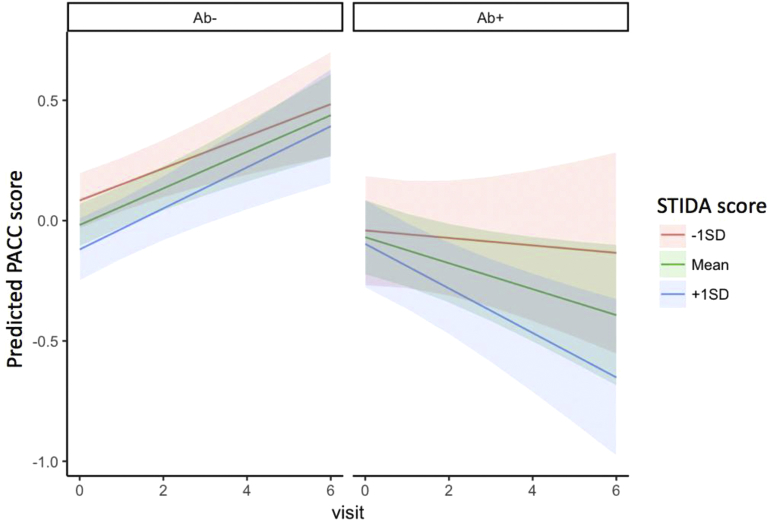
In the first set of longitudinal models, baseline STIDA score did not predict longitudinal PACC performance across participants (t = −1.3, P = .19). However, when we examined the impact of baseline Aβ status on the relationship between STIDA and PACC decline, the interaction term was significant (t = −2.18, P < .0001) such that higher STIDA score was more strongly associated with longitudinal PACC decline in Aβ+ individuals compared to Aβ− individuals (Fig. 1). Findings were also significant for all models when Aβ was used as a continuous measure (t = −3.13, P = .0018). GDS was not a significant independent predictor in the model and did not impact overall findings of SCC by Aβ to predict PACC. Similarly, adding APOE ε4 carrier status as an independent predictor in the model was not significant and did not impact overall findings of SCC to predict PACC.
Fig. 1.
STIDA predicting PACC decline separated by Aβ group. Visit occurs on an annual basis. Abbreviations: PACC, Preclinical Alzheimer Cognitive Composite; SD, standard deviation; STIDA, Structured Telephone Interview for Dementia Assessment.
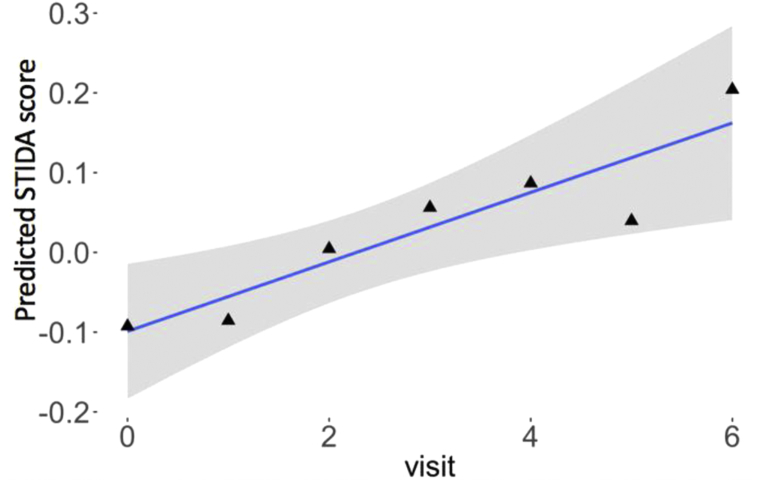
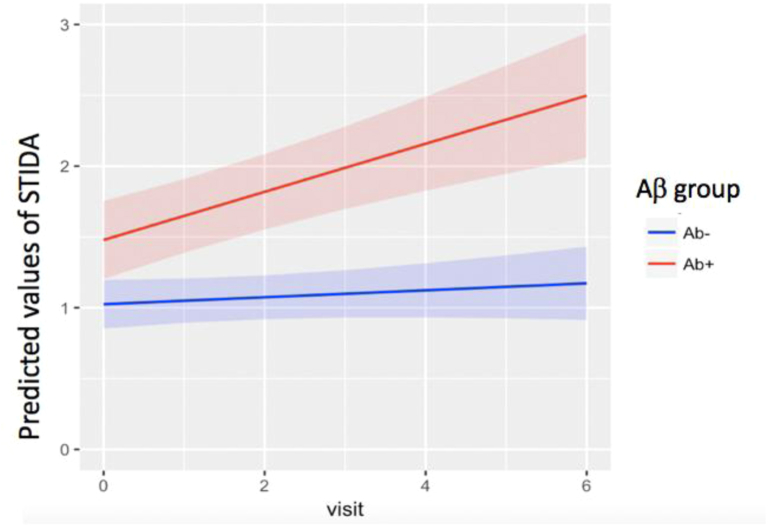
In the second set of models, we examined the longitudinal trajectory of the STIDA over the course of the study, controlling for covariates. Overall, STIDA score significantly increased over time (t = 2.24, P = .025) (Fig. 2). The time by Aβ group interaction was significant (t = 3.52, P = .0005), such that Aβ+ individuals demonstrated a greater increase in STIDA score over time compared to Aβ− individuals (Fig. 3). Taken in a different way, there was an annual increase in STIDA score of 0.03 in Aβ− individuals compared to 0.14 in Aβ+ individuals. When using amyloid as a continuous measure, the results were consistent with analyses using Aβ group, such that the interaction term with amyloid and time significantly predicted an increase in STIDA score (t = 2.52, P = .017). When GDS was added as an independent predictor, it did not significantly predict longitudinal SCC. When APOE ε4 carrier status was included in the model, APOE ε4 carrier status was not a significant predictor, but Aβ positivity remained a significant predictor of increasing SCC.
Fig. 2.
Greater endorsement on STIDA over the course of the study. Visit occurs on an annual basis. Abbreviation: STIDA, Structured Telephone Interview for Dementia Assessment.
Fig. 3.
Longitudinal STIDA by baseline Aβ group. Visit occurs on an annual basis. Abbreviations: STIDA, Structured Telephone Interview for Dementia Assessment; Aβ group, amyloid group.
4. Discussion
In the present study, we found that higher self-report of cognitive complaints (i.e., STIDA) at baseline was associated with longitudinal cognitive decline on a neuropsychological composite sensitive to change in preclinical AD (i.e., PACC) in the setting of elevated Aβ. In addition, we found that SCC showed an overall increase during the study and that this increase was most evident in individuals who were Aβ+ at baseline.
Only a few previous studies have investigated the role of SCC in predicting longitudinal outcomes in the context of AD biomarker positivity. One previous study [15] demonstrated that in clinically normal individuals who were Aβ+, greater SCC predicted greater rates of clinical progression to mild cognitive impairment or dementia, although they did not find evidence of higher rates of cognitive decline in this relatively small sample. In studies examining memory clinic patients who had memory concerns but were cognitively unimpaired on testing, one found that reduced glucose metabolism in the right precuneus at baseline predicted memory decline [16] and another found steeper cognitive decline was related to evidence of preclinical AD based on cerebrospinal fluid biomarkers [17]. Our results in the present study are in keeping with these findings, but unique in that our sample includes individuals recruited from the community who were not selected on the basis of SCC or from clinics where patients were evaluated for cognitive concerns.
Very limited studies have investigated the trajectory of SCC with longitudinal subjective report. The few studies that have compared longitudinal SCC with longitudinal cognitive performance found alignment between these measures in cognitively unimpaired individuals [35], [36]. Not surprisingly, however, longitudinal self-report trajectories and neuropsychological performance were shown to diverge as individuals reached dementia, when anosognosia becomes quite common [37]. In the present study, we had the advantage of examining the natural course of SCC progression in individuals thought to be in the preclinical stages of AD based on Aβ biomarker evidence. Our findings are in support of the notion that SCC does in fact reflect progression of AD, as an increase in SCC was observed in Aβ+ individuals, even after controlling for the impact of age and other covariates.
A few limitations to the present study are worth highlighting. The SCC measure used in the present study was brief; however, we were nonetheless able to observe change over time on this measure. Furthermore, its brevity makes it more appropriate for clinical settings as in lengthy AD prevention trials. In addition, our sample was community based and participants were not required to have baseline SCC to participate in the study. While we, nonetheless, observed a longitudinal increase in SCC symptoms in our sample, it will be important to evaluate if this effect is even stronger in individuals who report high levels of SCC, such as from a memory clinic setting [38].
In the context of elevated amyloid, we provide evidence for the potential added value of SCC assessment to predict and track cognitive decline, as well as to understand disease progression from the person's own perspective that may impact everyday functioning. Longitudinal monitoring of SCC has the potential to offer insight into clinically meaningful therapeutic effects of interventions being tested in clinical trials that cannot be fully realized by objective cognitive test measures alone. Importantly, our findings suggest that longitudinal assessment of SCC in the setting of preclinical AD biomarkers may be particularly valuable for tracking progression of the earliest symptomatic changes of AD.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. While there is growing consensus that subjective cognitive complaints (SCCs) is a useful marker within the context of preclinical Alzheimer's disease (AD), no studies have examined longitudinal SCC report in clinically normal older individuals with elevated amyloid.
-
2.
Interpretation: We demonstrate that SCC report increases longitudinally in individuals with elevated amyloid. Our findings suggest that longitudinal assessment of SCCs in the setting of preclinical AD biomarkers may be particularly valuable for tracking progression of the earliest symptomatic changes of AD.
-
3.
Future directions: Secondary AD prevention trials are in search of sensitive tools to track clinically meaningful treatment effects in response to interventions. This article provides support for using SCC questionnaires as an outcome measure in prevention trials.
Acknowledgments
R. Amariglio was involved with the study concept, drafting of the article, and analysis of the data. She is supported by Alzheimer's Association AARG-17-529011 and NIH grant K23AG044431. She is a coinvestigator for Eisai, Eli Lilly, Biogen, and Merck. These relationships are not related to the content in the article.
R. Buckley was involved with the revising of the article, study concept, and analysis of the data. She is supported by the NHMRC Dementia Research Fellowship (APP1105576).
E. Mormino was involved with the study concept and analysis of the data. She received funding from NIH grant NIA K01-AG051718 and the Stanford Neuroscience Institute.
G. Marshall was involved in the revising of the article. He has served as a paid consultant for Halloran Consulting Group and Grifols. He is a site principal investigator or coinvestigator for Janssen Research & Development, LLC, Eisai Inc., Eli Lilly and Company, Novartis, AbbVie, Merck, and Biogen clinical trials. These relationships are not related to the content in the article. G. Marshall receives research support for NIH grants R01 AG027435 and P01 AG036694.
K. Johnson was involved in the revising of the article and the interpretation of data. He has served as a paid consultant for Biogen, GE Healthcare, Janssen, Piramal, Genentech, Novartis, Lilly, Lundbeck, Roche, Merck, and Genzyme. He is a site principal investigator or coinvestigator for Lilly/Avid, Biogen, Merck, Eisai, Lilly, clinical trials. These relationships are not related to the content in the article. K. Johnson receives research support from NIH grants R01EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435, and R01 AG037497.
D. Rentz was involved in the revising of the article and interpretation of data. She received research support from the NIH grants P01 AG036694, U01 AG024904, R01 AG027435, R01 AG037497, and P50 AG005134, F-Prime investigator-initiated grant. She is a consultant for Eli Lilly, Janssen Pharmaceuticals, Biogen IDEC, and on the scientific advisory board for Neurotrack. These relationships are not related to the content in the article.
R. Sperling was involved in the revising of the article, study design, and interpretation of data. She has served as a paid consultant for Abbvie, Biogen, Genentech, Bracket, Roche, Sanofi, Lundbeck, Otsuka, Merck, Pfizer, General Electric. She is a site principal investigator/coinvestigator for grants from the National Institutes of Health, Alzheimer's Association, Harvard Neurodiscovery Center, Fidelity Neurosciences, Eli Lilly, and Janssen. She receives clinical research funding from Eli Lilly and Janssen. R. Sperling receives research support for NIH grants P01 AG036694, U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435. These relationships are not related to the content in the article.
References
- 1.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack C., Bennett D., Blennow K., Carrillo M., Dunn B., Haeberlein S. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amariglio R.E., Becker J.A., Carmasin J., Wadsworth L.P., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amariglio R.E., Mormino E.C., Pietras A.C., Marshall G.A., Vannini P., Johnson K.A. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology. 2015;85:56–62. doi: 10.1212/WNL.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F., Feyen L., Freymann K., Tepest R., Maier W., Heun R. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Mosconi L., De Santi S., Brys M., Tsui W.H., Pirraglia E., Glodzik-Sobanska L. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saykin A.J., Wishart H.A., Rabin L.A., Santulli R.B., Flashman L.A., West J.D. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley R.F., Hanseeuw B., Schultz A.P., Vannini P., Aghjayan S.L., Properzi M.J. Region-specific association of subjective cognitive decline with tauopathy independent of global beta-Amyloid burden. JAMA Neurol. 2017;74:1455–1463. doi: 10.1001/jamaneurol.2017.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dik M.G., Jonker C., Comijs H.C., Bouter L.M., Twisk J.W., van Kamp G.J. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57:2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- 10.Dufouil C., Fuhrer R., Alperovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 11.Hohman T.J., Beason-Held L.L., Lamar M., Resnick S.M. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology. 2011;25:125–130. doi: 10.1037/a0020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaup A.R., Nettiksimmons J., LeBlanc E.S., Yaffe K. Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology. 2015;85:1852–1858. doi: 10.1212/WNL.0000000000002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reisberg B., Shulman M.B., Torossian C., Leng L., Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., van Belle G., Crane P.K., Kukull W.A., Bowen J.D., McCormick W.C. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 15.Buckley R.F., Maruff P., Ames D., Bourgeat P., Martins R.N., Masters C.L. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement. 2016;12:796–804. doi: 10.1016/j.jalz.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Scheef L., Spottke A., Daerr M., Joe A., Striepens N., Kolsch H. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 17.van Harten A.C., Smits L.L., Teunissen C.E., Visser P.J., Koene T., Blankenstein M.A. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81:1409–1416. doi: 10.1212/WNL.0b013e3182a8418b. [DOI] [PubMed] [Google Scholar]
- 18.Knopman D.S., Jack C.R., Jr, Wiste H.J., Weigand S.D., Vemuri P., Lowe V. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Amariglio R.E., Rentz D.M. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71:1379–1385. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mormino E.C., Papp K.V., Rentz D.M., Donohue M.C., Amariglio R., Quiroz Y.T. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated amyloid-beta. Alzheimers Dement. 2017;13:1004–1012. doi: 10.1016/j.jalz.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Yesavage J.A. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 24.Wechsler D. The Psychological Corporation, Harcourt Brace Jovanovich, Inc; New York: 1987. Wechsler Memory Scale Revised Manual. [Google Scholar]
- 25.Amariglio R.E., Townsend M.K., Grodstein F., Sperling R.A., Rentz D.M. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59:1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go R.C., Duke L.W., Harrell L.E., Cody H., Bassett S.S., Folstein M.F. Development and validation of a structured telephone interview for dementia assessment (STIDA): the NIMH Genetics Initiative. J Geriatr Psychiatry Neurol. 1997;10:161–167. doi: 10.1177/089198879701000407. [DOI] [PubMed] [Google Scholar]
- 27.Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grober E., Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13–36. [Google Scholar]
- 29.Wechsler D. The Psychological Corporation; San Antonio: 1981. WAIS-R manual: Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- 30.Sperling R.A., LaViolette P.S., O'Keefe K., O'Brien J., Rentz D.M., Pihlajamaki M. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker J.A., Hedden T., Carmasin J., Maye J., Rentz D.M., Putcha D. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson K.A., Schultz A., Betensky R.A., Becker J.A., Sepulcre J., Rentz D. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price J.C., Klunk W.E., Lopresti B.J., Lu X., Hoge J.A., Ziolko S.K. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 34.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Ward A., Huijbers W. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amariglio R.E., Donohue M.C., Marshall G.A., Rentz D.M., Salmon D.P., Ferris S.H. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer's Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015;72:446–454. doi: 10.1001/jamaneurol.2014.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parisi J.M., Gross A.L., Rebok G.W., Saczynski J.S., Crowe M., Cook S.E. Modeling change in memory performance and memory perceptions: findings from the ACTIVE study. Psychol Aging. 2011;26:518–524. doi: 10.1037/a0022458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snitz B.E., Small B.J., Wang T., Chang C.C., Hughes T.F., Ganguli M. Do subjective memory complaints lead or follow objective cognitive change? A Five-Year Population Study of Temporal Influence. J Int Neuropsychol Soc. 2015;21:732–742. doi: 10.1017/S1355617715000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snitz B.E., Wang T., Cloonan Y.K., Jacobsen E., Chang C.H., Hughes T.F. Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimers Dement. 2018;14:734–742. doi: 10.1016/j.jalz.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]