Abstract
Autism spectrum disorder (ASD) is a highly heterogeneous neurodevelopmental disorder characterized by impaired social interactions, restrictive interests, and repetitive stereotypic behaviors. Among the various mechanisms underlying the pathogenesis of ASD, dysfunctions of dopaminergic signaling and mitochondria have been hypothesized to explain the core symptoms of children with ASD. However, only a few studies focusing on the pathological association between dopaminergic neurons (DN) and mitochondria in ASD have been performed using patient-derived stem cells and in vitro differentiated neurons. Stem cells from human exfoliated deciduous teeth (SHED) are neural crest-derived mesenchymal stem cells present in the dental pulp of exfoliated deciduous teeth; these cells can differentiate into dopaminergic neurons (DN) in vitro. This study aimed to investigate the pathological association between development of DN and mitochondria in ASD by using SHED as a disease- or patient-specific cellular model. The SHED obtained from three children with ASD and three typically developing children were differentiated into DN, and the neurobiology of these cells was examined. The DN derived from children with ASD showed impaired neurite outgrowth and branching, associated with decreased mitochondrial membrane potential, ATP production, number of mitochondria within the neurites, amount of mitochondria per cell area and intracellular calcium level. In addition, impaired neurite outgrowth and branching of ASD-derived DN were not improved by brain-derived neurotrophic factor (BDNF), suggesting impairment of the BDNF signaling pathway in ASD. These results imply that intracerebral dopamine production may have decreased in these children. The earliest age at which deciduous teeth spontaneously exfoliate in humans, and SHED can be noninvasively collected, is approximately 6 years. Our results suggest that in vitro analysis of SHED-derived DN obtained from children with ASD provides neurobiological information that may be useful in determining treatment strategies in the early stages of ASD.
Abbreviations: ASD, Autism spectrum disorder; ASD-DN, DN differentiated from a child with ASD; Ctrl-DN, DN differentiated from a typically developing child; DN, Dopaminergic neurons; MMP, Mitochondrial membrane potential; SHED, Stem cells from human exfoliated deciduous teeth
Keywords: Autism spectrum disorder, Dopaminergic neurons, Mitochondria, Stem cells from human exfoliated deciduous teeth
Highlights
-
•
Dental pulp stem cells of autistic patient differentiate into dopaminergic neurons.
-
•
These neurons show impaired neurite development compared with those from controls.
-
•
This impairment is associated with mitochondrial dysfunction.
-
•
Dental pulp stem cells may help establish treatment strategies against autism.
1. Introduction
Autism spectrum disorder (ASD) is a highly heterogeneous neurodevelopmental disorder [1]. The core symptoms of this disorder include impaired social interaction, restrictive interests, and repetitive stereotypic behaviors. Previous studies have reported abnormalities in a wide variety of genetic, environmental, and neurobiological factors related to the differentiation, growth, and function of the central nervous system of patients with ASD [2], [3], [4]. However, a common model for the pathogenesis of ASD has not yet been established, possibly because ASD exists as a spectrum, and has highly heterogeneous variants.
In silico analysis suggests that various genes associated with the dopaminergic pathway contribute to the pathogenesis of ASD, supporting experimental evidences and the dopamine hypothesis [5], [6]. On the other hand, mitochondrial dysfunction has been reported in the analysis of various tissues and postmortem brains of patients with ASD [7], [8]. However, only a few studies focusing on the neuropathological association between dopaminergic neurons (DN) and mitochondria in ASD have been conducted, since the direct examination of neurons in vivo is invasive and restricted.
Stem cells from human exfoliated deciduous teeth (SHED) are neural crest-derived mesenchymal multipotent stem cells obtained from the dental pulp of exfoliated deciduous teeth [9]. Some groups have succeeded in differentiating SHED into DN in vitro [10], [11]. The earliest age for spontaneous exfoliation of deciduous teeth in humans is approximately 6 years [12]. This age corresponds to the early stage of ASD, when it is important to determine the optimal treatment strategies for children suspected to have, or those diagnosed with, ASD [13], [14], [15].
The purpose of this study was to clarify the pathological association between development of DN and mitochondria in ASD by using SHED as a disease- or patient-specific cellular model for neurobiological analysis. For this purpose, we obtained SHED from three children with ASD, and examined the neurobiological features of DN differentiated from the SHED, comparing them with those differentiated from SHED obtained from three typically developing children.
2. Materials and methods
2.1. Isolation and culture of SHED
Experiments using human samples were reviewed and approved by the Kyushu University Institutional Review Board for Human Genome/Gene Research (permission number: 678-00), and were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the patients’ guardians. Deciduous teeth were collected from three typically developing children (4, 6, and 7 years old, respectively) and three children with ASD (5, 6, and 7 years old, respectively). None of these 3 ASD children showed comorbidities with chromosomal defects, epilepsy or syndromic phenotypes of tuberous sclerosis, Rett syndrome and other Mendelian disorders. The SHED were isolated as previously described [16], and were cultured in Alpha Modification of Eagle's Medium (Sigma-Aldrich, MO, USA) containing 15% fetal bovine serum (Sigma-Aldrich), 100 µM L-ascorbic acid 2-phosphate (Wako Pure Chemical Industries, Osaka, Japan), 2 mM L-glutamine (Life Technologies, NY, USA), 250 µg/mL fungizone (Life Technologies), 100 U/mL penicillin, and 100 µg/mL streptomycin (Life Technologies), at 37 °C, in an atmosphere containing 5% CO2. The cells were used for further experiments as a heterogeneous cell population, according to the experimental procedures reported by previous studies [9], [10], [11].
2.2. Differentiation of SHED into DN
Differentiation of SHED into DN was induced as described previously [11], but with minor modifications (brain derived neurotrophic factor [BDNF] was excluded except for the experiments shown in Fig. 5). In the first step, 1.5 × 105 SHED were plated in a 6-well culture plate, in the culture medium described above, and incubated overnight at 37 °C, in an atmosphere containing 5% CO2, and were then cultured in serum-free Dulbecco's Modified Eagle's Medium (DMEM, Sigma-Aldrich) supplemented with 20 ng/mL epidermal growth factor (Sigma-Aldrich), 20 ng/mL basic fibroblast growth factor (Peprotech, NJ, USA), and 1% N2 supplement (Life Technologies) for 2 days, at 37 °C, in an atmosphere containing 5% CO2. In the second step, DMEM was replaced with neurobasal medium (Life Technologies) supplemented with 2% B27 supplement (Life Technologies), 1 mM dibutyryladenosine 3,5-cyclic monophosphate (Sigma-Aldrich), 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), and 200 μM ascorbic acid (Nacalai Tesque, Kyoto, Japan), and cells were incubated for 5 days, at 37 °C, in an atmosphere containing 5% CO2. During the second step, no supplementing factors were added. In the experiment shown in Fig. 5, the differentiation of SHED was performed in the presence or absence of 50 ng/mL BDNF in the second step.
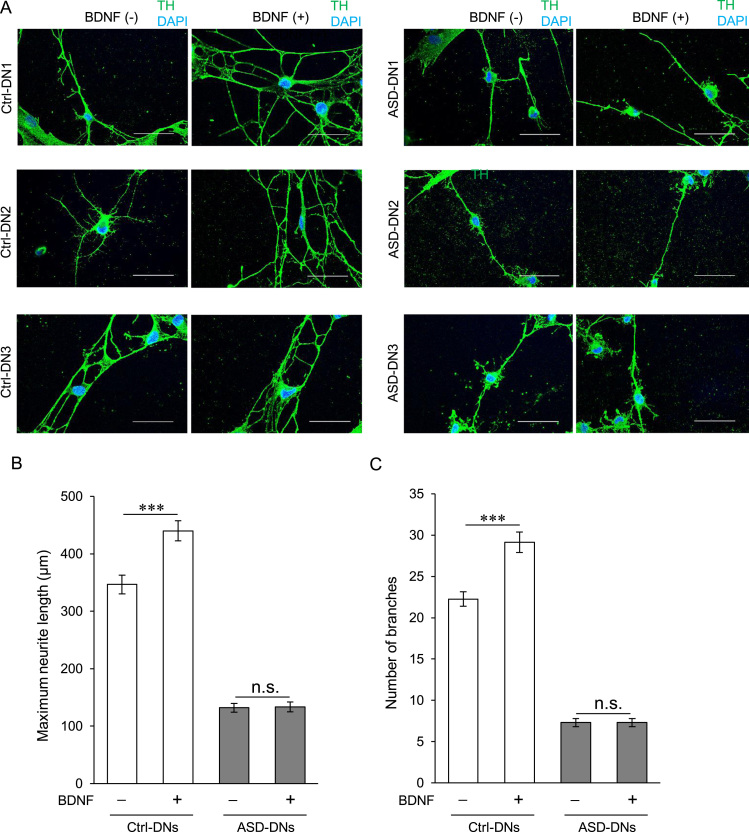
Fig. 5.
Effect of BDNF supplementation on neurite development in ASD-DNs. (A) SHEDs were differentiated into DNs in the presence or absence of BDNF. The DNs were then immunostained with anti-TH antibody and counterstained with DAPI. Scale bar = 50 µm. (B, C) Maximum neurite length (B) and total number of branches per cell (C) of DN were measured. The mean ± SEM from 30 cells from each of the three Ctrl-DNs and ASD-DNs are shown. ***P < 0.001. DN, Dopaminergic neurons; ASD, Autism spectrum disorder; TH, tyrosine hydroxylase; Ctrl-DNs, DN differentiated from stem cells derived from exfoliated deciduous teeth of typically developing children; ASD-DNs, DN differentiated from stem cells derived from exfoliated deciduous teeth of children with ASD; DAPI, 4ˊ,6-diamidino-2-phenylindole dihydrochloride; SEM, standard error of the mean; n.s., not significant.
2.3. Immunocytochemistry
The SHED cultured on a cover glass were fixed with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS, pH 7.4) for 10 min, and were subsequently permeabilized with 0.1% Triton X-100 for 5 min. The cells were blocked with 2% bovine serum albumin in PBS for 20 min. Following this, the cells were incubated with one of the following primary antibodies, for 90 min: anti-Tom20 (Santa Cruz Biotechnology, CA, USA), anti-tyrosine hydroxylase (TH; Proteintech, IL, USA), anti-β-Tubulin III (Sigma-Aldrich). The cells were subsequently incubated with Alexa Fluor-conjugated secondary antibodies (Life Technologies). After staining with secondary antibodies in the dark for 60 min, the nuclei were counterstained with 4ˊ,6-diamidino-2-phenylindole dihydrochloride (DAPI; Dojindo, Kumamoto, Japan). The cover glasses were then mounted on slides, using the ProLong Diamond mounting medium (Life Technologies).
2.4. Analysis of neurite outgrowth and branching in cultured DN
Fluorescence images of DN stained with TH and DAPI were acquired using a Nikon C2 confocal microscope (Nikon, Tokyo, Japan), followed by measurement of the maximum length and total number of neurite branches of each double-positive cell. This measurement was performed using the Neurite Outgrowth module of the MetaMorph software (Molecular Devices, CA, USA) [17].
2.5. Measurement of Tom20-stained area per cell area
Fluorescence images of DN stained with Tom20, TH, and DAPI were acquired using a Nikon C2 confocal microscope (Nikon). The Tom20-stained and TH-stained area of each DN were measured as the mitochondrial and cell area, respectively. The measurement of each area was performed using the Multi Wavelengths Cell Scoring module of the MetaMorph software (Molecular Devices).
2.6. Measurement of mitochondrial membrane potential
Mitochondrial membrane potential (MMP) was measured by staining with JC-1 (Life Technologies), as previously described [16]. Populations of 10,000 cells were analyzed for each sample, and green and red JC-1 signals were detected using the FL1 and FL2 channels, respectively, of a FACSCalibur (BD Bioscience, CA, USA) flow cytometer. The geometric means of FL1 and FL2 were measured using the CellQuest software (BD Bioscience), and the FL2/FL1 ratio was calculated. A higher FL2/FL1 ratio implied high MMP.
2.7. Analysis of intracellular ATP levels
The cells were harvested in ice-cold PBS. To measure intracellular ATP levels, CellTiter-Glo Luminescent Cell Viability Assay (Promega, WI, USA) was used. The ATP luminescence signals were divided by the number of cells.
2.8. Measurement of intracellular calcium levels
In order to measure intracellular calcium (Ca2+) levels, the cells were cultured in µ-dishes (ibidi, Munich, Germany), and then incubated with 2 µM Fluo-4 AM (Life Technologies), 0.05% (w/v) Pluronic F-127 (Sigma-Aldrich), and 500 µM probenecid (Sigma-Aldrich) in Hanks’ Balanced Salt Solution for 1 h, at 37 °C. Fluorescent images of Fluo-4 signal were acquired using a Nikon C2 confocal microscope (Nikon). The fluorescence intensity of Fluo-4 per area, for each cell, was measured using the NIS-Elements software (Nikon).
2.9. Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
The expression of transcription factors that are involved in the specification of the midbrain dopaminergic lineage, pituitary homeobox 3 (PITX3) [18], [19], and nuclear receptor related 1 protein (NURR1) [20], [21] were measured using RT-qPCR. Total RNA extraction and RT-qPCR were performed as previously described [16]. The sequences of the primer sets used in this study were as follows: PITX3, 5′-CCTACGAGGAGGTGTACCCC-3′ (forward) and 5′-AGGCGAATGGAAAGGTCTTGG-3′ (reverse); NURR1, 5′-GCACTTCGGCAGAGTTGAATGA-3′ (forward) and 5′-GGTGGCTGTGTTGCTGGTAGTT-3′ (reverse); and GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-ATGGTGGTGAAGACGCCAGT-3′ (reverse). The relative expression levels of the target genes were analyzed using the comparative threshold cycle method by normalizing them to GAPDH expression levels.
2.10. Statistical analyses
Statistical analyses were performed with the Student's t-test, using SPSS software version 20 (IBM, IL, USA). Values are presented as means ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
3. Results
3.1. Neurite development of DN differentiated from SHED derived from children with ASD
All SHED derived from the three typically developing children and those derived from the three children with ASD were differentiated into cells positive for TH, a DN marker, after being subjected to the differentiation-induction procedure without BDNF (Fig. 1A). There was no significant difference between these two groups in the mRNA expression levels of other midbrain DN markers, PITX3 and NURR1, as well as the ratio of TH-positive cells after differentiation (Fig. 1B and C). However, the maximum neurite length and total number of branches per cell were lower for DN differentiated from SHED derived from children with ASD (ASD-DNs) than for those from the typically developing children (Ctrl-DNs) (Fig. 1A); quantitative analysis showed that this difference was statistically significant (Fig. 1D and E). These data suggest that SHED derived from children with ASD may differentiate into DN, but may not achieve adequate neurite development.
Fig. 1.
Neurite development of DN derived from children with ASD. (A) TH expressing cells are shown for each of the three Ctrl-DNs (1–3) and three ASD-DNs (1–3). Cells were immunostained with anti-TH antibody and counterstained with DAPI. Scale bar = 50 µm. (B) The mRNA expression of PITX3 and NURR1 were measured using quantitative reverse transcription polymerase chain reaction. The relative expression of each gene was calculated using the 2-ΔΔCt method. Graphs show the mean ± SEM from three experiments. (C) The percentage of TH-positive cells were measured. The mean ± SEM from 30 cells from each of the three Ctrl-DNs and ASD-DNs are shown. (D, E) Maximum neurite length (D) and total number of branches per cell (E) of DN were measured. The mean ± SEM from 30 cells from each of the three Ctrl-DNs and ASD-DNs are shown. ***P < 0.001. DN, Dopaminergic neurons; ASD, Autism spectrum disorder; TH, tyrosine hydroxylase; Ctrl-DNs, DN differentiated from stem cells derived from exfoliated deciduous teeth of typically developing children; ASD-DNs, DN differentiated from stem cells derived from exfoliated deciduous teeth of children with ASD; DAPI, 4ˊ,6-diamidino-2-phenylindole dihydrochloride; SEM, standard error of the mean; n.s., not significant.
3.2. Decreased mitochondrial activity and distribution within neurites of ASD-DNs
Neurite development requires large amounts of ATP, mainly supplied by mitochondria. The MMP and ATP production were measured to examine the association between mitochondrial activity and inadequate neurite development in ASD-DNs. Both MMP and ATP levels were significantly lower in ASD-DNs than in Ctrl-DNs (Fig. 2A and B). Neurite development is closely related to mitochondrial recruitment [22]. Thus, we next examined mitochondrial distribution within neurites (Fig. 3A and B). Quantitative analysis showed that the percentage of mitochondria-containing neurites was significantly lower in ASD-DNs than in Ctrl-DNs (Fig. 3C). Tom20-stained area per cell area was significantly lower in ASD-DNs than in Ctrl-DNs (Fig. 3D). These results suggest that the impaired neurite development observed in ASD-DNs is associated with decreased mitochondrial activity and distribution within neurites as well as decreased amount of mitochondria within cells.
Fig. 2.
Decreased mitochondrial activity in ASD-DNs. (A) Ctrl-DNs and ASD-DNs were stained with JC-1, and red and green fluorescent signals were analyzed using flow cytometry. The ratio of red/green was calculated. The mean ± SEM from each three experiments of Ctrl-DNs and ASD-DNs are shown in the graph. *P < 0.05. (B) The ATP levels were measured by luminescence assay. The ATP luminescence signals were divided by the number of cells. Data represent the mean ± SEM from five experiments each for the three Ctrl-DNs and ASD-DNs. *P < 0.05. ASD-DNs, DN differentiated from stem cells derived from exfoliated deciduous teeth of children with autism spectrum disorder; Ctrl-DNs, DN differentiated from stem cells derived from exfoliated deciduous teeth of typically developing children; DN, Dopaminergic neurons; SEM, standard error of the mean.
Fig. 3.
Decreased mitochondrial distribution within neurites of ASD-DNs. (A, B) Ctrl-DNs and ASD-DNs were stained with anti-Tom20 (mitochondrial marker) and anti-β-Tubulin III (neuronal marker) antibodies and counterstained with DAPI; Ctrl-DN1 (A) and ASD-DN1 (B) are shown as representative examples. Scale bar = 50 µm. Details of the boxed region of the merged image are shown in the right panel. Scale bar = 10 µm. (C) The percentage of mitochondria-containing neurites is shown in the graph. The mean ± SEM values obtained after the analysis of 30 cells from each of the three Ctrl-DNs and ASD-DNs are shown. ***P < 0.001. (D) Tom20-stained area/cell area was measured. The mean ± SEM values obtained after the analysis of 30 cells from each of the three Ctrl-DNs and ASD-DNs are shown. ***P < 0.001. ASD-DNs, Dopaminergic neurons differentiated from stem cells derived from exfoliated deciduous teeth of children with autism spectrum disorder; Ctrl-DNs, Dopaminergic neurons differentiated from stem cells derived from exfoliated deciduous teeth of typically developing children; DAPI, 4ˊ,6-diamidino-2-phenylindole dihydrochloride; SEM, standard error of the mean.
3.3. Decreased intracellular calcium levels in ASD-DNs
Calcium plays an important role in neurite development [23]. Calcium metabolism and homeostasis are important mitochondrial functions. The amount of intracellular Ca2+ was significantly lower in ASD-DNs than in Ctrl-DNs (Fig. 4A–D). This suggests that decreased neurite development in ASD-DNs is associated with decreased mitochondria-mediated Ca2+ metabolism.
Fig. 4.
Decreased intracellular calcium concentration in ASD-DNs. (A, B) Ctrl-DNs and ASD-DNs were stained with the calcium indicator dye Fluo-4. Confocal images of Fluo-4 fluorescence of Ctrl-DN1 (A) and ASD-DN1 (B) are shown as representative examples. Scale bar = 50 µm. (C) Fluorescent intensity indicator. (D) The fluorescence intensity per area was measured. The mean ± SEM values from obtained after the analysis of 30 cells from each of the three Ctrl-DNs and ASD-DNs are shown. ***P < 0.001. ASD-DNs, Dopaminergic neurons differentiated from stem cells derived from exfoliated deciduous teeth of children with autism spectrum disorder; Ctrl-DNs, Dopaminergic neurons differentiated from stem cells derived from exfoliated deciduous teeth of typically developing children; SEM, standard error of the mean.
3.4. Effect of BDNF on neurite outgrowth and branching in ASD-DNs
It is known that BDNF promotes neurite outgrowth and branching during neuronal development. We tested whether the decreased neurite development of ASD-DNs observed in the BDNF-free protocol improved upon BDNF supplementation (Fig. 5A–C). Consistent with previous reports, BDNF supplementation was effective in producing maximum neurite outgrowth and total number of branches per cell in Ctrl-DNs. However, no apparent effects of BDNF were observed in ASD-DNs, suggesting impairment of the signaling pathway downstream of BDNF involved in neurite outgrowth and branching.
4. Discussion
In the present study, we differentiated SHED into DN using an induction medium modified from the technique suggested by Fujii et al. [11]; BDNF was not supplemented in our medium, except for the experiment shown in Fig. 5. It is known that BDNF promotes neuronal maturation by autocrine or paracrine mechanisms, impairments in BDNF signaling are involved in the neuropathogenesis of Rett syndrome which involves autistic symptoms [24], [25], [26]. Therefore, a BDNF-supplemented medium may mask the impairment of these mechanisms. We found that the SHED derived from the three children with ASD tested in this study could differentiate into DN without BDNF, but the neurite development was impaired after differentiation, and this was associated with mitochondrial dysfunction, including decreased MMP, ATP levels, mitochondrial transport, and Ca2+ levels. In addition, supplementation of BDNF did not ameliorate the impaired neurite development of ASD-DNs.
Neurite development involves ATP-dependent morphological and functional alterations, such as cell membrane enlargement, cytoskeletal rearrangement, and transport of various substances to the neurite terminals [27]. Therefore, it is important to recruit active mitochondria to the neurite growth cones. The decreased MMP observed in ASD-DNs in this study may have resulted in an insufficient ATP production for neurite development as well as unsuccessful recruitment of mitochondria to the neurites. Kinesin, a motor protein, plays an important role in the recruitment of mitochondria from the cell body to the neurites [28]; Miro1 is one of the factors that binds mitochondria to kinesin, and this binding is inhibited by decreased MMP levels [29]. In addition, the decreased amount of mitochondria in ASD-DNs may also have been involved in the insufficient ATP supply, although the mechanism was not clarified here.
In this study, the intracellular Ca2+ concentration in the ASD-DNs was decreased, suggesting the role of mitochondrial dysfunction in Ca2+ deregulation. Intracellular Ca2+ plays important roles in the regulation of signaling molecules, such as Ca2+/calmodulin-dependent protein kinases (CaMKs), for neuronal development and function [23]. In primary cultures of rat cortical neurons, it has been shown that BDNF-induced neurite outgrowth is promoted by CaMKIIβ-mediated LIM kinase 1 activation [30]. It is likely, therefore, that reduction of intracellular Ca2+ may attenuate neurite development via the BDNF-CaMKIIβ-LIM kinase 1 pathway. This may be supported by our observation that supplementation of BDNF did not show any effect on neurite development of ASD-DNs. In addition, BDNF activates the mitochondrial respiratory complex I and promotes mitochondrial biogenesis via peroxisome proliferator-activated receptor gamma coactivator 1-alpha, suggesting that the interaction between BDNF and mitochondrial function may be involved in neurite development [31], [32].
In this study, there are several limitations to understanding the underlying neuropathology of ASD. First, the neurobiological characteristics of ASD-DNs that we described here do not necessarily reflect the activity and function of DN of the patients we tested. In the brain, the growth and function of DN is modified by interaction with other types of neurons or glial cells [33]. Second, it is impossible to generalize the impaired neurite development of DN to the whole ASD population because ASD shows a broad spectrum, including highly heterogeneous genetic backgrounds. Third, it is not clear whether mitochondrial dysfunction is primary, due to mutations of the genes that directly regulate the function, or secondary, due to the other genetic or functional defects, such as impaired BDNF signaling. To address these concerns, further studies are required, involving co-culture of SHED with other types of cells, more number of subjects, and mutation analysis of mitochondrial and nuclear genes.
In conclusion, we demonstrate that DN differentiated from SHED from children with ASD show impaired neurite development, associated with mitochondrial dysfunction. These results suggest that intracerebral dopamine production may have decreased in these patients. Such findings may, thus, contribute to the development of optimal individualized therapeutic strategies for the treatment of patients in the early stages of ASD.
Acknowledgments
We thank all the members of the Department of Pediatric & Special Needs Dentistry at Kyushu University Hospital for valuable suggestions, technical support, and materials. We appreciate the technical assistance provided by the Research Support Center at the Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences. This work was supported by the Japan Society for the Promotion of Science (KAKENHI Grant nos. JP25670877, JP16K15839, and JP17K17334).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.09.004.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.09.004.
Contributor Information
Hiroki Kato, Email: kato@dent.kyushu-u.ac.jp.
Keiji Masuda, Email: kemasuda@dent.kyushu-u.ac.jp.
Appendix A. Transparency document
Supplementary material
Appendix A. Supplementary material
Supplementary materialSupplemental Fig. 1. Flow cytometry plots for mitochondrial membrane potential of all other subjects
Supplementary materialSupplemental Fig. 2. Mitochondrial localization in dopaminergic neurons of all other subjects
Supplementary materialSupplemental Fig. 3. Calcium levels in dopaminergic neurons of all other subjects
References
- 1.Harris J.C. New classification for neurodevelopmental disorders in DSM-5. Curr. Opin. Psychiatry. 2014;27:95–97. doi: 10.1097/YCO.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 2.Folstein S.E., Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat. Rev. Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 3.Walsh C.A., Morrow E.M., Rubenstein J.L. Autism and brain development. Cell. 2008;135:396–400. doi: 10.1016/j.cell.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Torre-Ubieta L., Won H H., Stein J.L., Geschwind D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016;22:345–361. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen M., Roth A., Kyzar E.J., Poudel M.K., Wong K., Stewart A.M., Kalueff A.V. Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD) Neurochem. Int. 2014;66:15–26. doi: 10.1016/j.neuint.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Pavăl D. A dopamine hypothesis of autism spectrum disorder. Dev. Neurosci. 2017;39:355–360. doi: 10.1159/000478725. [DOI] [PubMed] [Google Scholar]
- 7.Lombard J. Autism: a mitochondrial disorder? Med. Hypotheses. 1998;50:497–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths K.K., Levy R.J. Evidence of mitochondrial dysfunction in autism: biochemical links, genetic-based associations, and non-energy-related mechanisms. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/4314025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Wang X., Sun Z., Wang X., Yang H., Shi S., Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–1383. doi: 10.1089/scd.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii H., Matsubara K., Sakai K., Ito M., Ohno K., Ueda M., Yamamoto A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for parkinsonian rats. Brain Res. 1613;2015:59–72. doi: 10.1016/j.brainres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Ten Cate A.R. Chapter XI. Tooth eruption. In: Bhaskar S.N., editor. Orban's Oral histology and Embryology. Tenth edition. The C. V. Mosby; St Louis: 1986. pp. 361–374. [Google Scholar]
- 13.Zwaigenbaum L., Bryson S., Garon N. Early identification of autism spectrum disorders. Behav. Brain Res. 2013;251:133–146. doi: 10.1016/j.bbr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Anagnostou E., Zwaigenbaum L., Szatmari P., Fombonne E., Fernandez B.A., Woodbury-Smith M., Brian J., Bryson S., Smith I.M., Drmic I., Buchanan J.A., Roberts W., Scherer S.W. Autism spectrum disorder: advances in evidence-based practice. CMAJ. 2014;186:509–519. doi: 10.1503/cmaj.121756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elder J.H., Kreider C.M., Brasher S.N., Ansell M. Clinical impact of early diagnosis of autism on the prognosis and parent-child relationships. Psychol. Res. Behav. Manag. 2017;10:283–292. doi: 10.2147/PRBM.S117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato H., Pham T.T.M., Yamaza H., Masuda K., Hirofuji Y., Han X., Sato H., Taguchi T., Nonaka K. Mitochondria regulate the differentiation of stem cells from human exfoliated deciduous teeth. Cell Struct. Funct. 2017;116:105–116. doi: 10.1247/csf.17012. [DOI] [PubMed] [Google Scholar]
- 17.Leach M.K., Naim Y.I., Feng Z.Q., Gertz C.C., Corey J.M. Stages of neuronal morphological development in vitro – an automated assay. J. Neurosci. Methods. 2011;199:192–198. doi: 10.1016/j.jneumeth.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Saucedo-Cardenas O., Quintana-Hau J.D., Le W.D., Smidt M.P., Cox J.J., De Mayo F., Burbach J.P., Conneely O.M. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zetterström R.H., Solomin L., Jansson L., Hoffer B.J., Olson L., Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 20.Nunes I., Tovmasian L.T., Silva R.M., Burke R.E., Goff S.P. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc. Natl. Acad. Sci. Usa. 2003;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Munckhof P., Luk K.C., Ste-Marie L., Montgomery J., Blanchet P.J., Sadikot A.F., Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- 22.Vaarmann A., Mandel M., Zeb A., Wareski P., Liiv J., Kuum M., Antsov E., Liiv M., Cagalinec M., Choubey V., Kaasik A. Mitochondrial biogenesis is required for axonal growth. Development. 2016;143:1981–1992. doi: 10.1242/dev.128926. [DOI] [PubMed] [Google Scholar]
- 23.Takemoto-Kimura S., Suzuki K., Horigane S.I., Kamijo S., Inoue M., Sakamoto M., Fujii H., Bito H. Calmodulin kinases: essential regulators in health and disease. J. Neurochem. 2017;141:808–818. doi: 10.1111/jnc.14020. [DOI] [PubMed] [Google Scholar]
- 24.Davies A.M., Wright E.M. Neurotrophic factors: neurotrophin autocrine loops. Curr. Biol. 1995;5:723–726. doi: 10.1016/s0960-9822(95)00144-8. [DOI] [PubMed] [Google Scholar]
- 25.Larimore J.L., Chapleau C.A., Kudo S., Theibert A., Percy A.K., Pozzo-Miller L. Bdnf overexpression in hippocampal neurons prevents dendritic atrophy caused by Rett-associated MECP2 mutations. Neurobiol. Dis. 2009;34:199–211. doi: 10.1016/j.nbd.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampathkumar C., Wu Y.J., Vadhvani M., Trimbuch T., Eickholt B., Rosenmund C. Loss of MeCP2 disrupts cell autonomous and autocrine BDNF signaling in mouse glutamatergic neurons. Elife. 2016;5:e19374. doi: 10.7554/eLife.19374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng A., Hou Y., Mattson M.P. Mitochondria and neuroplasticity. ASN Neuro. 2010;2:e00045. doi: 10.1042/AN20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxton W.M., Hollenbeck P.J. The axonal transport of mitochondria. J. Cell Sci. 2012;125:2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y.L., Selkoe D., Rice S., Steen J., LaVoie M.J., Schwarz T.L. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito A., Miyajima K., Akatsuka J., Kondo H., Mashiko T., Kiuchi T., Ohashi K., Mizuno K. CaMKIIβ-mediated LIM-kinase activation plays a crucial role in BDNF-induced neuritogenesis. Genes Cells. 2013;18:533–543. doi: 10.1111/gtc.12054. [DOI] [PubMed] [Google Scholar]
- 31.Markham A., Cameron I., Franklin P., Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur. J. Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 32.Jodeiri Farshbaf M., Ghaedi K., Megraw T.L., Curtiss J., Shirani Faradonbeh M., Vaziri P., Nasr-Esfahani M.H. Does PGC1α/FNDC5/BDNF elicit the beneficial effects of exercise on neurodegenerative disorders? Neuromol. Med. 2016;18:1–15. doi: 10.1007/s12017-015-8370-x. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert J., Man H.Y. Fundamental elements in autism: from neurogenesis and neurite growth to synaptic plasticity. Front. Cell. Neurosci. 2017;11:359. doi: 10.3389/fncel.2017.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary materialSupplemental Fig. 1. Flow cytometry plots for mitochondrial membrane potential of all other subjects
Supplementary materialSupplemental Fig. 2. Mitochondrial localization in dopaminergic neurons of all other subjects
Supplementary materialSupplemental Fig. 3. Calcium levels in dopaminergic neurons of all other subjects