Abstract
Introduction
Caregivers of individuals with dementia are at heightened risk for stress-related mental and physical illnesses, and this problem is growing. There is a critical need to develop effective interventions for caregivers. This study tested whether a 2-day intervention improved psychological health in caregivers of individuals with dementia.
Methods
Family caregivers (N = 104) were randomly assigned to a 2-day intervention or wait-list control group. The intervention uses techniques aimed at fostering self-care for caregivers and improving communication between caregivers and individuals with dementia. Self-reported caregiver burden, stress, anxiety, and depression were measured at 1, 3, and 6 months after intervention.
Results
Most participants (91.5%) completed the entire study. The intervention significantly reduced perceived stress for up to 6 months (Β = -2.84, t = -2.68, P = .008) and was considered by nearly all respondents to be helpful for managing challenging behaviors.
Discussion
A low-cost, brief intervention shows promise for producing lasting improvements in caregiver's psychological health.
Keywords: Alzheimer's disease, Memory impairment, Mental health, Improvisation, Mindfulness, Validation, Acceptance, Psychological well-being
Highlights
-
•
A 2-day intervention reduced stress in family caregivers of persons with dementia.
-
•
Family caregivers reported less stress up to 6 months after intervention.
-
•
This brief and affordable intervention can improve caregivers' psychological health.
1. Background
Dementia is a serious public health concern and economic burden that is expected to worsen as the population ages in the absence of effective treatments and preventive strategies [1], [2]. Most individuals with dementia are cared for by a family member, most often a spouse or adult child [3]. Family caregivers of individuals with dementia often experience significant psychological distress, including higher levels of chronic stress, depression, and anxiety. They also report greater subjective cognitive concerns and sleep disturbance compared to noncaregivers and caregivers of individuals with other health conditions [4], [5], [6], [7]. These psychological difficulties, particularly stress, have been associated with increased systemic inflammation, pain, and cardiovascular disease in caregivers [8], [9], [10]. In addition, worsening psychological and physical health in caregivers can adversely impact the individual with dementia—for example, they increase the likelihood of institutionalization of the person with dementia, which is associated with decreased psychological and physical health and increased mortality in individuals with dementia [11], [12], [13], [14]. Thus, there is an urgency to develop empirically tested interventions that promote well-being in family caregivers [1].
A number of studies have tested the efficacy of a variety of programs (e.g., psychoeducation, mindfulness training, cognitive behavioral therapy), in improving psychological well-being for caregivers. A meta-analysis that evaluated 30 controlled studies suggested that structured programs, such as those that teach caregivers practical skills in the care of patients, led to better outcomes (e.g., reduced psychological morbidity) [15]. However, many of these programs are time consuming (e.g. weekly sessions) and costly, which represent significant barriers to participation for most caregivers. Therefore, there is a need for brief and inexpensive interventions that can lead to lasting reductions in caregiver distress.
Here we examine for the first time a new 2-day intervention, known as “Meeting Alzheimer's: Effective Communication Connection and Care—Experiential Dementia Training,” in improving psychological well-being in family caregivers of individuals with dementia over a 6-month period. This program aims to teach caregivers practical skills for self-care and care for individuals with dementia through the use of combined techniques drawn from psychotherapeutic interventions and improvisational theater. We hypothesized that the 2-day intervention would decrease depression symptomatology, anxiety, perceived stress, and caregiver burden.
2. Method and materials
2.1. Participants
Participants were the primary family caregivers of individuals formally diagnosed with dementia by a medical practitioner. Based on the available data, 62% of the individuals with dementia cared for by the participants were diagnosed with dementia of the Alzheimer's type; other diagnoses included frontotemporal dementia, Lewy Body dementia, and mixed dementia. Caregivers were excluded if they were nonfluent in English, currently participating in another intervention for caregivers, had a history of a severe psychiatric disorder, or had a severe disability or disease that posed a problem in their caregiving (e.g., cancer, Parkinson's disease). Caregivers were recruited from the community between 11/2014 and 12/2016 via churches, respite care centers, senior centers, online, campus e-mail, and local organizations. The recruitment flyer invited family caregivers to participate in a “dementia care research study.” Fifty-two percent of participants were caring for a parent, 45% of participants were caring for a spouse, and 3% of participants were caring for a sibling with dementia. Demographics and other sample characteristics are reported in Table 1.
Table 1.
Demographics and other sample characteristics
| Wait-list control (n = 42) |
Intervention (n = 62) |
|
|---|---|---|
| M (SD) | ||
| Caregiver | ||
| Age | 63.4 (10.9) | 62.5 (9.9) |
| Sex (% women) | 79 | 69 |
| Years of education | 15.7 (2.8) | 15.6 (2.9) |
| Years spent caring | 3.0 (2.6) | 4.3 (4.5) |
| Living with individual (%) | 59 | 53 |
| Baseline BDI | 11.7 (7.1) | 11.8 (7.7) |
| Baseline BAI | 7.7 (7.2) | 8.1 (8.0) |
| Baseline PSS | 16.3 (5.6) | 16.0 (6.5) |
| Baseline CBI | 34.3 (12.2) | 34.9 (15.3) |
| Individual with dementia | ||
| Age | 78.6 (9.5) | 78.2 (10.8) |
| FAST stage | 5.7 (1.3) | 5.7 (1.2) |
Abbreviations: BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; CBI, Caregiver Burden Inventory; FAST, the Reisberg Functional Assessment Staging Tool; PSS, Perceived Stress Scale.
2.2. Measures
Stage of dementia was assessed with the Functional Assessment Staging Tool (FAST) [16]. The FAST scale consists of seven major functional levels (1–7), which are concordant with the corresponding global level of functional capacity and cognition of the Global Deterioration Scale, with a higher number corresponding to greater impairment.
Perceived stress was assessed with the Perceived Stress Scale (PSS) [17]. The PSS is a 10-item scale that measures the degree to which one appraises situations in their life to be stressful. The scale is designed to measure how uncontrollable, unpredictable, and overloaded individuals find their lives. The scale comprises two factors: perceived helplessness and perceived self-efficacy [18]. A higher score (range 0–48) indicates greater perceived stress.
Caregiver burden was assessed with the Caregiver Burden Inventory (CBI) [19]. The CBI is a 24-item scale that evaluates five dimensions of caregiver burden: time dependence, developmental, physical, social, and emotional burdens. A higher score (range 0–96) indicates greater burden.
Depression was assessed with the Beck Depression Inventory-II (BDI-II) [20]. The BDI-II is a 21-item scale that evaluates both affective and somatic components of depression. A higher score (range 0–63) indicates greater levels of depression.
Anxiety was assessed with the Beck Anxiety Inventory (BAI) [21]. The BAI is a 21-item scale that evaluates cognitive and somatic components of anxiety. A higher score (range 0–63) indicates greater levels of anxiety.
In addition, caregivers completed a questionnaire created by the authors to gather additional information related to caregiving. This questionnaire included items such as whether the caregiver was currently residing with the individual with dementia, the amount of time spent weekly caring for the individual, and an opportunity to provide open-ended comments about their experience as a caregiver. After completing the intervention arm of the study, participants completed additional items related to their perception and use of the skills that were introduced during the intervention.
2.3. Procedure
Approval was obtained from the University of Iowa's Institutional Review Board. Individuals were screened over the phone or in-person, and those who were eligible were randomly assigned to the intervention or the wait-list control group. All participants were informed at the time of enrollment that they would participate in the intervention but were given different instructions about the dates and procedure of the study, depending on the group to which they were assigned. Participants provided consent in accordance with the Declaration of Helsinki and were remunerated for participating. Sessions for seven intervention groups and five control groups were held throughout the course of the study. Data collection at 1, 3, and 6 months was completed through the mail.
2.3.1. Wait-list control group
Participants in the control group participated in a 1.5-hour session to obtain informed consent and complete the FAST, BDI-II, BAI, PSS, CBI, and a demographics form. Participants completed these measures and the caregiver questionnaire again after 1, 3, and 6 months. Participants in the control group participated in the intervention at 6 months.
2.3.2. Intervention group
The intervention took place over 2 days. Participants provided informed consent at the beginning of the first day. At this time, they completed the same measures as participants in the control group. Participants again completed these measures and the caregiver questionnaire 1, 3, and 6 months after intervention.
2.4. Intervention
Family caregivers participated in a manualized 2-day intervention. The intervention was delivered by one of the authors (J.A.) to facilitate consistency across sessions. This author was not involved in recruitment, group assignment, scheduling, data collection, or data analysis. Each workshop comprised a combination of individuals who were assigned to the intervention group as well as wait-list controls (who had been participating in the control group for 6 months). On the day of the workshop, the only difference between individuals who were assigned to the intervention group as opposed to the control group is that individuals in the intervention group were consented upon arrival and subsequently completed their first set of questionnaires. To reduce the possibility of knowing whether participants were in the control or intervention group, the person who delivered the intervention was always asked to arrive after all of the paperwork was completed. Thus, she would each time arrive to a room with a similar number of caregivers ready to start the intervention. The intervention combined techniques from multiple therapeutic interventions including mindfulness, behavior management training, and validation therapy. It covered multiple topics including (1) psychoeducation about dementia, (2) self-care for caregivers, (3) using verbal and nonverbal language to communicate effectively with individuals with dementia, (4) identifying and validating emotions in individuals with dementia, (5) using mindfulness skills to notice the current needs of the individuals with dementia, and (6) managing difficult behaviors. Many of these topics were taught using active and engaging exercises drawn from improvisational theater and creative drama.
Specifically, caregivers participated in mindfulness exercises that aimed to help them (1) learn how to identify and understand their own emotions as well as those of the individual with dementia and (2) meet the individual they were caring for in the present moment to engage fully with them. Other exercises included teaching caregivers to recognize and validate the emotions of the individual with dementia using verbal and nonverbal communication. Caregivers also learned skills to manage difficult behaviors in the individual with dementia and were provided a chance to practice the skills using role play and improvisation techniques. They were also able to practice these skills with the individual they were caring for between the first and second session. They later discussed their successful and unsuccessful attempts to implement the skills with the other caregivers and collaboratively generated alternative approaches.
2.5. Statistical analyses
Statistical analyses were performed using R (version 3.4.1) and SPSS. To assess for group differences in our dependent variables (PSS, CBI, BDI-II, BAI) over time, data were submitted to a linear mixed-effects model using R's linear mixed-effects package (lme4) [22]. Mixed-effects modeling was selected over repeated-measures ANOVA to more accurately model individual differences in the data. Mixed-effects modeling allows for the separation of random effects (e.g., inter-subject variability) from fixed effects (e.g., group differences), which makes it a more powerful statistical analysis technique [23]. The starting model for each analysis included (1) fixed effects for intercepts, linear slope (average change between baseline and 1 month), and quadratic slope (acceleration in rate of change over time), (2) fixed effects of intervention group on linear slope and quadratic slope, and (3) random subject-specific effects on intercept, linear slope, and quadratic slope. Random effects of subject on intercept, linear slope, or quadratic slope were discarded if likelihood ratio tests revealed that they did not contribute significantly to the model.
All discrete predictors (e.g., group, sex) were effect-coded. Potential covariates were selected a priori and included caregiver age, caregiver education, caregiver sex, patient age, stage of dementia at baseline (as determined by the FAST), and living status (whether or not the caregiver was living with the patient at baseline). Covariates were added to the model one at a time and were not included in the final model if they did not account for a significant amount of variance. Item-level missing data were handled using mean imputation if less than 20% of the items in a scale were missing. If greater than 20% of a scale's items were missing, the scale was counted as missing and was excluded from the analyses. This resulted in the exclusion of one BAI score at baseline, two CBI scores at baseline, three CBI scores at 1 month, two CBI scores at 3 months, and one CBI score at 6 months. As four statistical models were conducted, a Bonferroni correction was applied, and each individual model was tested at α = .0125.
3. Results
3.1. Attrition
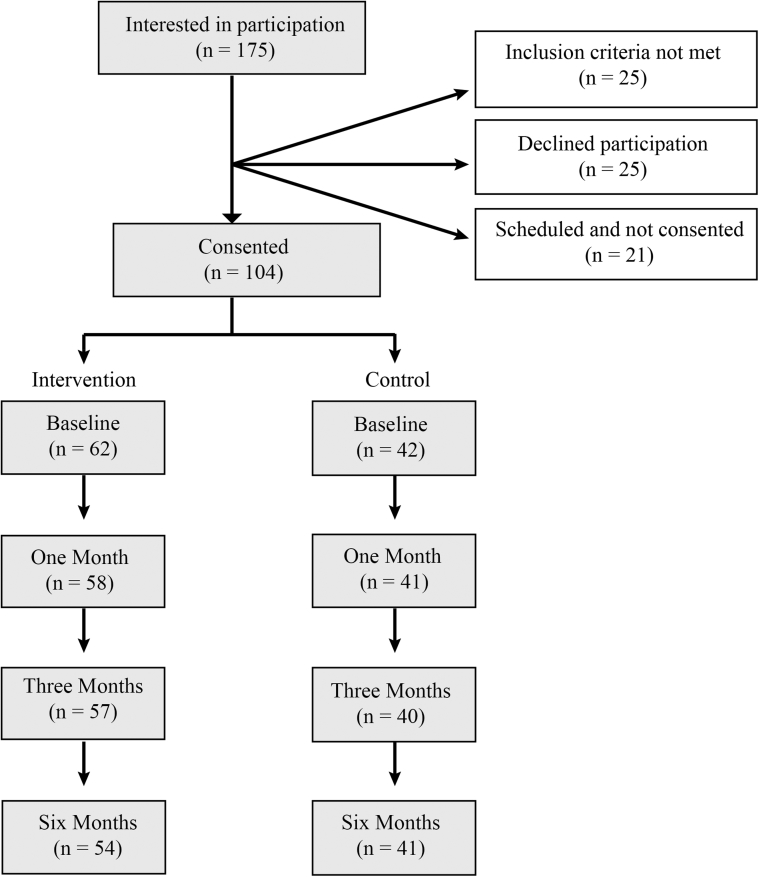
Of the 104 caregivers enrolled, 94 completed questionnaires at all four time points in the study (Fig. 1). Five caregivers elected to discontinue the study (four at 1 month and one at 6 months; reasons for discontinuing were not disclosed). One caregiver returned the questionnaires at 1 and 6 months, but not at 3 months. Four caregivers were ineligible to complete the intervention due to the death of the individual with dementia they were caring for (one at 1 month and three at 6 months).
Fig. 1.
Flowchart of study participation. One hundred seventy-five family caregivers expressed interest in participating in the study. Of those, 104 participants were consented and enrolled. Participants who were “scheduled but not consented” canceled their participation in advance or simply did not attend; 95 participants completed participation in the study. Five caregivers elected to discontinue the study and four discontinued following the death of the person with dementia.
3.2. Perceived stress
The maximal random effects model justified by the data included a random effect for intercept and linear slope for participants (Table 2). There was not a significant difference between groups at baseline, t(115.1) = 0.17, P = .864, d = 0.03. A significant main effect was detected for the linear slope for time, t(234.0) = −2.80, P = .006, d = 0.37, which indicates that on average, perceived stress decreased significantly between baseline and 1 month. A significant quadratic slope for time, t(188.4) = 2.73, P = .007, d = 0.40, suggests that the overall rate of decrease in stress increased over time.
Table 2.
Full fixed effects structure
| Effects | Description | β | SE | t-value | Cohen's d | P-value |
|---|---|---|---|---|---|---|
| Perceived stress | ||||||
| Group | Intervention or control | 0.110 | 0.64 | 0.17 | 0.03 | .864 |
| Time | Time linear slope | −0.768 | 0.28 | −2.80 | 0.37 | .006* |
| Quadratic time | Time quadratic slope | 0.115 | 0.04 | 2.73 | 0.40 | .007* |
| Group × time | Interaction | -0.716 | 0.28 | -2.61 | 0.34 | .010* |
| Group × quad time | Interaction | 0.105 | 0.04 | 2.48 | 0.36 | .014 |
| Perceived stress: perceived self-efficacy | ||||||
| Group | Intervention or control | 0.349 | 0.31 | 1.13 | 0.18 | .261 |
| Time | Time linear slope | 0.062 | 0.17 | 0.35 | 0.04 | .723 |
| Quadratic time | Time quadratic slope | <0.001 | 0.03 | 0.01 | <0.01 | .989 |
| Group × time | Interaction | -0.467 | 0.17 | -2.69 | 0.32 | .008* |
| Group × quad time | Interaction | 0.068 | 0.03 | 2.48 | 0.29 | .014 |
| Perceived stress: perceived helplessness | ||||||
| Group | Intervention or control | -0.242 | 0.45 | -0.54 | 0.11 | .589 |
| Time | Time linear slope | -0.835 | 0.23 | -3.59 | 0.73 | <.001* |
| Quadratic time | Time quadratic slope | 0.115 | 0.03 | 3.29 | 0.68 | .001* |
| Group × time | Interaction | -0.247 | 0.23 | -1.06 | 0.22 | .290 |
| Group × quad time | Interaction | 0.036 | 0.03 | 1.03 | 0.21 | .304 |
| Caregiver burden | ||||||
| Group | Intervention or control | 0.498 | 1.31 | 0.38 | 0.07 | .704 |
| FAST baseline | Baseline dementia stage | 3.351 | 1.02 | 3.27 | 0.66 | .001* |
| Time | Time linear slope | -1.455 | 0.53 | -2.75 | 0.37 | .006* |
| Quadratic time | Time quadratic slope | 0.214 | 0.08 | 2.62 | 0.39 | .010* |
| Group × time | Interaction | -0.448 | 0.53 | -0.85 | 0.11 | .397 |
| Group × quad time | Interaction | 0.070 | 0.08 | 0.85 | 0.13 | .394 |
| Depression | ||||||
| Group | Intervention or control | 0.260 | 0.75 | 0.35 | 0.06 | .729 |
| Time | Time linear slope | -1.064 | 0.37 | -2.85 | 0.39 | .005* |
| Quadratic time | Time quadratic slope | 0.145 | 0.06 | 2.50 | 0.38 | .014 |
| Group × time | Interaction | -.414 | 0.37 | -1.11 | 0.15 | .269 |
| Group × quad time | Interaction | .053 | 0.06 | 0.90 | 0.14 | .367 |
| Anxiety | ||||||
| Group | Intervention or control | 0.572 | 0.75 | 0.76 | 0.15 | .447 |
| Time | Time linear slope | -1.010 | 0.39 | -2.60 | 0.53 | .011* |
| Quadratic time | Time quadratic slope | 0.163 | 0.06 | 2.71 | 0.58 | .008* |
| Group × time | Interaction | -0.154 | 0.39 | -0.40 | 0.08 | .693 |
| Group × quad time | Interaction | 0.027 | 0.06 | 0.45 | 0.10 | .651 |
NOTE: Group × time = interaction between group and the linear slope of time. Group × quad time = interaction between group and the quadratic slope of time. The interaction terms (in bold) indicate potential effects of the intervention over time. * indicates significant at α = .0125.
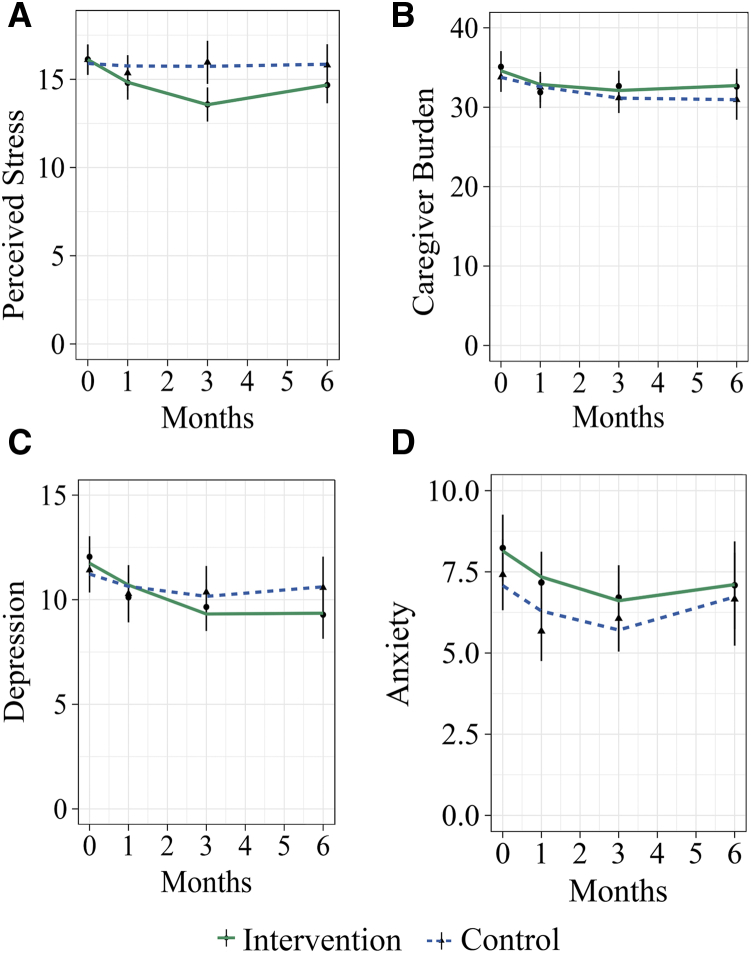
The perceived stress analysis revealed two significant interactions. First, a significant interaction between intervention group and the linear slope for time suggests that overall, individuals in the intervention group showed a faster rate of improvement (decreased perceived stress) between baseline and 1 month relative to the control group, t(234.0) = -2.61, P = .010, d = 0.34. Second, there was a marginally significant interaction between intervention group and the quadratic slope for time, t(188.4) = 2.48, P = .014, d = 0.36. This indicates that the reduction in perceived stress tended to accelerate more quickly in the intervention group than in the control group (Fig. 2A).
Fig. 2.
Self-reported psychological health after the intervention. Model results for (A) perceived stress, (B) caregiver burden, (C) anxiety, and (D) depression. Graphs depict actual and model predicted data over the course of the study. Individual data points represent group means with corresponding vertical bars representing standard error. Note that actual data were only available at 0 (baseline), 1, 3, and 6 months; lines represent model predicted data over the course of 6 months.
Follow-up analyses were conducted to examine whether this effect was observed across both subscales embedded within the PSS: perceived self-efficacy and perceived helplessness [18].
3.2.1. Perceived self-efficacy
The maximal random-effects model justified by the data included a random intercept for participants (Table 2). This model revealed a significant interaction between group and the linear slope for time, suggesting that overall, individuals in the intervention group showed a faster rate of increased perceived self-efficacy between baseline and 1 month relative to the control group, t(287.6) = −2.69, P = .008, d = 0.32. There was a marginally significant interaction between group and the quadratic slope for time, t(286.7) = 2.48, P = .014, d = 0.29, indicating that the improvement in self-efficacy tended to accelerate more quickly in the intervention group than in the control group.
3.2.2. Perceived helplessness
The maximal random-effects model justified by the data included a random effect for intercept, linear slope, and quadratic slope for participants (Table 2). There was not a significant interaction between intervention group and the linear slope for time t(97.0) = −1.06, P = .290, d = 0.22, or between group and the quadratic slope for time t(94.2) = 1.03, P = .304, d = 0.21. This indicates that we were unable to detect a significant effect of the intervention on the perceived helplessness component of the PSS.
3.3. Caregiver burden
The maximal random-effects structure justified by the data included a random effect for intercept and linear slope for participants (Table 2). There was not a significant difference between groups at baseline, t(109.6) = 0.382, P = .704, d = 0.07. A significant main effect was detected for the linear slope of time, t(220.0) = -2.75, P = .006, d = 0.37, indicating that on average, caregiver burden decreased significantly each month. A significant quadratic slope for time, t(178.9) = 2.62, P = .010, d = 0.39, suggests that the overall rate of decrease in burden increased over time. A significant main effect of stage of dementia at baseline, t(99.0) = 3.27, P = .001, d = 0.66, indicates that participants caring for someone in a more advanced stage of dementia reported a higher level of burden at baseline. There was not a significant interaction between group and the linear slope of time, t(220.0) = −0.85, P = .397, d = 0.11, or between group and the quadratic slope of time, t(178.9) = 0.85, P = .394, d = 0.13, indicating that we were unable to detect a significant effect of the intervention on caregiver burden (Fig. 2B).
3.4. Depression
The maximal random-effects structure supported by the data included a random effect for intercept and linear slope for participants (Table 2). There was not a significant difference between groups at baseline, t(119.3) = 0.35, P = .729, d = 0.06. A significant main effect was observed for the linear slope of time, t(209.8) = -2.85 P = .005, d = 0.39, indicating that on average, depression decreased significantly between baseline and 1 month. A marginally significant quadratic slope for time, t(168.2) = 2.50 P = .014, d = 0.39, suggests that the overall rate of decrease in depression tended to accelerate over time. There was not a significant interaction between group and the linear slope of time, t(209.8) = −1.11, P = .269, d = 0.15, or between group and the quadratic slope of time, t(168.2) = 0.90, P = .367, d = 0.14, indicating that we were unable to detect a significant effect of the intervention on depression (Fig. 2C).
3.5. Anxiety
The maximal random effects structure supported by the data included a random-effect for intercept, linear slope, and quadratic slope for participants (Table 2). There was not a significant difference between groups at baseline, t(101.8) = 0.76, P = .447, d = 0.15. A significant main effect was detected for the linear slope of time, t(94.8) = −2.60, P = .011, d = 0.53, indicating that on average, anxiety decreased significantly between baseline and 1 month. A significant quadratic slope for time, t(85.9) = 2.71, P = .008, d = 0.58, indicates that the overall rate of decrease in anxiety accelerated as each month progressed. There was not a significant interaction between group and the linear slope of time, t(94.8) = −0.40, P = .693, d = 0.08, or between group and the quadratic slope of time, t(85.9) = 0.45, P = .651, d = 0.10, indicating that we were unable to detect a significant effect of the intervention on anxiety (Fig. 2D).
3.6. Adherence and perception of intervention
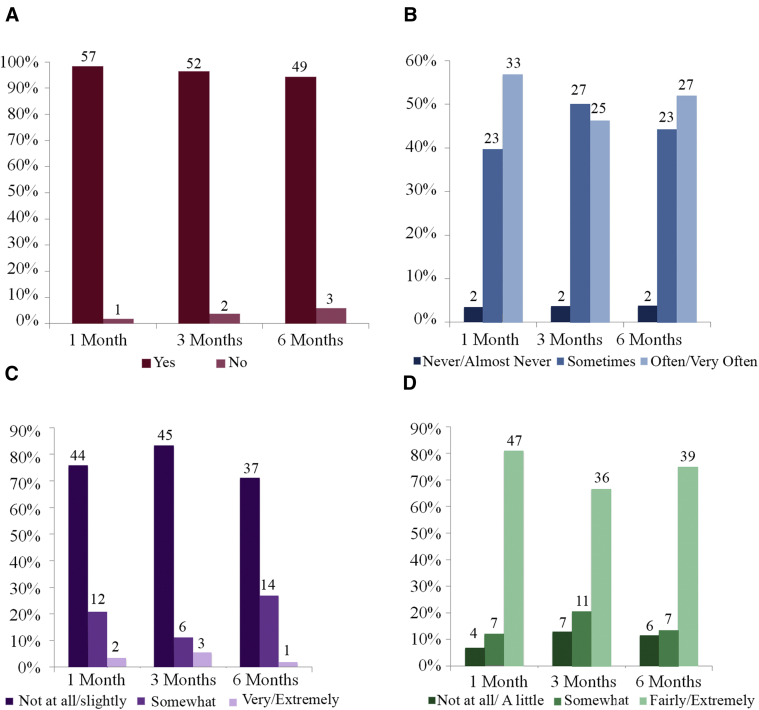
Nearly all of caregivers (98%) reported using the skills from the intervention 1 month later; 94% reported continuing to use these skills 6 months later (Fig. 3A). Fifty-two percent of caregivers reported using the skills often/very often 6 months after the intervention (Fig. 3B). Six months after the intervention, 71% of caregivers reported that it was not at all difficult or only slightly difficult to use the skills from the intervention (Fig. 3C). Furthermore, most caregivers (75%) reported finding the skills that they learned helpful 6 months after the intervention (Fig. 3D). Caregivers reported that they were able to use these skills to reconnect with the person they were caring for and to better manage distressing behaviors (e.g., agitation, confusion). Open-ended responses regarding caregivers' perception of the intervention are provided in Table 3.
Fig. 3.
Qualitative descriptions of the intervention. Graphs depict the percentage of caregivers who endorsed each response. The number of caregivers who endorsed each response is depicted above each bar. These questions only apply to caregivers assigned to the intervention group. Participants were asked (A) Have you used the skills learned in the workshop, (B) How often do you use the skills you learned in the workshop (C) How difficult has it been to implement the skills you learned during the workshop and (D) How helpful have you found the skills you learned in the workshop.
Table 3.
Caregiver's perception of the intervention
| “Caregiving is hard, stressful, and very emotionally draining—it's like my entire life has been turned upside down—I had thought retirement would be fun, easy and enjoyable—it is not. My physical health suffers as well as my mental health… This workshop was very, very good and gave me many tools and much info to make caregiving better. I recommend it to everyone!” |
| “I am so grateful for the workshop, I learned lots of wonderful skills to help me get through helping Mom at the hospital.” |
| “It made me handle my mother better—even if only slightly, or better said—maybe it made me handle myself better.” |
| “The workshop has given me a new outlook concerning my husband's care—I try to see his point of view in dealing with day-to-day activities” |
| “I believe that even at the early stage, that “stepping into the reality” is key to all of this! I will live on in this realm!” |
| “Your seminar was most helpful with getting rid of reality therapy, slowing down, and just going with the flow” |
| “I will try to use some of the techniques I learned in the workshop. I think it has helped quite a bit.” |
| “I thought the workshop was great! If I would have had it earlier on, it would have been more helpful! I had already been taking care of my dad for 1 and a half years when I went to the workshop, so I felt like I was familiar with a lot of info.” |
| “The workshop has given me some new and different techniques in dealing with my husband. These behaviors have made it easier to deal with him. Thank you!” |
| “The workshop is well intentioned but seemed more gender specific and geared toward a touchy feely.” |
| “Really need a support group in this area for FTD caregivers. Need more information on how to deal with a loved one with FTD on a daily basis.” |
| “I found the workshop very helpful even though my husband has aphasic and cannot speak much anymore. It did change my attitude in trying to help and communicate with him even without language.” |
| “I am so glad that I attended this workshop. It was a very enlightening event for me. I have such a more positive outlook on this journey with my wonderful wife. [My wife's] grace is amazing and even though this is not the path I would have chosen, I think my love for her as grown exponentially with this new challenge.” |
| “Thank you for letting me participate in this project. By thinking about my feelings it helps me cope better with my situation.” |
4. Discussion
The population of individuals with dementia in the United States is expected to almost triple by 2060 [1], which will lead to a significant increase in the number of caregivers. Therefore, it is imperative to identify ways to improve caregivers' quality of life and well-being, as caring for individuals with dementia often leads to detriments in caregivers' physical and mental health, which can also indirectly impact the patient's well-being [4], [5], [6], [7], [12], [13]. The present study investigated a novel 2-day intervention in decreasing symptoms of depression, anxiety, burden, and perceived stress in family caregivers of individuals with dementia. Findings show a significant decrease in perceived stress that persists up to 6 months after intervention, over and above what was observed in the control group. Upon further examination, we found that this effect appears to be specific to the component of perceived self-efficacy on the PSS, and not perceived helplessness, suggesting that this intervention bolsters participants' confidence in their ability to care for the individual with dementia. This increase in confidence is also reflected in caregiver's qualitative feedback regarding the intervention (Table 3). We did not find significant changes in symptoms of depression, anxiety, or burden, although there was a visible trend toward decreased depression symptomatology in the intervention group. It is possible that the reason we did not observe changes on depression and anxiety is because, even if the caregiver can cope better with stress, observing their loved one decline in the absence of a cure may continue to cause mild levels of sadness, hopelessness, loss of energy, or nervousness, all of which are core symptoms of depression and anxiety and are arguably a “normal” reaction to the context. Notably, depression and anxiety were also largely below clinically significant levels. A future study could examine whether a decrease is seen in those with greater levels of depression and anxiety. Finally, nearly all caregivers reported that the skills that they learned in the intervention were easy to implement and helpful (see Table 3).
Caring for an individual with dementia has been characterized as a severe, long-term chronic stressor [24]. Perceived stress describes one's experienced level of stress as a function of an objective stressor. Among other factors, perceived stress may vary as a function of the caregiver's coping skills [17]. Greater perceived stress is associated with negative emotional, physiological, and behavioral responses that increase the individual's risk for psychological and physical conditions (e.g., depression, cardiovascular disease, pain) [9], [10], [25], [26]. The stress/health model of dementia caregiving suggests that reducing perceived stress can lead to positive changes in the caregiver's emotional and behavioral response to caregiving, ultimately leading to improved psychological and physical health outcomes. Therefore, the current 2-day intervention could potentially serve as a way to prevent or reduce stress, and as a consequence, it may improve psychological and physical well-being in caregivers.
Several components of the intervention may have contributed to a lasting reduction in perceived stress, including mindfulness practices. Prior studies suggest that mindfulness effectively reduces caregiver perceived stress and depression [24], [27], [28], and a recent meta-analysis revealed that mindfulness-based interventions led to a moderate decrease in caregiver burden and a large decrease in depression for individuals caring for a loved one with dementia [29]. It has been hypothesized that mindfulness-based interventions may provide caregivers an opportunity to notice their unhelpful reactions or thought patterns in response to caregiving and detach from “autopilot” [30]. Furthermore, nonjudgmental acceptance, a key element of mindfulness, may also help caregivers engage in a full and rich life despite the stress and demands associated with caregiving for someone with a neurodegenerative disease [28], [29]. In the present study, mindfulness exercises were designed to help caregivers identify and understand their own emotions as well as those of the individual with dementia, and engage fully in the present moment with the individual for whom they are caring. Thus, it is plausible that the mindfulness component of our intervention significantly contributed to decreased perceived stress in the participants.
The behavior management training was likely also critical for reducing stress, particularly since most of the reported change on the PSS was seen on items related to self-efficacy. The intervention provides caregivers skills they can use to manage difficult behaviors in the individual with dementia, which has been shown to be particularly effective at reducing stress [31]. In the current intervention, caregivers learned and practiced skills to manage difficult behaviors, using role play and improvisation techniques.
Finally, the current intervention focused on improving communication through exercises that included teaching caregivers how to identify and validate the emotions of the individual with dementia (rather than, e.g., trying to orient the individual to our current reality, which can be frustrating and counterproductive). This is particularly important as prior work has shown that individuals with dementia, particularly those with impaired episodic memory, experience emotions for an extended time even when they can no longer remember the emotion-inducing event [32]. Thus, validating the emotions of the individual with dementia may provide a meaningful way for caregivers to connect with them while minimizing negative emotions that could lead to conflicts.
A major advantage of the current intervention is that it takes place over the course of only 2 days. Brief interventions are cost-effective [33] and can be more accessible than multiweek interventions, particularly for individuals from rural communities [34]. In addition, brief interventions increase the likelihood of treatment completion, which is often a significant obstacle for mental health treatment [35]. In fact, several caregivers anecdotally reported that it was easier to find respite care for a single weekend than to find care for a weekly appointment, making this structure more feasible. Although it has been debated whether a brief intervention can have a lasting impact for dementia caregivers [15], the present study showed that a 2-day intervention can significantly reduce perceived stress for up to 6 months. Furthermore, the present study also included caregivers of individuals with dementia of different etiologies (although most of them had probable Alzheimer's disease) and stages, as well as of a variety of ages, levels of education, and years spent caring for an individual with dementia. This suggests that the intervention may be helpful for different types and severity of dementia, and for caregivers with different backgrounds. More research is needed to confirm the generalizability of these findings to different etiologies and stages of dementia.
A limitation of the present study is that it had a relatively small sample size (N = 104). This limited our ability to investigate possible individual differences such as severity of dementia, living status, and the relationship to the individual with dementia, all of which may influence individuals' responsiveness to an intervention [36]. In addition, three individuals with dementia were admitted to a nursing home over the course of the study (1 intervention, 2 controls). The small sample size limited our ability to investigate the impact of nursing home admission on measures of psychological well-being. It is possible that the present study did not include outcome measures that may have been better suited to detect potential benefits of the intervention, such as a measure of changes in the quality of life for the individual with dementia. In addition, future work may examine more closely the aspects of perceived stress that are targeted by the intervention. Furthermore, it is likely that our sample mostly included individuals currently experiencing stress related to caregiving. Future work would be needed to investigate the effectiveness of this intervention in individuals who are not experiencing such distress. It is also possible that demand characteristics played a role in caregiver's responses. However, this seems unlikely as the intervention's effects were specific to perceived stress and did not reflect widespread improvement across all outcome measures. However, future studies may consider comparing the intervention group to an active control group to minimize potential demand characteristics, as well as to discern whether the reduction in stress is the result of individual components of the intervention or their unique combination. Future studies may also consider testing this intervention in a more ethnically and racially diverse sample.
As the number of family members caring for individuals with dementia rises [3], there will be a growing need for affordable, brief, and effective interventions that can reduce stress in dementia caregivers. This intervention applies unique, engaging exercises and improvisation techniques to provide caregivers an opportunity to learn and practice skills designed to reduce stress and improve their interactions with individuals with dementia. This is critical as dementia caregivers are more likely to use skills they learned through the use of active techniques than those they learn through passive didactics [37]. This approach is easy to implement and can be performed by individuals who are not licensed mental health professionals. Its unique and brief structure makes “Meeting Alzheimer's” a compelling intervention that may be especially accessible for caregivers.
Research in context.
-
1.
Systematic review: The authors reviewed previous literature via usual methods (e.g., PubMed). Prior studies have shown that weekly programs for caregivers of individuals with dementia can improve psychological health in caregivers. However, brief interventions offer a more cost-effective and accessible alternative to weekly interventions.
-
2.
Interpretation: Family caregivers of individuals with dementia completed a 2-day intervention known as “Meeting Alzheimer's.” Compared to participants assigned to a wait-list control group, individuals in the intervention group reported decreased perceived stress up to 6 months after intervention, regardless of their age, sex, education, or the disease severity of the individual with dementia. These findings suggest that a brief intervention can lead to a lasting reduction in caregiver stress.
-
3.
Future directions: Future studies may consider testing the intervention in a larger and more ethnically diverse sample, as well as comparing the intervention group to an active control group or a weekly intervention.
Acknowledgments
The authors thank all the caregivers who participated in this study, without whom this research would not have been possible. The authors also thank the Alzheimer's Association, Iowa City Senior Center, churches in the Iowa City area, Oak Park Place, and everyone else who assisted with recruitment and Pathways for offering to care for the individual with dementia during the workshops. The authors thank Jonah Heskje, Monica Acevedo-Molina, Michelle Knirr, and Seima Al-Momani for their work in recruitment and data entry, as well as Teresa Treat for providing guidance with statistical analyses. This work was supported by the National Science Foundation Graduate Research Fellowship Program awarded to E.G-V. (1048957), the Fraternal Order of Eagles, and Kiwanis International.
Footnotes
J.A. serves on the board of directors for Healing Moments. This author remained blind to group throughout the study and was not involved with data analysis or interpretation.
References
- 1.Brookmeyer R., Abdalla N., Kawas C.H., Corrada M.M. Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement. 2018;14:121–129. doi: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer's Association 2017 Alzheimer's disease facts and figures. Alzheimer's Demen. 2017;13:325–373. [Google Scholar]
- 4.Campbell P., Wright J., Oyebode J., Job D., Crome P., Bentham P. Determinants of burden in those who care for someone with dementia. Int J Geriatr Psychiatry. 2008;23:1078–1085. doi: 10.1002/gps.2071. [DOI] [PubMed] [Google Scholar]
- 5.Sörensen S., Duberstein P., Gill D., Pinquart M. Dementia care: mental health effects, intervention strategies, and clinical implications. Lancet Neurol. 2006;5:961–973. doi: 10.1016/S1474-4422(06)70599-3. [DOI] [PubMed] [Google Scholar]
- 6.van der Lee J., Bakker T.J., Duivenvoorden H.J., Dröes R.-M. Multivariate models of subjective caregiver burden in dementia: a systematic review. Ageing Res Rev. 2014;15:76–93. doi: 10.1016/j.arr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Ma M., Dorstyn D., Ward L., Prentice S. Alzheimers' disease and caregiving: a meta-analytic review comparing the mental health of primary carers to controls. Aging Ment Health. 2017:1–11. doi: 10.1080/13607863.2017.1370689. [DOI] [PubMed] [Google Scholar]
- 8.Mausbach B.T., Roepke S.K., Ziegler M.G., Milic M., von Känel R., Dimsdale J.E. Association between chronic caregiving stress and impaired endothelial function in the elderly. J Am Coll Cardiol. 2010;55:2599–2606. doi: 10.1016/j.jacc.2009.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roepke S.K., Allison M., Von Känel R., Mausbach B.T., Chattillion E.A., Harmell A.L. Relationship between chronic stress and carotid intima-media thickness (IMT) in elderly Alzheimer's disease caregivers. Stress. 2012;15:121–129. doi: 10.3109/10253890.2011.596866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiecolt-Glaser J.K., Preacher K.J., MacCallum R.C., Atkinson C., Malarkey W.B., Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aneshensel C.S., Pearlin L.I., Levy-Storms L., Schuler R.H. The transition from home to nursing home mortality among people with dementia. J Gerontol Ser B Psychol Sci Soc Sci. 2000;55:S152–S162. doi: 10.1093/geronb/55.3.s152. [DOI] [PubMed] [Google Scholar]
- 12.Eska K., Graessel E., Donath C., Schwarzkopf L., Lauterberg J., Holle R. Predictors of institutionalization of dementia patients in mild and moderate stages: a 4-year prospective analysis. Demen Geriatr Cogn Disord extra. 2013;3:426–445. doi: 10.1159/000355079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman M.A., Kramer J.H. Factors affecting decisions to institutionalize demented elderly. Gerontologist. 1991;31:371–374. doi: 10.1093/geront/31.3.371. [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K., Fox P., Newcomer R., Sands L., Lindquist K., Dane K. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 15.Brodaty H., Green A., Koschera A. Meta analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc. 2003;51:657–664. doi: 10.1034/j.1600-0579.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 16.Sclan S.G., Reisberg B. Functional assessment staging (FAST) in Alzheimer's disease: reliability, validity, and ordinality. Int Psychogeriatr. 1992;4:55–69. doi: 10.1017/s1041610292001157. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385–396. [PubMed] [Google Scholar]
- 18.Roberti J.W., Harrington L.N., Storch E.A. Further psychometric support for the 10-item version of the perceived stress scale. J Coll Couns. 2006;9:135–147. [Google Scholar]
- 19.Novak M., Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798–803. doi: 10.1093/geront/29.6.798. [DOI] [PubMed] [Google Scholar]
- 20.Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Beck Depression Inventory-ii (bdi-ii) [Google Scholar]
- 21.Beck A., Epstein N., Brown G., Steer R. An inventory for measuring clinical anxiety: Psychometrie properties. J Consulting Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 22.Bates D., Maechler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 23.Magezi D.A. Linear mixed-effects models for within-participant psychology experiments: an introductory tutorial and free, graphical user interface (LMMgui) Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain F.A., Nazarian N., Lavretsky H. Feasibility of Central Meditation and Imagery Therapy for dementia caregivers. Int J Geriatr Psychiatry. 2014;29:870–876. doi: 10.1002/gps.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz R., Martire L.M. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12:240–249. [PubMed] [Google Scholar]
- 26.Damjanovic A.K., Yang Y., Glaser R., Kiecolt-Glaser J.K., Nguyen H., Laskowski B. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oken B.S., Fonareva I., Haas M., Wahbeh H., Lane J.B., Zajdel D. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J Altern Complement Med. 2010;16:1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z., Chen Q.-l., Sun Y.-Y. Mindfulness training for psychological stress in family caregivers of persons with dementia: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging. 2017;12:1521. doi: 10.2147/CIA.S146213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins R.N., Kishita N. The Effectiveness of Mindfulness-and Acceptance-Based Interventions for Informal Caregivers of People With Dementia: A Meta-Analysis. Gerontologist. 2018:1–17. doi: 10.1093/geront/gny024. [DOI] [PubMed] [Google Scholar]
- 30.Mackenzie C.S., Poulin P.A. Living with the dying: Using the wisdom of mindfulness to support caregivers of older adults with dementia. Int J Health Promotion Education. 2006;44:43–47. [Google Scholar]
- 31.Selwood A., Johnston K., Katona C., Lyketsos C., Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord. 2007;101:75–89. doi: 10.1016/j.jad.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Guzmán-Vélez E., Feinstein J.S., Tranel D. Feelings without memory in Alzheimer's disease. Cogn Behav Neurol. 2014;27:117–129. doi: 10.1097/WNN.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson P., Gregg J., Dahl J., Lundgren T. Springer; New York, NY: 2004. ACT in medical settings. A practical guide to acceptance and commitment therapy; pp. 295–314. [Google Scholar]
- 34.Arcury T.A., Gesler W.M., Preisser J.S., Sherman J., Spencer J., Perin J. The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health Serv Res. 2005;40:135–156. doi: 10.1111/j.1475-6773.2005.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wierzbicki M., Pekarik G. A meta-analysis of psychotherapy dropout. Prof Psychol Res Pr. 1993;24:190–195. [Google Scholar]
- 36.Van Mierlo L.D., Meiland F.J., Van der Roest H.G., Dröes R.M. Personalised caregiver support: effectiveness of psychosocial interventions in subgroups of caregivers of people with dementia. Int J Geriatr Psychiatry. 2012;27:1–14. doi: 10.1002/gps.2694. [DOI] [PubMed] [Google Scholar]
- 37.Chee Y.K., Gitlin L.N., Dennis M.P., Hauck W.W. Predictors of adherence to a skill-building intervention in dementia caregivers. J Gerontol Ser A: Biol Sci Med Sci. 2007;62:673–678. doi: 10.1093/gerona/62.6.673. [DOI] [PubMed] [Google Scholar]