Abstract
Introduction
Transcranial direct current stimulation (tDCS) has been recently shown to improve language outcomes in primary progressive aphasia (PPA) but most studies are small and the influence of PPA variant is unknown.
Methods
Thirty-six patients with PPA participated in a randomized, sham-controlled, double-blind, within-subject crossover design for 15 daily sessions of stimulation coupled with written naming/spelling therapy. Outcome measures were letter accuracy of treated and untreated words immediately after and at 2 weeks and 2 months posttreatment.
Results
tDCS treatment was more effective than sham: gains for treated words were maintained 2 months posttreatment; gains from tDCS also generalized to untreated words and were sustained 2 months posttreatment. Different effects were obtained for each PPA variant, with no tDCS advantage for semantic variant PPA.
Discussion
The study supports using tDCS as an adjunct to written language interventions in individuals with logopenic or nonfluent/agrammatic PPA seeking compensatory treatments in clinical settings.
Keywords: Primary progressive aphasia, Language, Dementia, Treatment, Alzheimer's disease, Frontotemporal dementia, svPPA, nfvPPA, lvPPA, Naming, Spelling
Highlights
-
•
Anodal tDCS over the left IFG enhances effects of written naming therapy in PPA.
-
•
tDCS helps written naming benefits generalize in logopenic and nonfluent PPA.
-
•
No generalization occurs for semantic variant PPA with left IFG tDCS.
-
•
Behavioral gains sustain for 2 months after tDCS but rescind after sham.
-
•
tDCS is a potentially useful adjunct to language therapy in PPA.
1. Introduction
The study of the human connectome has shown that the brain is organized in major hubs [1]. One major hub is the left inferior frontal gyrus (IFG), traditionally referred to as Broca's area. The contribution of the left IFG to language production has long been known, but its importance has been recently reappraised using structural and functional connectivity techniques [1], [2], [3]. Seeley et al. [4] put forth the “network degeneration hypothesis” and demonstrated that degeneration happens according to spatial patterns determined by brain hubs that are distinct for each neurodegenerative syndrome. Three of the syndromes they compared were typical Alzheimer's disease (AD), semantic variant primary progressive aphasia (svPPA), and nonfluent/agrammatic primary progressive aphasia (nfvPPA). The principal treatments for these primary progressive aphasia (PPA) variants are speech and language therapy. These language therapy effects may be augmented by transcranial direct current stimulation (tDCS), as we discuss later, and it is therefore important to clarify mechanisms such as by exploring how effects relate to the functional connectivity of the relevant brain hubs.
In the present study we asked the following question: what are the effects of stimulating the principal language hub, that is, the left IFG? The main PPA variants offer an experimental field to address this question: in nfvPPA, the left IFG is the main locus of cortical atrophy; in logopenic variant primary progressive aphasia (lvPPA) atrophy is most pronounced in the left supramarginal and angular gyri, regions to which the IFG connects by way of the dorsal language stream (in terms of Hickok and Poeppel [5]) through the superior longitudinal fasciculus III or the arcuate fasciculus [3]; finally, in svPPA the main atrophy hub is the left anterior temporal lobe, which connects to the IFG through the ventral language stream in terms of Hickok and Poeppel [5], and in particular the uncinate fasciculus (or temporofrontal extreme capsule fasciculus).
Evidence from poststroke aphasia indicates that language therapy effects might be enhanced by tDCS—a safe, easily-applied, and well-tolerated application of low voltage electrical current to the brain through surface electrodes [6], [7], [8], [9]. Recently, language intervention studies have reported that tDCS may be an effective tool for augmenting the benefits of therapy in PPA [10], [11], [12]. Two groups (including ours) had initially evaluated the use of tDCS alongside language therapy in PPA [13], [14], [15]. Cotelli et al. [14] showed that in nfvPPA, tDCS over the left dorsolateral prefrontal cortex, alongside language therapy targeting oral naming, resulted in greater and longer-lasting improvements than sham tDCS plus language therapy. Our group, using tDCS over the left IFG alongside written naming therapy, found similar results and generalization of the gains to untreated “outcomes” that persisted at 2 months after intervention [15].
Although studies [13], [16], [17], [18], [19], [20], [21] provide proof-of-concept for the efficacy of tDCS in PPA, these small studies do not yet provide sufficient evidence for sustainability of gains and generalization to untreated items. One difficulty in studying tDCS efficacy in PPA is its phenotypic diversity. Recent diagnostic criteria distinguish three variants based on their clinical profiles and link to distinct atrophy patterns, at least in initial stages [12]. No study has systematically addressed the differential effects of tDCS in these variants. The study we report here evaluated in each variant the effects of tDCS delivered over the left IFG and had sufficient statistical power to evaluate these variant-specific effects.
The main outcome we used is spelling performance (letter accuracy). For example, “seed” spelled “SIED” would have three correct letters. Letter accuracy was measured for sham and tDCS conditions for each individual before, during, and after treatment (immediately after, and 2 weeks and 2 months posttreatment). The rationale for targeting spelling performance is that written communication has become increasingly important in society, owing to the ubiquity of email, text messaging, and social media. Spell check improves performance accuracy if the spelling is close or phonologically plausible (e.g., feal for feel). Written naming/spelling are also important forms of alternative communication when speech or language production is markedly impaired—as is typical of nfvPPA [22]. All PPA variants show spelling deficits [23], [24]; therefore written production is a broadly applicable rehabilitation target. Furthermore, meta-analyses [25], [26] and experimental imaging studies [27] have shown the left IFG to be critical for spelling. We implemented a generic treatment that has been shown to improve spelling in all PPA variants [28], [29]. Our statistical methodology, that is, generalized estimation regression [30], was intended to address the progressiveness of neurodegeneration and account for carryover effects in crossover designs.
2. Materials and methods
2.1. Participants
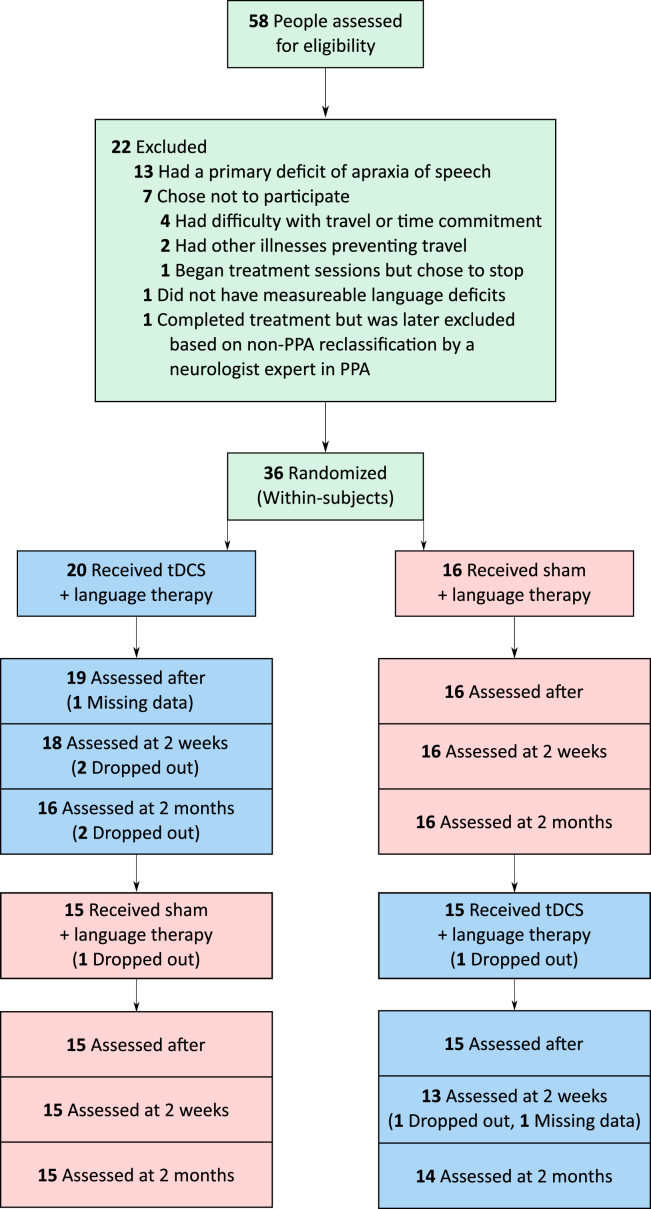
Thirty-six individuals with PPA were enrolled: 14 with nfvPPA, 12 with lvPPA, and 10 with svPPA. Inclusion criteria were native English speakers, minimum high-school education, premorbidly proficient spellers, absence of developmental disorders, absence of nondegenerative neurologic disorders (e.g., stroke), neurodegenerative dementia with focal and progressive language deficits (those with deficits primarily in other cognitive domains were excluded), and formal criteria-based diagnosis of PPA [12] at a specialized center. The diagnostic evaluation included cognitive-language testing, neurologic examination, and neuroimaging (magnetic resonance imaging or positron emission tomography). The variant was determined according to formal criteria [12]. The three groups were matched for sex, age, education, symptom duration, language severity (as defined in the Frontotemporal Dementia (FTD) Clinical Dementia Rating subscale), and overall severity according to the FTD Clinical Dementia Rating (see Fig. 1 and Supplementary Tables 1A and 1B) [31], [32], [33].
Fig. 1.
Participant flow chart and time course of interventions and evaluations for the two groups of participants in the crossover design.
2.2. Study design
Participants were recruited from Johns Hopkins clinics and referrals from diagnostic centers. All participants received anodal tDCS over the left IFG paired with language/spelling therapy, and sham stimulation paired with language/spelling therapy, using stratified randomization of the order of the stimulation conditions within each variant, in a within-subjects crossover design (clinicaltrials.gov identifier: NCT02606422). Participants took part in up to 15 consecutive therapy sessions for each stimulation condition, five sessions per week; the average number of sessions was 14 (standard deviation = 1.6), the median 15. The two treatments (sham and active tDCS) were separated by 2 months (in two cases this interval was 4 months because of health issues). All participants received tDCS at either phase 1 or 2 of treatment. Tables 1 and 2 summarize participant characteristics.
Table 1.
Means (standard deviations) for baseline tasks grouped by first-phase condition (N = 36)
| Task | tDCS first | Sham first | F (1, 34) | P value |
|---|---|---|---|---|
| Letter fluency (sum of words generated in one minute each for F, A, and S) [34] | 16.58 (10.22) | 11.81 (11.12) | 1.744 | .196 |
| Semantic fluency (sum of words generated in one minute each for fruits, animals, and vegetables) [34] | 13.84 (11.23) | 11.29 (7.68) | 0.538 | .469 |
| Object naming (BNT, 30 total) [35] | 13.50 (11.82) | 13.38 (9.97) | 0.001 | .974 |
| Action naming (HANA, 35 total) [36] | 15.00 (12.09) | 14.94 (9.46) | 0.000 | .987 |
| Digit span forward (9 total) [37] | 4.23 (2.09) | 4.13 (1.88) | 0.022 | .882 |
| Digit span backward (9 total) [37] | 2.88 (1.90) | 2.47 (1.66) | 0.453 | .506 |
| JHU sentence anagrams (10 total, in-house test) | 5.61 (3.43) | 5.57 (3.98) | 0.001 | .976 |
| Object semantics (PPT, 15 total) [38] | 13.6 (2.51) | 13.63 (2.39) | 0.439 | .513 |
| Action semantics (KD, 15 total) [39] | 12.69 (2.58) | 11.75 (3.34) | 0.792 | .381 |
| Sentence repetition (NACC, 37 words total) [40] | 27.50 (9.04) | 25.00 (9.78) | 0.547 | .466 |
| Syntactic comprehension (SOAP, 40 total) [41] | 27.14 (7.29) | 27.57 (7.76) | 0.023 | .882 |
| Verbal learning (RAVLT Delayed Recall, 15 total) [42] | 5.75 (2.19) | 3.71 (2.63) | 2.684 | .125 |
| Spelling words (JHU, % correct) [43] | 86.52 (17.58) | 80.98 (16.31) | 0.778 | .385 |
| Spelling nonwords (JHU, % correct) [43] | 76.13 (20.79) | 71.54 (21.66) | 0.324 | .574 |
Abbreviation: tDCS, transcranial direct current stimulation; BNT, Boston Naming Test; HANA, Hopkins Assessment for Naming Actions; JHU, Johns Hopkins University; PPT, Pyramids and Palm Trees; KD, Kissing and Dancing; NACC, National Alzheimer's Coordinating Center; SOAP, Subject-relative, Object-relative, Active, and Passive; RAVLT, Rey Auditory Verbal Learning Test.
Table 2.
Means (standard deviations) for baseline tasks grouped by variant (N = 36)
| Task | lvPPA | nfvPA | svPPA | F (2, 33) | P value |
|---|---|---|---|---|---|
| Letter fluency (sum of words generated in one minute each for F, A, and S) | 16.00 (10.16) | 10.21 (5.85) | 18.50 (14.95) | 2.025 | .149 |
| Semantic fluency (sum of words generated in one minute each for fruits, animals, and vegetables) | 18.00 (12.00) | 12.08 (8.38) | 7.33 (5.52) | 3.416 | .046 |
| Object naming (BNT, 30 total) | 18.18 (10.26) | 17.54 (9.81) | 2.90 (3.04) | 10.672 | .000 |
| Action naming (HANA, 35 total) | 18.73 (10.66) | 17.54 (10.33) | 6.67 (7.11) | 4.584 | .018 |
| Digit span forward (9 total) | 3.42 (1.59) | 3.82 (1.67) | 5.60 (2.17) | 4.481 | .019 |
| Digit span backward (9 total) | 2.29 (0.92) | 2.32 (1.55) | 3.70 (2.52) | 2.367 | .109 |
| Spatial span forward (9 total) [44] | 3.65 (0.97) | 3.12 (1.80) | 3.61 (2.23) | 0.342 | .713 |
| Spatial span backward (9 total) | 3.15 (1.25) | 2.96 (1.66) | 3.50 (2.28) | 0.253 | .778 |
| JHU Sentence anagrams (10 total) | 7.00 (2.49) | 4.54 (3.67) | 5.56 (4.39) | 1.338 | .278 |
| Object semantics (PPT, 15 total) | 13.82 (2.44) | 13.75 (2.63) | 12.30 (2.06) | 1.323 | .281 |
| Action semantics (KD, 15 total) | 12.82 (2.64) | 12.83 (2.73) | 10.67 (3.39) | 1.798 | .184 |
| Sentence repetition (NACC, 37 words total) | 26.45 (7.13) | 21.60 (11.84) | 30.80 (6.83) | 2.707 | .084 |
| Syntactic comprehension (SOAP, 40 total) | 28.44 (6.11) | 25.55 (7.61) | 28.63 (8.78) | 0.526 | .597 |
| Verbal learning (RAVLT Delayed Recall, 15 total) | 5.67 (3.14) | 4.20 (2.28) | 4.25 (2.06) | 0.544 | .594 |
| Spelling words (JHU, % correct) | 81.35 (21.16) | 84.45 (15.12) | 87.12 (14.4) | 0.274 | .762 |
| Spelling nonwords (JHU, % correct) | 76.69 (16.54) | 59.04 (20.17) | 84.52 (20.42) | 4.014 | .031 |
Abbreviations: lvPPA, logopenic variant primary progressive aphasia; nfvPPA, nonfluent/agrammatic primary progressive aphasia; svPPA, semantic variant primary progressive aphasia; BNT, Boston Naming Test; HANA, Hopkins Assessment for Naming Actions; JHU, Johns Hopkins University; PPT, Pyramids and Palm Trees; KD, Kissing and Dancing; NACC, National Alzheimer's Coordinating Center; SOAP, Subject-relative, Object-relative, Active, and Passive; RAVLT, Rey Auditory Verbal Learning Test.
Evaluations occurred before, immediately after, and at 2 weeks posttreatment and 2 months posttreatment for each phase of the study. We used a crossover design to facilitate recruitment and reduce effects of individual variability, an important feature of PPA. However, these designs complicate interpretation because of the inevitable disease progression during the washout period. The statistical analysis accounted for both potential carryover effects and language deterioration over time (see Section 2.5).
In the tDCS and sham conditions, two sets of materials were used: treated items (practiced at each treatment session) and untreated items never practiced but evaluated before treatment and at follow-ups. Evaluations measured letter accuracy in treated words for tDCS over sham and retention of gain effects, and in untreated words for generalization effects. All evaluations were carried out by trained personnel blind to the treatment condition.
2.3. tDCS methods
tDCS was delivered using Soterix Transcranial Direct Current Stimulator Clinical Trials Model 1500. Current was delivered at 2 mA intensity (estimated current density 0.08 mA/cm2) for 20 minutes in the tDCS condition and 30 seconds in the sham condition. Nonmetallic, conductive rubber electrodes covered with saline-soaked 5 × 5 cm sponges were used to minimize the possibility of chemical reactions at the skin/electrode interface. For both tDCS and sham interventions, the electrical current was ramped up at stimulation onset, eliciting a transient (typically 30 seconds) tingling sensation. After ramping in the sham condition, current intensity was decreased to 0 mA. Stimulation (for both conditions) started at the same time as language therapy. These procedures successfully blind participants to the assigned stimulation condition [45]. Language therapy continued for another 20 to 25 minutes.
The therapist was blind to the stimulation condition. Participants were asked to report their general pain level once or twice during each session with the Wong-Baker FACES Pain Rating Scale (www.WongBakerFACES.org).
The anodal site of tDCS application was the left frontal lobe, as in Tsapkini et al. [15], corresponding to the F7 electrode, using the EEG 10-20 electrode position system [46]. Electrode patches were 5 cm × 5 cm (2.54 cm/inch), covering the entire left IFG [25], [26], [47], [48]. In addition, the IFG was individually coregistered to pretreatment magnetic resonance imaging scans using a fiducial marker. We used anodal stimulation to increase brain excitation [49], which has been shown to augment language rehabilitation in previous studies [7]. The reference electrode, the cathode, was placed on each participant's right cheek (Supplementary Fig. 1).
2.4. Written naming/spelling therapy
We combined the spell-study-spell procedure [29] in our previous PPA treatment studies [15], [50] with an oral and written naming paradigm [28]. Given the possibility of different spelling deficits in each variant [24] (but see [23]), we developed individualized treated and untreated word sets, while keeping the same procedures and outcome measures.
The participant was shown a picture on the computer, asked to orally name it, and then to write the name. If the patient could not name the picture (orally or in writing), (s)he was asked to describe it, what it does, and so forth, to evaluate and reinforce semantic knowledge as in semantic feature analysis treatment [28]. If (s)he still could not produce the word orally, (s)he was provided with the correct word and asked to repeat it three times. Likewise, if the patient could not write it or wrote it incorrectly, the clinician provided the correct spelling in a spell-study-spell procedure, that is, the clinician wrote the correct word, reviewed each letter's sound, then asked the patient to copy the word three times [28].
Treated and untreated sets (10–30 words depending on case severity) were matched in length and frequency. Four evaluations were administered for each therapy phase: before therapy, immediately after the end of therapy, 2 weeks posttherapy, and 2 months posttherapy. Letter accuracy was determined based on a scoring system [43] that considered letter deletions, additions, substitutions, and movements. Interrater reliability was managed as follows: each item was scored and a second person performed ratings and noted discrepancies. Interrater reliability was 95%. Discrepancies were discussed to generate a consensus score.
2.5. Statistical analyses
Demographics and other patient characteristics were compared between variants, and between treatment order (tDCS or sham first), using t and F tests for continuous variables, and Fisher's exact tests for categorical variables (see Tables 1 and 2 and Supplementary Tables 1A and 1B).
The primary outcome measures were the percentage of correct letters in treated and untreated word lists. These outcomes were evaluated using the general crossover formulation [51], allowing for each follow-up time point, effects of treatment (sham vs. tDCS), phase (1 or 2), and for their interaction (see Supplementary Table 2 for additional specifications). Specifically, the data used for each patient i were the following: the order of treatments (orderi = sham in phase 1 and tDCS in phase 2, or the reverse order); the change in letter accuracy immediately after sham minus before sham, denoted by δYi,sham, after; 2 weeks after sham minus before sham, denoted by δYi,sham,2w, and 2 months after sham minus before sham, denoted by δYi,sham,2m; and the analogous changes under tDCS (δYi,tDCS, after; δYi,tDCS,2w; and δYi,tDCS,2m, respectively). For each follow-up time point, for example, immediately after, the data (orderi, δYi,sham, after, δYi,tDCS, after) were analyzed to estimate the parameters of the general crossover formulation [51], as in Supplementary Table 2. Of interest in this formulation are the intervention effects, δ(T vs. S),p1 for phase 1, δ(T vs. S),p2 for phase 2, and the overall average of these effects, δ(T vs. S),aver. Estimates of these effects, standard errors, and confidence intervals were obtained using the generalized estimating equation method with robust estimation of the variance of the estimates [30]. This robust method accounts for the possible correlation among the repeated outcomes across times and phases within an individual [30]. P values are exact (nonparametric) and were obtained by comparing the generalized estimating equation estimates to their distribution calculated through permutation of the order-of-intervention assignment (tDCS then sham, or reverse) across patients [52]. The sample of 36 participants was estimated to have more than 80% power to detect an effect size larger than 0.40 for the average intervention effect δ(T vs. S),aver for treated items, and larger than 0.55 for untreated items. For subsamples of 12 participants, which are relevant to the variant subsamples, the corresponding detectable effect sizes were 0.65 and 0.90, respectively.
3. Results
3.1. Tolerability
Electrical stimulation was well tolerated. The maximum pain rating per session was averaged across sessions and participants. The FACES mean pain rating for tDCS was 2.21 (standard deviation 2.48, range 0–10); the mean rating for sham was 2.14 (standard deviation 2.13, range 0–10). No episodes of intolerability occurred, nor were there other adverse effects. During debriefing at the end of the study participants guessed correctly the order of stimulation conditions just 53% of the time, equivalent to chance.
3.2. Effects of tDCS compared with sham
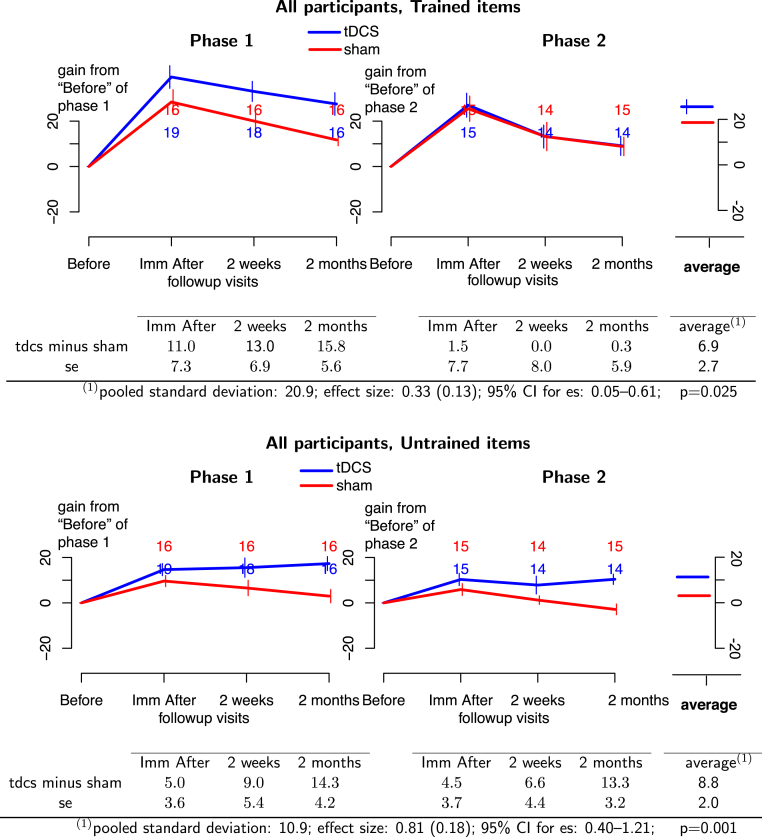
Across phases, the average gain of tDCS over sham was significant for treated items (effect size = 0.33; 95% CI: 0.05–0.61; P = .025) and untreated items (effect size = 0.81; 95% CI: 0.40–1.21; P = .001). In the entire cohort (Fig. 2, top panel), tDCS significantly improved retention of treated items with a moderate effect size and improved generalization of therapy gains to untreated items (Fig. 2, bottom panel) at 2 months with a large effect size (see Fig. 2). For treated items, the effect was numerically larger in the first phase, although the difference between phases was not significant. For treated items in phase 1 and for untreated items in both phases, the difference between gains in tDCS and sham conditions increased with time. In other words, initial gains were retained in the tDCS condition but not in the sham condition, indicating that tDCS seemed to alter the rate of decline in written naming.
Fig. 2.
(Top panel) Treated items for all 36 PPA participants. (Bottom panel) Untreated items for all 36 PPA participants. Phase 1 of treatment is on the left and phase 2 is on the right. Blue lines, tDCS effects; red lines, sham effects. The graphs depict the absolute therapy gain in terms of the percentage of change from baseline condition. Bars at each time point represent 1 standard deviation from the mean. The numbers at each follow-up time indicate the number of participants at that time. Average effects and P values of the GEE statistic are calculated in the last column of the tables. Abbreviations: GEE, generalized estimating equation; PPA, primary progressive aphasia; tDCS, transcranial direct current stimulation.
3.3. Effects of tDCS by PPA variant
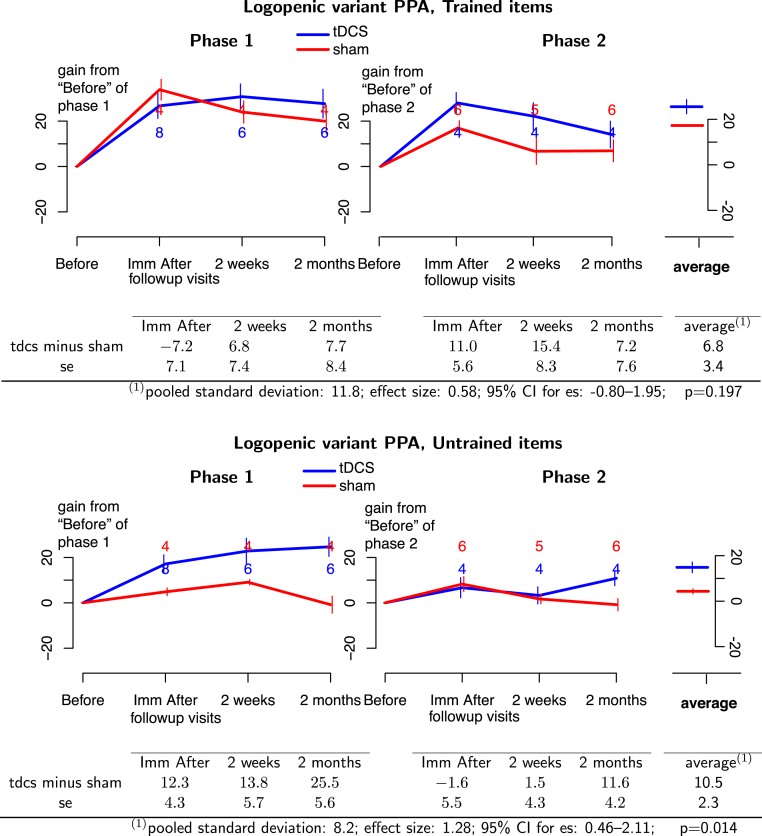
For the lvPPA group, tDCS plus language therapy was more beneficial than sham plus language therapy for treated items with a considerable effect size that did not reach statistical significance (effect size = 0.58; 95% CI: −0.80 to 1.95; P = .197). On the other hand, a large and significant tDCS advantage was found for untreated items, indicating generalization of gains (effect size = 1.28; 95% CI: 0.46–2.11; P = .014, see Fig. 3).
Fig. 3.
(Top panel) Treated items for lvPPA participants. (Bottom panel) Untreated items for lvPPA participants. Blue lines, tDCS effects; red lines, sham effects. Phase 1 of treatment is on the left and phase 2 is on the right. The graphs depict the absolute therapy gain in terms of the percentage of change from baseline condition. Bars at each time point represent 1 standard deviation from the mean. The numbers at each follow-up time indicate the number of participants at that time. Average effects and P values of the GEE statistic are calculated in the last column of the tables. Abbreviations: GEE, generalized estimating equation; lvPPA, logopenic variant primary progressive aphasia; tDCS, transcranial direct current stimulation.
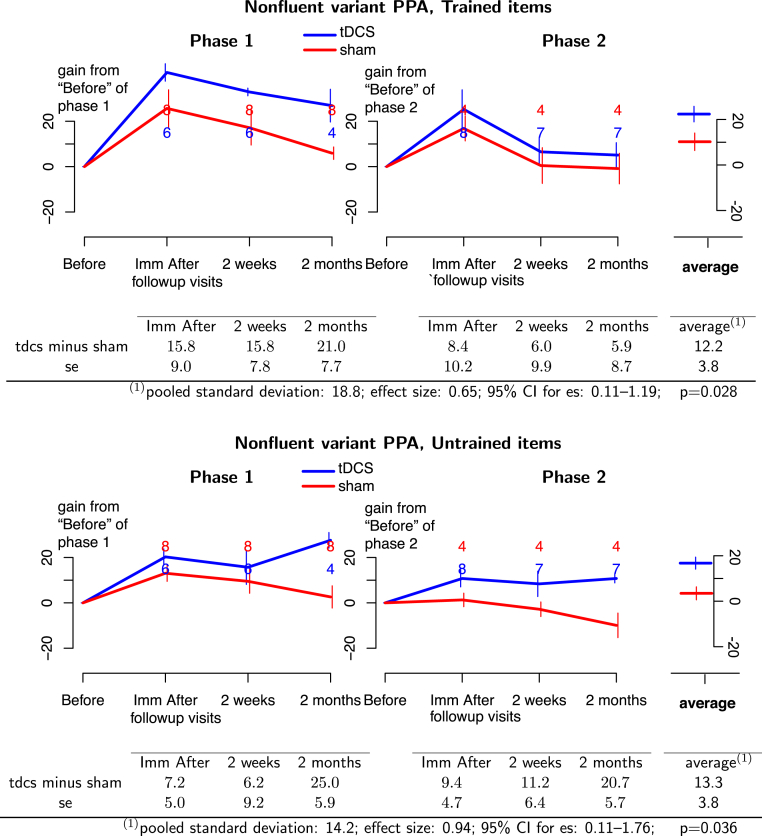
For the nfvPPA group, tDCS with language therapy was more beneficial than sham with language therapy for treated items with a considerable and significant effect size (effect size = 0.65; 95% CI: 0.11–1.19; P = .028). In addition, a large and significant tDCS advantage was found for generalization to untreated items: (effect size = 0.94; 95% CI: 0.11–1.76; P = .036, see Fig. 4).
Fig. 4.
(Top panel) Treated items for nfvPPA participants. (Bottom panel) Untreated items for nfvPPA participants. Phase 1 of treatment is on the left and phase 2 is on the right. Blue lines, tDCS effects; red lines, sham effects. The graphs depict the absolute therapy gain in terms of the percentage of change from baseline condition. Bars at each time point represent 1 standard deviation from the mean. The numbers at each follow-up time indicate the number of participants at that time. Average effects and P values of the GEE statistic are calculated in the last column of the tables. Abbreviations: GEE, generalized estimating equation; nfvPPA, nonfluent/agrammatic primary progressive aphasia; tDCS, transcranial direct current stimulation.
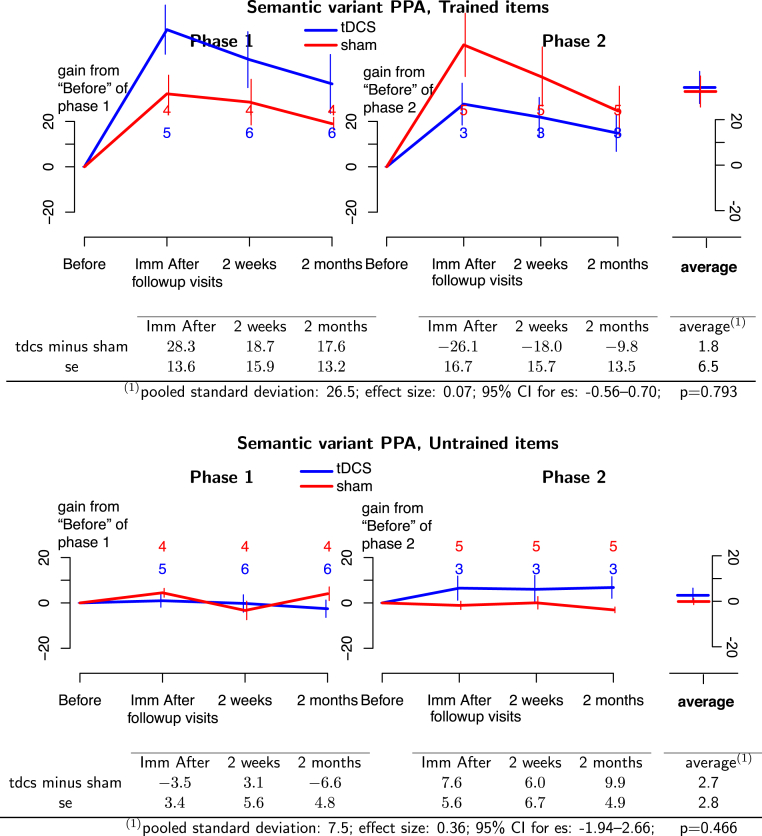
For the svPPA group, tDCS plus language therapy was not more beneficial than sham plus language therapy for treated items (effect size = 0.07; 95% CI: −0.56 to 0.70; P = .793). Although the comparisons between interventions were numerically opposite between phases for individual time points, no such comparison was statistically significant. No tDCS advantage was found for untreated items (effect size = 0.36; 95% CI: −1.94 to 2.66; P = .466, see Fig. 5). In short, in svPPA tDCS did not augment language therapy for treated or untreated items.
Fig. 5.
(Top panel) Treated items for svPPA participants. (Bottom panel) Untreated items for svPPA participants. Phase 1 of treatment is on the left and phase 2 is on the right. Blue lines, tDCS effects; red lines, sham effects. The graphs depict the absolute therapy gain in terms of the percentage of change from baseline condition. Bars at each time point represent 1 standard deviation from the mean. The numbers at each follow-up time indicate the number of participants at that time. Average effects and P values of the GEE statistic are calculated in the last column of the tables. Abbreviations: GEE, generalized estimating equation; svPPA, semantic variant primary progressive aphasia; tDCS, transcranial direct current stimulation.
4. Discussion
In this study we tested the hypothesis that tDCS, as an adjunct to language therapy, will improve treatment outcomes in PPA. We compared the effects of anodal tDCS administered over the left IFG on written naming letter accuracy in three variants of PPA. To our knowledge, this is the largest cohort of patients with PPA studied for variant-specific tDCS effectiveness. Overall, tDCS combined with written word production/spelling treatment improved letter accuracy more than sham and treatment gains were retained 2 months after treatment and generalized to untreated items. We also observed that tDCS efficacy differed across variants.
4.1. Differential tDCS effects across PPA variants
Our findings concur with tDCS efficacy reported for different PPA variants in other studies and also provide new information. Using tDCS in an oral naming intervention in eight nfvPPA participants, Cotelli et al. [14] found that therapy effects generalized and lasted longer when therapy was paired with tDCS. Our replication of these findings is important because of several methodological differences: Cotelli et al. used a between-subjects design and an oral naming intervention, and stimulated a different but nearby area—the dorsolateral prefrontal cortex. By confirming retention and generalization of treatment with a crossover design, we increase confidence that anodal tDCS over the left frontal cortex improves naming therapy effects in nfvPPA.
The benefits of tDCS over sham in lvPPA—a variant usually associated with AD pathology—are consistent with reported benefits of tDCS in participants with AD. One study [53] showed increased effects of tDCS over sham in visual recognition memory after five daily sessions of bilateral tDCS over the middle temporal cortex. Another study showed benefits of tDCS over the inferior parietal cortex in oral naming rehabilitation in AD and FTD (mixed PPA variants), along with generalization of treatment effects [17]. The present study covers an important gap in studying tDCS effects on language rehabilitation in lvPPA, a variant presenting with early word-retrieval deficits, dysfluency, and spelling impairments (thereby resembling nfvPPA), but having relatively preserved grammar and a high probability of AD (rather than FTD) pathology.
Teichmann et al. [20] reported that a single session of anodal tDCS over left temporal areas and cathodal tDCS over right temporal areas improved comprehension accuracy and processing speed in svPPA immediately after intervention [20]. Several differences between their study and ours may account for the different outcomes in svPPA. In particular, they used comprehension accuracy as the outcome of interest, rather than a production task, and the measure was derived from a forced-choice task that offered a 50% chance of success.
4.2. Sustainability of tDCS effects
In the present study we used a 2-month interval between stimulation phases, among the longest in tDCS studies in neurodegenerative syndromes, and still observed a carryover effect. The washout period for tDCS is not well understood in general, nor in PPA in particular [54]. Indeed, it is possible that tDCS effects carry over into the subsequent sham condition for some patients, resulting in an inflated sham performance in the second phase. This is suggested by results for treated items where the phase 1 effect for tDCS compared with sham is not apparent in phase 2 (see Fig. 2), which suggests that positive effects for patients receiving tDCS in phase 1 lasted more than 2 months and carried over to phase 2 (sham condition). Therefore it is necessary to investigate tDCS effects using longer washout periods between stimulation phases, but it is important to consider as well that total washout may not be desirable in neurodegenerative syndromes. The finding that tDCS augmentation of language therapy effects persisted for 2 months after each treatment phase represents a desirable durability of benefit. Another goal for future work would be to measure the duration of these effects.
4.3. Implications for future neuromodulation studies (tDCS and transcranial magnetic stimulation)
We used a tDCS treatment combined with language therapy instead of other neuromodulation techniques, such as transcranial magnetic stimulation (TMS). One might ask whether the effects observed here are specific to tDCS or are likely to be observed with other neuromodulation methods. To our knowledge, there are no published studies of TMS in PPA, but there are recent TMS studies in AD [55], [56].
Comparative studies of the effectiveness of tDCS and TMS in PPA and other conditions would be of great interest, given differences in their mechanisms of action. TMS involves direct neuronal stimulation whereas tDCS does not, which may explain the disparate physiological after-effects documented in a recent study by Cirillo et al. [57]. TMS can be administered with better spatial and temporal resolution than tDCS, but the advantage in temporal resolution from chronometric single-pulse TMS is not considered notable because such single-pulse protocols are not used for therapeutic purposes. TMS protocols designed for enduring therapeutic effects (such as the TMS depression protocol) involve several minutes of repetitive stimulation (thus delivering temporal resolution similar to tDCS protocols). Furthermore, “tDCS is a gentler” approach compared with TMS: subjectively, the tingling sensation of tDCS is much more tolerable than the salient tapping sensation of TMS. Also, repetitive TMS, especially at higher frequencies, is capable of inducing seizures, as noted in recent safety guidelines [58]. In contrast, the most serious adverse effect known for tDCS is skin burns, which can largely be avoided if one does not abrade the skin before electrode placement. Regarding seizures, guidelines exist for limits of TMS frequency, train duration, intensity, and intertrain interval to avoid seizure induction. However, these guidelines have mostly been developed for the healthy brain (relative to the neurodegeneration context); the limits may differ for compromised brains or in patients taking certain types of medication [58]. Even when a study is well within safety limits (such as with single-pulse TMS), there are healthy control subjects who become anxious during the procedures, or dizzy or nauseous and cannot continue. It is also much more difficult to blind the subject to sham TMS. Although sham coils exist, they replicate the clicking sound of TMS but do not adequately replicate the peripheral sensation of the stimulation on the scalp. Consequently, subjects who receive real TMS first in a crossover design may notice the difference in sensation during a subsequent sham TMS sensation and become unblinded to their condition. With regard to applicability and readiness of the methodology for a clinical setting, tDCS is a small device that is easy to use in the speech-language therapist's clinical practice without requiring sophisticated neuronavigation techniques and knowledge, unlike TMS. Without neuronavigation, as in a recent TMS study in AD [55], the spatial resolution of TMS is greatly diminished [56]. Another problem noted regarding the aforementioned study of TMS in AD was that TMS was not compared with sham and not combined with any cognitive training either simultaneously or sequentially [56]. Cognitive, language, or even motor training delivered simultaneously or immediately after tDCS or TMS seems crucial for enhancing neuromodulation effects as noted in a recent consensus article [59]. Simultaneous cognitive or language training is, however, hard to perform during TMS because of the distracting sensation and adverse effects it causes. These disadvantages of TMS would easily be outweighed by any large improvements in therapeutic effectiveness, so comparative studies are certainly warranted.
This study provides useful information for planning confirmatory trials with the same or alternative designs. In general, the power for a design to detect an effect size, say , is approximately , where is the (standard error)2 of the estimator for the effect size, and is the cumulative distribution of the normal. For our design, the effect size (se) for untrained items was 0.81 (0.18) with n = 36. This means that so is 1.2. It follows from the power relation that n = 15 would provide 80% power for detecting the same value of the observed effect size. For trained items, the effect size (se) was 0.33 (0.13), and the aforementioned calculation gives n = 43. If, however, we focus only on period 1, the effect size is 0.78 (0.35) for untrained items and 0.77 (0.31) for trained items. Therefore, and using analogous calculations as previously mentioned, a design with only one period would need 48 subjects to have 80% power for untrained items and 56 subjects to have 80% power for trained items.
Another confirmatory design could use two periods but focus enrollment on subjects of a particular severity. Similar calculations as previously mentioned show that a design that would enroll only low severity participants would require n = 16 to detect the observed effect sizes of 0.47 (trained) and 1.23 (untrained) items; a design that would enroll only medium or high severity participants would require n = 82 to detect the observed effect sizes ≥0.21 for trained and ≥0.57 for untrained items.
4.4. Limitations of this study
We chose to stimulate the left IFG for all PPA variants given its key role in written word production [25]. The choice to stimulate the same area, the left IFG for all PPA variants, can be viewed as a limitation but has provided important insights for tDCS rehabilitation approaches based on recent knowledge regarding structural and functional connectivity [1], [2], [3] and a network degeneration hypothesis [4]. Stimulating a major brain hub for both the dorsal and the ventral language streams [5] was beneficial for the retention and generalization of therapy gains. The variant that seemed to benefit most from tDCS delivered over the left IFG was nfvPPA, for which the left IFG is the main locus of atrophy; in lvPPA the left IFG is not the locus of atrophy but belongs to the same dorsal language stream—and it is noteworthy that these cases experienced generalization of therapy gains in the tDCS condition. In contrast, in svPPA, where the left IFG is not the main locus of atrophy but belongs to the ventral language stream, the tDCS condition showed only retention of gains in the first phase of treatment. Thus another future objective would be to probe the role of other language hubs or the effects of delivering stimulation over the loci of atrophy for the lvPPA and svPPA phenotypes.
It is also to be noted that the electrode patches covered a large cortical area, possibly impacting BA 44, 45, and 47. This makes it difficult to precisely target specific language areas that occupy just about 4 to 6 cm2 of brain tissue, and means that there may have been some stimulation of areas contiguous to the intended target. Other techniques with better spatial resolution, such as TMS, could shed light on the effects of more precise targeting of areas of interest within the language network.
Finally, we note that we did not assess the effect of the therapy on functional communication skills, for example, whether patients improved in writing text messages or notes. Future studies should address tDCS impact on functional communication with questionnaires and measurements that evaluate everyday communication.
5. Conclusions
This study, using a within-subjects crossover sham-controlled design, provides novel evidence that the effect of anodal tDCS over the left IFG delivered alongside language therapy enhances spelling accuracy differentially for each PPA variant. Importantly, the beneficial effects of using a very simple montage over the broader left IFG show that tDCS can be readily used to augment language therapy in PPA and has the potential to slow the decline in treated functions in a neurodegenerative syndrome.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature (PubMed, Google Scholar, conference proceedings) extensively. Our and other groups have recently presented preliminary data describing the effects of transcranial direct current stimulation (tDCS) in primary progressive aphasia (PPA). Previous work is appropriately cited. In the present article, we report the most extensive evidence of tDCS over the left inferior frontal gyrus in PPA.
-
2.
Interpretation: Overall, this clinical trial showed that tDCS over the left inferior frontal gyrus coupled with written naming helps therapy gains sustain longer and generalize to untreated items. However, effects differed by PPA variant: logopenic and nonfluent variants showed generalization but the semantic variant did not.
-
3.
Future directions: The PPA variant may be an important factor in determining tDCS outcomes in PPA. Future research questions could address the following: (1) the role of structural and functional connectivity in tDCS effects; and (2) the effects of tDCS in other language hubs or nonhub areas of the brain.
Acknowledgments
The authors are grateful to our participants for their unfailing commitment and interest in our study. The authors also thank referring physicians. The authors would also like to acknowledge the kind contribution of Dr Marom Bikson's group for simulating the current flow for our montage.
Funding: This work was supported by grants from the Science of Learning Institute at Johns Hopkins University and by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through award R01 DC014475 to K.T. A.H. was supported by NIH (NIDCD) through awards R01 DC05375, R01 DC011317, and P50 DC014664; B.R. was supported by NIDCD P50 006740. C.U.O. was supported by the Jane Tanger Black Fund for Young-Onset Dementias and the Nancy H. Hall Fund for Geriatric Psychiatry.
Footnotes
Conflict of interest: None to report.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2018.08.002.
Supplementary data
Supplementary Fig. 1.
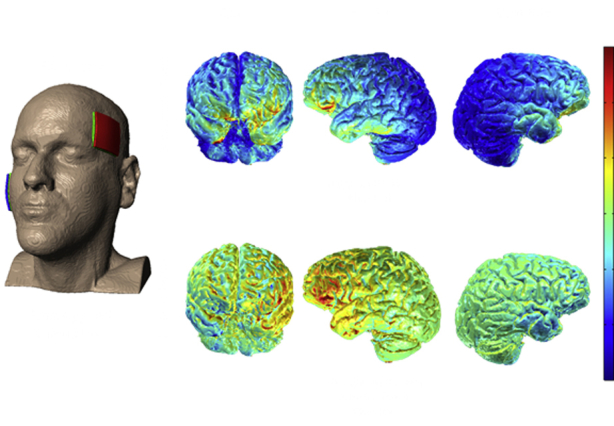
Simulation of current flow over the left IFG as targeted by 5 × 5 cm sponges. (Image courtesy of Dr. Marom Bikson).
References
- 1.Sporns O., Honey C.J., Kötter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey S., Campbell J.S., Pike G.B., Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catani M., Piccirilli M., Cherubini A., Tarducci R., Sciarma T., Gobbi G. Axonal injury within language network in primary progressive aphasia. Ann Neurol. 2003;53:242–247. doi: 10.1002/ana.10445. [DOI] [PubMed] [Google Scholar]
- 4.Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickok G., Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Floel A., Rosser N., Michka O., Knecht S., Breitenstein C. Noninvasive brain stimulation improves language learning. J Cogn Neurosci. 2008;20:1415–1422. doi: 10.1162/jocn.2008.20098. [DOI] [PubMed] [Google Scholar]
- 7.Fridriksson J., Richardson J.D., Baker J.M., Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011;42:819–821. doi: 10.1161/STROKEAHA.110.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaug G., Renga V., Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland R., Crinion J. Can tDCS enhance treatment of aphasia after stroke? Aphasiology. 2012;26:1169–1191. doi: 10.1080/02687038.2011.616925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesulam M., others Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 11.Mesulam M.-M., Rogalski E.J., Wieneke C., Hurley R.S., Geula C., Bigio E.H. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10:554–569. doi: 10.1038/nrneurol.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotelli M., Manenti R., Alberici A., Brambilla M., Petesi M., Cosseddu M. Using transcranial direct current stimulation (tDCS) to treat agrammatic variant of primary progressive aphasia. Dement Geriatr Cogn Disord. 2012;34:164–165. [Google Scholar]
- 14.Cotelli M., Manenti R., Petesi M., Brambilla M., Cosseddu M., Zanetti O. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis. 2014;39:799–808. doi: 10.3233/JAD-131427. [DOI] [PubMed] [Google Scholar]
- 15.Tsapkini K., Frangakis C., Gomez Y., Davis C., Hillis A.E. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: preliminary results and challenges. Aphasiology. 2014;28:1112–1130. doi: 10.1080/02687038.2014.930410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gervits F., Ash S., Coslett H.B., Rascovsky K., Grossman M., Hamilton R. Transcranial direct current stimulation for the treatment of primary progressive aphasia: an open-label pilot study. Brain Lang. 2016;162:35–41. doi: 10.1016/j.bandl.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roncero C., Kniefel H., Service E., Thiel A., Probst S., Chertkow H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer's disease and frontotemporal dementia. Alzheimers Dement Transl Res Clin Interv. 2017;3:247–253. doi: 10.1016/j.trci.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manenti R., Bianchi M., Cosseddu M., Brambilla M., Rizzetti C., Padovani A. Anodal transcranial direct current stimulation of parietal cortex enhances action naming in Corticobasal Syndrome. Front Aging Neurosci. 2015;7:49. doi: 10.3389/fnagi.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung J., Bauer A., Grossman M., Hamilton R.H., Coslett H.B., Reilly J. Semantic feature training in combination with transcranial direct current stimulation (tDCS) for progressive anomia. Front Hum Neurosci. 2017;11:253. doi: 10.3389/fnhum.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teichmann M., Lesoil C., Godard J., Vernet M., Bertrand A., Levy R. Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann Neurol. 2016;80:693–707. doi: 10.1002/ana.24766. [DOI] [PubMed] [Google Scholar]
- 21.McConathey E.M., White N.C., Gervits F., Ash S., Coslett H., Grossman M. Baseline performance predicts tDCS-mediated improvements in language symptoms in primary progressive aphasia. Front Hum Neurosci. 2017;11:347. doi: 10.3389/fnhum.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillis A.E., Tuffiash E., Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cogn Neurosci. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- 23.Sepelyak K., Crinion J., Molitoris J., Epstein-Peterson Z., Bann M., Davis C. Patterns of breakdown in spelling in primary progressive aphasia. Cortex. 2011;47:342–352. doi: 10.1016/j.cortex.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shim H., Hurley R.S., Rogalski E., Mesulam M. Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia. 2012;50:1929–1935. doi: 10.1016/j.neuropsychologia.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purcell J.J., Turkeltaub P.E., Eden G.F., Rapp B. Examining the central and peripheral processes of written word production through meta-analysis. Front Psychol. 2011;2:239. doi: 10.3389/fpsyg.2011.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planton S., Jucla M., Roux F.E., Demonet J.F. The “handwriting brain”: a meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex. 2013;49:2772–2787. doi: 10.1016/j.cortex.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 27.DeMarco A.T., Wilson S.M., Rising K., Rapcsak S.Z., Beeson P.M. Neural substrates of sublexical processing for spelling. Brain Lang. 2017;164:118–128. doi: 10.1016/j.bandl.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beeson P.M., Egnor H. Combining treatment for written and spoken naming. J Int Neuropsychol Soc. 2006;12:816–827. doi: 10.1017/S1355617706061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapp B., Glucroft B. The benefits and protective effects of behavioural treatment for dysgraphia in a case of primary progressive aphasia. Aphasiology. 2009;23:236–265. doi: 10.1080/02687030801943054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang K.-Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 31.Broe M., Hodges J.R., Schofield E., Shepherd C.E., Kril J.J., Halliday G.M. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–1011. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- 32.Knopman D.S., Kramer J.H., Boeve B.F., Caselli R.J., Graff-Radford N.R., Mendez M.F. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J Neurol. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benton A.L., Hamsher K.S., Sivan A.B. 3rd ed. Psychological Assessment Resources (PAR); Lutz, FL: 1994. Multilingual aphasia examination. [Google Scholar]
- 35.Kaplan E., Goodglass H., Weintraub S., Goodglass H. Lea & Febiger; Philadelphia: 1983. Boston naming test. [Google Scholar]
- 36.Breining B.L., Tippett D.C., Davis C., Posner J., Sebastian R., Oishie K. Paper Presented at Clinical Aphasiology Conference; Monterey, CA: 2015. Assessing dissociations of object and action naming in acute stroke. [Google Scholar]
- 37.Wechsler D. Psychological Corporation; San Antonio: 1981. Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- 38.Howard D., Patterson K.E. Thames Valley Test Company; 1992. The Pyramids and Palm Trees Test: a test of semantic access from words and pictures. [Google Scholar]
- 39.Bak T.H., Hodges J.R. Kissing and dancing—a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. J Neurolinguistics. 2003;16:169–181. [Google Scholar]
- 40.Hillis A. University of Washington; Washington: 2015. Sentence Repetition Test. NACC UDS-FTLD Neuropsychol. Battery Instr. Form C1-F. 3.0. [Google Scholar]
- 41.Love T., Oster E. On the categorization of aphasic typologies: the SOAP (a test of syntactic complexity) J Psycholinguist Res. 2002;31:503–529. doi: 10.1023/a:1021208903394. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt M. Western Psychological Services; Los Angeles, CA: 1996. Rey auditory verbal learning test: a handbook. [Google Scholar]
- 43.Goodman R.A., Caramazza A. Johns Hopkins University; Baltimore, MD: 1985. The Johns Hopkins University Dysgraphia Battery. [Google Scholar]
- 44.Corsi P.M. Human memory and the medial region of the brain. Diss Abstr Int. 1972;34:819B. [Google Scholar]
- 45.Gandiga P.C., Hummel F.C., Cohen L.G. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Homan R.W. The 10-20 electrode system and cerebral location. Am J EEG Technol. 1988;28:269–279. [Google Scholar]
- 47.Price C.J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 48.Purcell J.J., Napoliello E.M., Eden G.F. A combined fMRI study of typed spelling and reading. Neuroimage. 2011;55:750–762. doi: 10.1016/j.neuroimage.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Datta A., Baker J.M., Bikson M., Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011;4:169–174. doi: 10.1016/j.brs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsapkini K., Hillis A.E. Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behav Neurol. 2013;26:55–66. doi: 10.3233/BEN-2012-110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones B., Kenward M.G. vol. 98. CRC Press; New York: 2003. (Design and analysis of cross-over trials). [Google Scholar]
- 52.Rosenbaum P.R. Conditional permutation tests and the propensity score in observational studies. J Am Stat Assoc. 1984;79:565–574. [Google Scholar]
- 53.Boggio P.S., Ferrucci R., Mameli F., Martins D., Martins O., Vergari M. Prolonged visual memory enhancement after direct current stimulation in Alzheimer's disease. Brain Stimul. 2012;5:223–230. doi: 10.1016/j.brs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Brunoni A.R., Nitsche M.A., Bolognini N., Bikson M., Wagner T., Merabet L. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alcalá-Lozano R., Morelos-Santana E., Cortés-Sotres J.F., Garza-Villarreal E.A., Sosa-Ortiz A.L., Gonzalez-Olvera J.J. Similar clinical improvement and maintenance after rTMS at 5 Hz using a simple vs. complex protocol in Alzheimer's disease. Brain Stimul. 2018;11:625–627. doi: 10.1016/j.brs.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen J.-P., Suarez A., Le Saout E., Meignier M., Nizard J., Lefaucheur J.-P. Combining cognitive training and multi-site rTMS to improve cognitive functions in Alzheimer's disease. Brain Stimul. 2018;11:651–652. doi: 10.1016/j.brs.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Cirillo G., Di Pino G., Capone F., Ranieri F., Florio L., Todisco V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017;10:1–18. doi: 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reis J., Robertson E., Krakauer J.W., Rothwell J., Marshall L., Gerloff C. Consensus: “can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.