Abstract
In 2001, the Institute of Medicine issued a challenge to the American health care system to improve the quality of care by focusing on six major areas: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity. The patient-centered model of care directly addresses important limits of surgical care of the lumbar spine, i.e., the lack of effective methods for increasing patient participation and engagement in post-operative follow-up. Recent evidence indicates that post-surgical outcomes are better among those with higher patient activation. We therefore developed an intervention based on the principles of motivational interviewing to increase patient activation: the Functional Recovery in Lumbar Spine Surgery Health Behavior Change Counseling (HBCC) intervention. The HBCC was designed to maximize post-operative engagement and participation in physical therapy and home exercise, to improve functional recovery, and to decrease pain in individuals undergoing elective lumbar spine surgery. From December 2009 through October 2012, 120 participants were recruited and divided into two groups: those receiving (intervention group, 60) and not receiving (control group, 60) the HBCC intervention. The current manuscript provides a detailed description of the theoretical framework and study design of the HBCC and describes the implementation of this health behavior intervention in a university-based spine service. The HBCC provides a model for conducting health behavioral research in a real-world setting.
Keywords: Lumbar spine, Spine surgery, Patient activation, Motivational interviewing, Physical therapy, Rehabilitation
1. Introduction
Spine surgery is one of the most common inpatient procedures in the United States [1]. Surgical rates have risen dramatically during the past two decades [1,2], especially among those more than 60 years of age or with degenerative spine conditions. Despite advances in surgical techniques, however, spine surgery outcomes are highly variable and at times poor [3]. Traditionally studied individual characteristics (demographic, physiologic, social, and negative psychologic variables such as depression) do not completely account for this varied outcome [4]. Recent research has highlighted the importance of patient participation in, and taking responsibility for, personal health and recovery [5,6].
The North American Spine Society [7] has recommended post-operative physical therapy after surgery for degenerative conditions of the lumbar spine. The muscles of the back become weakened through pre-operative deconditioning [8] or as a result of surgical incisions through muscle groups [9]. Among the primary objectives of physical therapy are to retrain muscles, to increase strength, and to improve endurance. Adherence to physical therapy after lumbar spine surgery can be influenced by the presence of depressive symptoms and by attitudes, such as motivation to participate in physical therapy.
Patient activation, defined as an individual’s propensity to engage in adaptive health behaviors that may lead to improved health outcomes, has been identified as a potentially important factor in this process [5]. Hibbard et al. [5] conceptualized patient activation and provided a framework for understanding its many aspects: health locus of control, self-efficacy in the self-management behaviors, and readiness to change health-related behaviors. An activated patient forms a partnership with his or her health care provider. The important role of the patient in the management of disease is recognized and promoted in the Collaborative Care model [10]. This model incorporates the patient engaging in activities that promote health and prevent adverse events, interacting with health professionals, adhering to prescribed treatment plans, monitoring emotional and physical health, and managing the effects of illness on the ability to function [11], i.e., it promotes patient activation (Fig. 1).
Fig. 1.
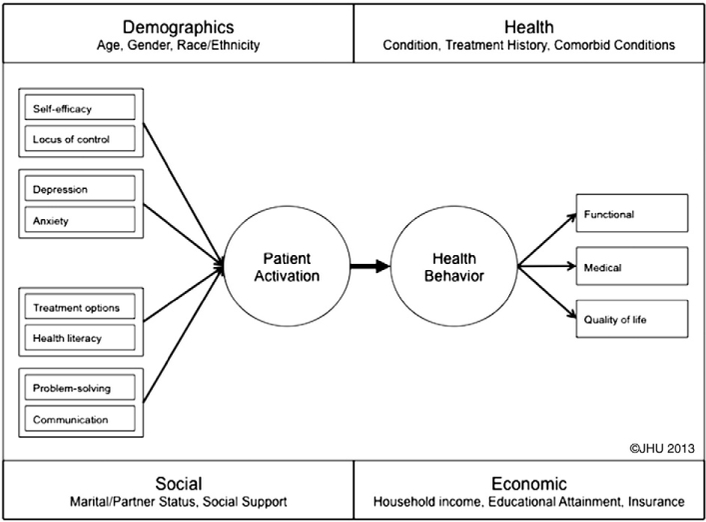
Development and maintenance of patient activation. Copyright 2013 The Johns Hopkins University. Used with permission.
The concept of patient activation directly addresses a shortcoming in the current models for surgical care of the lumbar spine: the lack of effective methods for increasing patient participation in post-operative care, particularly for those who are at risk for poor outcomes. To improve chances of a successful recovery from surgery and return to work, therefore, patients must be encouraged to take an active role in their care, to communicate with their health care providers, and to make necessary behavioral changes. These goals can be achieved by empowering the patient through increasing patient activation and self-efficacy (a person’s ability to complete tasks and to reach goals). The patient-centered care model supports active involvement of patients and their families in decision-making about treatment options. Patient-centered care is critical to remediating the lack of effective methods for increasing patient participation in post-operative care [12,13]. Recent evidence has shown that patient-centered interventions improve outcomes[14].
In pursuit of this goal, we developed a new tool to increase patient activation and, thus, engagement and participation in physical therapy (PT) and home exercise after lumbar spine surgery: the Functional Recovery in Lumbar Spine Surgery (FRiLSS) Health Behavior Change Counseling (HBCC) intervention. The purpose of this report was to describe the HBCC intervention and the study design and rationale. We hypothesized that, compared with individuals who were provided with standard pre-operative care, individuals who underwent a brief HBCC intervention would show: 1) higher patient activation and self-efficacy; 2) greater participation and engagement in post-operative PT; and 3) substantial reduction in post-operative pain, improvement in post-operative functional status, and reduction in post-operative disability.
2. Design and methods
2.1. HBCC intervention: design and development (completed)
The brief HBCC intervention was designed to facilitate patient activation, self-efficacy, and condition-specific knowledge. This paradigm uses motivational interviewing (MI), a collaborative, person-centered form of guidance to elicit and strengthen motivation for change [15]. Developed to address poor treatment outcomes among chronic drinkers [16], MI has been successfully applied to many health behavior problems across diverse patient populations, including weight management for those with obesity [17] and compliance among those with substance abuse problems [18] or human immunodeficiency virus or acquired immune deficiency syndrome [19].
The rationale for the intervention was our previous work [20,21] showing the role that patient activation has on health behavior, the work of the co-investigators in related intervention programs designed to enhance functional outcomes after limb loss [22], and the existent literature showing effective brief interventions based on MI [23–25].
The delivery of this brief HBCC intervention program was based on counseling work by Miller and Rollnick [16] to develop a concrete set of techniques that manifest the principles and practice of MI in a brief encounter. Their original work resulted in a structured session lasting 40 min that has been shown to be effective in reducing problem drinking behavior [16]. The utility of a brief HBCC intervention consisting of a single in-person session with telephone contact has been shown to lead to increased health and functional status among those with multiple sclerosis [26]. Strong empirical evidence exists that a variety of health care professionals of varying levels of training can successfully learn MI counseling via 1- to 2-day training workshops [27].
The development of the HBCC intervention proceeded in two phases: Phase 1, refinement of session content; and Phase 2, training of motivational interviewers.
Based on our previous work in the field, we had developed an a priori format and script for the HBCC intervention. Drs. Wegener and Skolasky worked closely with the research staff and the motivational interviewer to modify this format and script (Tables 1 and 2).
Table 1.
Intervention format for first telephone contacta.
| Note: Review materials from baseline assessment before starting intervention session. | |
|---|---|
| 1. | Greet patient and engage about upcoming surgery. Discuss expectations regarding procedure and what he/she hopes will change as a result of the surgery and rehabilitation. |
| 2. | Present overview of the session. The goals are to: |
| a. Explore the patient’s plan for participation in his/her recovery and how this plan matches up with his/her goals; and | |
| b. Identify concerns the patient may have and what he/she would want change about participating in his/her recovery. | |
| 3. | Indicate you have some information to share, if it’s OK with them. Briefly review the results of the baseline patient activation and his/her confidence to take part in physical therapy following surgery with the patient. “I’ve got some information that may be relevant to our discussion here. It may be similar to what your doctor discussed with you, but this will give us an opportunity to discuss it. Is it OK with you if we go over this a minute?” |
| a. Use importance scale (Likert scale; range, 0 to 10) to explore his/her beliefs about the importance of participation in rehabilitation and recovery process. (“From looking at your views on your role in health care, you indicate that you believe that taking an active role is important. Can you tell me some of the things that you currently do to take an active role in the management of your spine condition? What are you planning to do post-surgery to improve your outcome?”) | |
| b. Use confidence scale (Likert scale; range, 0 to 10) to explore his/her ability to follow through on rehabilitation plans. (“From looking at your assessment of your confidence to take part in physical therapy, you rated it as a 3 out of 7. Tell me what made you chose 3 instead of 0?”… and … “What would help you move from a 3 to a 7”?) | |
| c. Remember the keys to good feedback: | |
| i. Be objective (you are providing them with information that they can take or leave; you are NOT evaluating them); never argue; and | |
| ii. Follow-up with reflections and affirmations. | |
| d. The goal is to maximize change and commitment talk by: | |
| i. Using questions that elicit change talk, by asking for elaboration (if the patient gives a little, ask for more details); | |
| ii. Contrasting his/her goals and expectations for recovery with the level of participation they are planning; | |
| iii. Using the pros and cons technique; | |
| iv. Looking forward to what he/she wants to achieve; and | |
| v. Looking backward to previous successes he/she has had in managing health problems. | |
| e. Avoid attempting to convince him/her to change or argue with his/her perspective. | |
| 4. | Use the key question: “So given all that, how do you feel about participating in your physical therapy after surgery?” Then |
| a. Reflect his/her answer. | |
| b. Affirm the positive dimensions. | |
| c. Ask for commitment to participation. | |
Copyright 2013 The Johns Hopkins University. Used with permission.
Table 2.
Intervention format for subsequent telephone contacta.
| Note: Review materials from baseline assessment before starting intervention session. | |
|---|---|
| 1. | Greet patient and engage about the preceding surgery. Discuss the goals identified during the in-person pre-operative session. |
| 2. | Present overview of the session. The goals are to: |
| a. Explore the patient’s plan for participation in his/her recovery and how this plan matches up with his/her goals; and | |
| b. Identify concerns he/she may have and what he/she would want to change about his/her participation in his/her recovery. | |
| 3. | Indicate you have some information to share, if it’s OK with them. Briefly re-examine with the patient the results of the baseline patient activation and his/her patient’s confidence to take part in physical therapy after surgery. “I’ve got some information that may be relevant to our discussion here. It may be similar to what your doctor discussed with you, but this will give us an opportunity to discuss it. Is it OK with you if we go over this a minute?” |
| a. Use the importance scale (Likert scale; range, 0 to 10) to explore his/her beliefs about the importance of participation in rehabilitation and recovery process. (“From looking at your views on your role in health care, you indicate that you believe that taking an active role is important. Can you tell me some of the things that you currently do to take an active role in the management of your spine condition? What are you planning to do post-surgery to improve your outcome?”) | |
| b. Use the confidence scale (Likert scale; range, 0 to 10) to explore his/her ability to follow through on rehabilitation plans. (“From looking at your assessment of your confidence to take part in physical therapy, you rated it as a 3 out of 7. Tell me what made you chose 3 instead of 0?” … and … “What would help you move from a 3 to a 7”?) | |
| c. Remember the keys to good feedback: | |
| i. Be objective (you are providing them with information that they can take or leave; you are NOT evaluating them); never argue; and | |
| ii. Follow-up with reflections and affirmations. | |
| d. The goal is to maximize change and commitment talk by: | |
| i. Using questions that elicit change talk, by asking for elaboration (if he/she gives a little, ask for more details); | |
| ii. Contrasting his/her goals and expectations for recovery with the level of participation he/she is planning; | |
| iii. Using the pros and cons technique; | |
| iv. Looking forward to what he/she wants to achieve; and | |
| v. Looking backward to previous successes he/she has had in managing health problems. | |
| e. Avoid attempting to convince him/her to change or argue with his/her perspective. | |
| 4. | Use the key question: “So given all that, how do you feel about participating in your physical therapy after surgery?” Then: |
| a. Reflect their answer. | |
| b. Affirm the positive dimensions. | |
| c. Ask for commitment to participation. | |
Copyright 2013 The Johns Hopkins University. Used with permission.
To provide an effective brief HBCC intervention, it was necessary to train a set of individuals to serve as motivational interviewers (Phase 2). The ultimate goal was to have these individuals be health care providers (e.g., nurse practitioners and physician assistants) to maximize the “real-world” application of the current intervention. However, given that we were providing an HBCC session within a research context, we made use of trained motivational interviewers to streamline the delivery of the intervention and to minimize the amount of time that a participant would wait if we had only used health care providers. We provided appropriate training to both the health care providers and these motivational interviewers in the principles and practice of MI [28] (Tables 3 and 4). In these training sessions, the key strategies of MI (e.g., open-ended questions, reflective listening, affirmation, summarization, and elicitation of change talk) were presented and practiced. We conducted focused training sessions of approximately 12 h spread over 3 days in a 2-week period.
Table 3.
Training in motivational interviewing-based health behavior change counseling—fundamentalsa.
| Goal | The development of basic skills in MI to conduct a brief health behavior change counseling intervention for individuals at risk for poor participation in post-surgery rehabilitation for degenerative conditions of the lumbar spine. |
|---|---|
| Background | It has been established that it is possible to train health care providers to an acceptable level of proficiency in MI with a specific training sequence [28]. |
| Training/syllabus | We used a skills-based training strategy for interviewers and health care providers modeled on effective training, supplemented by ongoing monitoring of intervention integrity and booster sessions. The initial training was 12 h spread over 3 days in a 2-week period. The training covered MI principles, the style of MI, description and demonstration of MI methods, and skill-building practice. Interviews were taped and used to monitor treatment integrity and inform booster training practice. Each segment used multiple learning strategies: limited lecture (less than 20%), demonstration, practice (focusing on rehabilitation participation after spine surgery), and feedback (80%). As part of training, the interventionists completed the Helpful Responses Questionnaire before and after training. This questionnaire is designed to assess how trainees demonstrate listening and empathy in their interaction [28]. |
| Maintenance | To maintain intervention quality and integrity during intervention period, there were monthly 1-hour training “booster” sessions over the course of the intervention period. During these sessions, the interviewers reviewed the spirit and principles of MI, practiced the key strategies, and listened to audiotapes of intervention sessions between interventionists and patients. |
MI = motivational interviewing.
Copyright 2013 The Johns Hopkins University. Used with permission.
Table 4.
Training in motivational interviewing-based health behavior change counseling—session specificsa.
| Session | Section/hour title | Concepts addressed |
|---|---|---|
| 1 (hours 1–3) | MI as a style and spirit | Respect for individual’s autonomy and choice |
| Respect the individuals autonomy | ||
| Eliciting the individuals values and goals | ||
| Person-centered versus disorder-centered approach | ||
| Motivation as a state or stage, not a fixed character trait | ||
| Client defensiveness or resistance as a therapeutic process | ||
| Effect of therapist style on client behavior | ||
| Collaboration, not confrontation | ||
| Underlying principles of MI | Express empathy | |
| Develop discrepancy | ||
| Roll with resistance, avoiding argument | ||
| Support self-efficacy | ||
| Stages of patient activation | Believes active role is important | |
| Has the confidence and skills to take action | ||
| Taking action | ||
| Staying the course under times of stress | ||
| 2 (hours 4–7) | MI strategies: OARS | Open-ended questions |
| Affirmations | ||
| Reflective listening | ||
| Summaries | ||
| OARS practice | Types of reflections (simple, amplified, double-sided) | |
| Levels of reflection (repeat, rephrase, paraphrase) | ||
| Exploring ambivalence | Decision balance | |
| Developing discrepancy (exploring goals and values, looking forward) | ||
| The role of and rolling with resistance | What does it look and feel like? (arguing, interrupting, negating or “denial”, ignoring) | |
| What is it? (a cue to change strategies, a normal reaction to having freedoms, an interpersonal process) | ||
| Ways to manage resistance! (reflections, shift focus, reframe, agreement with a twist, emphasize personal choice and control, coming alongside) | ||
| 3 (hours 8–12) | The concept of motivation: confidence and importance Change talk | As related to patient activation |
| Methods of measuring (readiness ruler) | ||
| Recognizing change talk (desire, ability, reasons, needs, commitment level) | ||
| Eliciting change talk (evocative statements, elaboration) | ||
| Developing a change plan | Role of information and advice | |
| Menu options | ||
| Asking for commitment | ||
| Putting it into practice | Specific problems encountered in patients undergoing spine surgery |
MI = motivational interviewing; OARS = open-ended questions, affirmations, reflective listening, and summaries.
Copyright 2013 The Johns Hopkins University. Used with permission.
To prevent “drift” during the application of this intervention, we conducted monthly “booster” sessions. Dr. Skolasky worked closely with the motivational interviewers to review taped intervention sessions and to reinforce the key strategies of MI. During the “booster” sessions, Dr. Skolasky met with the interventionists to review a random sample of intervention sessions recorded on audiotape. During these sessions, the interviewers reviewed the principles and practice of MI, reviewed the key strategies, and listened to audiotapes of actual intervention sessions between interviewers and patients. The first session was a 1-hour session conducted during the second month of the intervention phase; it was designed to maintain the integrity of the brief HBCC intervention. Subsequent monthly sessions followed the same format.
2.2. Implementation (in process)
This ongoing prospective clinical trial uses a two-group, lagged control design (Fig. 2) to evaluate the brief HBCC intervention. Because the intervention is considered a health delivery system intervention, prospective individual randomization is not practicable. However, the two-group, lagged control design prevents contamination across patients by staff who are delivering the brief HBCC intervention. The study was reviewed and approved by our local Institutional Review Board. Patients complete written informed consent.
Fig. 2.
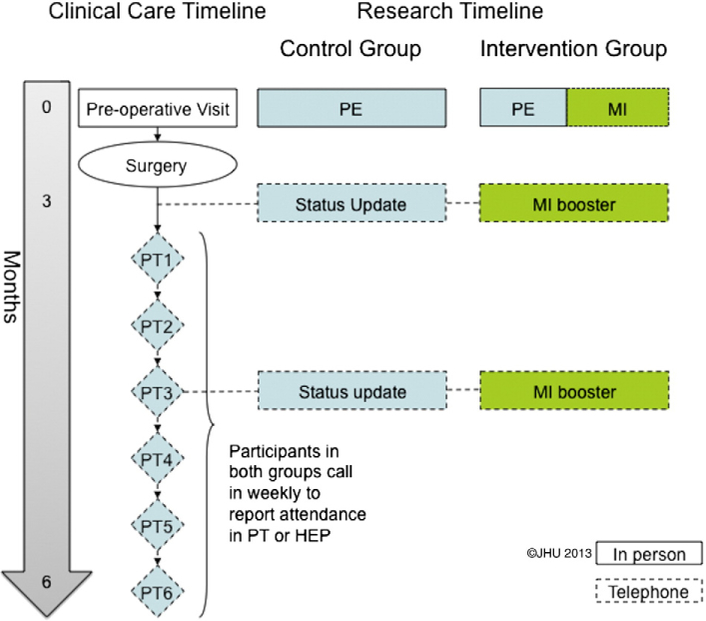
Schematic of control (standard care) and intervention (Health Behavior Change Counseling) groups. PT = physical therapy. HEP = home exercise program. Copyright 2013 The Johns Hopkins University. Used with permission. MI = motivational interviewing. PE = patient education. Solid line = in person. Dotted line = via telephone.
2.2.1. Participant eligibility and initial enrollment
Enrollment began in December 2009 and is completed.
All potential participants in our study are first evaluated in a specialty care clinic in orthopaedic spine surgery or neurosurgery. To be included, patients must be: 1) going to have surgical care for degenerative conditions (i.e., instability [e.g., spondylolisthesis] and deformity [e.g., scoliosis]) of the lumbar spine; 2) able to provide informed consent without proxy assistance; 3) at least 18 years of age; and 4) English-speaking. Specific exclusion criteria include previous surgery of the spine at the currently affected level and ongoing cognitive impairments. Patients with such previous surgery are excluded because recovery of function after revision surgery has a markedly different clinical course than after primary surgery [29].
At our institution, a patient who is a potential candidate for spine surgery is typically seen once before surgical intervention. During this pre-operative visit, the patient meets with the orthopaedic surgeon and a mid-level provider (e.g., a physicians’ assistant or nurse practitioner), undergoes an initial evaluation, discusses the surgical procedure and the recovery process, and receives a treatment plan that includes surgery. Although the implementation of the evaluation of this intervention at a single institution may limit the generalizability of our findings, we believe that it provides a proof-of-concept experience that can be used to guide a multicenter project.
Patients deemed surgical candidates are approached during the pre-operative clinic visit and asked to participate in the HBCC intervention study. After a discussion of the study rationale and procedures, patients are given an opportunity to have any questions addressed. Patients who agree to participate then provide informed consent.
As of October 2012, 120 participants have been enrolled. Based on date of enrollment, they were assigned to one of two study groups: control group (the first 60 did not receive the HBCC intervention) or intervention group (the second 60 received the intervention). Follow-up of both groups is ongoing.
Fourteen patients refused participation in this study. There were no significant differences between their sociodemographic characteristics or clinical presentations and those of the participants in this study.
2.2.2. Control group
To mirror the schema for the HBCC group and to control for attention, individuals in the control group are contacted via telephone twice after surgery. The first telephone call occurs approximately 3 months after surgery, the time when patients typically begin PT and/or home exercise programs (HEPs). The second telephone call occurs approximately 6 months after surgery. During each telephone call, the research staff discusses with the participant their progress after surgery. If the participant raises any questions, he or she is directed to speak with the surgeon. Each telephone call lasts approximately 30 min.
2.2.3. HBBC group
Participants in the HBCC group receive the brief counseling intervention, led by an interviewer trained in MI-based, in the form of a 1-hour pre-operative telephone interview. In the appointment, the interventionist focuses on increasing the participant’s perceived importance of PT or HEP in successful rehabilitation and confidence to follow through on PT or HEP treatment. The interventionist explores the participant’s beliefs about the importance of participation in rehabilitation, clarifies with the participant his or her specific reasons for attending rehabilitation, and helps the individual articulate the anticipated benefits. The interventionist uses these responses as a guide for additional questioning regarding how the patient will take an active role in the management of his or her spine condition and what he or she is planning to do after surgery to improve the outcome. The session ends with the interviewer reflecting the participant’s answers, affirming positive dimensions, and asking for a commitment to participation in rehabilitation.
Each participant in the HBCC intervention group is again contacted by telephone at 3 and 6 months after surgery. Each call serves to identify the barriers that the participant perceives in following through in PT or HEP and to increase the individual’s motivation and commitment to engage in such adaptive health behavior(s). These telephone calls, based on the principles of MI, use collaborative rather than directive communication and last approximately 30 min each.
2.2.4. Assessment of fidelity
Audio recordings are made for all telephone calls with all participants in both groups to assess the fidelity of the HBCC intervention. The integrity of the brief HBCC intervention is enhanced through our training schedule for motivational interviewers; however, standard procedures exist for the assessment of the quality of interventions based on MI. To measure the quality of a planned MI intervention, we use the Motivational Interviewing Treatment Integrity Code, version 2.0, which provides an estimate of how closely the planned intervention meets the principals and techniques that are part of the MI theory [30]. Quality will be assessed both overall and as a function of the motivational interviewer (health care provider versus trained motivational interviewer).
2.2.5. Outcome measures
We determine the success of the brief HBCC intervention by measuring, at regular clinic visit intervals (Table 5), its impact on intermediary, primary, and secondary outcomes.
Table 5.
Assessment schedule.
| Variable | Measure | Pre-operative | Post-operative | ||||
|---|---|---|---|---|---|---|---|
| 3 mos | 6 mos | 12 mos | 24 mos | ||||
| Intermediary outcomes | |||||||
| Patient activationa | Patient Activation Measure | x | x | ||||
| Self-efficacya | Modified Self Efficacy Scale | x | x | ||||
| Primary outcomes | |||||||
| Physical therapy attendancea | Attendance (number of sessions attended/sessions prescribed) | x | |||||
| Physical therapy engagementb | Hopkins Rehabilitation Engagement Rating Scale | x | |||||
| Home exercise attendancea | Attendance (number of sessions attended/sessions prescribed) | x | |||||
| Secondary outcomes | |||||||
| Pain intensitya | Numeric Rating Scale | x | x | x | x | x | |
| Health statusa | Short Form 12, version 2 | x | x | x | x | x | |
| Disabilitya | Oswestry Disability Index | x | x | x | x | x | |
| Correlates | |||||||
| Socio-demographic factorsa | Age, gender, race/ethnicity, primary language, marital status, household size | x | |||||
| Co-morbiditiesa | Elixhauser Comorbidity Measure | x | |||||
| Depressive symptomsa | Patient Health Questionnaire-9 | x | |||||
| Education/economic resourcesa | Education, household income | x | |||||
| Health habitsa | Smoking (frequency/amount), obesity (height and weight) | x | |||||
Data provided through patient self-report.
Data provided through physical therapist report.
Intermediary outcomes consist of patient activation and self-efficacy and are assessed at the pre-operative and 3-month post-operative visit. To assess patient activation, we use the Patient Activation Measure (PAM). Each participant completes this 13-item questionnaire that addresses key cognitive and psychologic factors [31], rating their agreement on individual test items on a scale of strongly disagree to strongly agree. Scores on the PAM are continuous measures ranging from 0 (no activation) to 100 (high activation). A previous report of the use of this questionnaire has revealed an average of 55 points (range of 40 to 80) [5]. The use of the PAM in a cohort of individuals about to undergo spine surgery for degenerative conditions of the lumbar spine has been shown to provide a reliable (intra-class correlation coefficient of 0.87) and valid assessment of patient activation [32].
Self-efficacy to participate in PT or HEP is measured using an instrument designed to assess an individual’s confidence to perform required exercises/tasks, an instrument adapted from the Arthritis Self-Efficacy Scale [33]. There is substantial literature that shows state-dependent customized measures of self-efficacy are useful in predicting behavior [34–40]. Each item is presented as a question (e.g., How certain are you that you can regulate your activity so as to be active without aggravating your back condition?). The respondents rate each belief on the 10-point Likert scale [41].
Primary outcomes consist of attendance and engagement in PT or HEP. Attendance is based on self-report assessments collected weekly during the first 6 weeks of rehabilitation. Rehabilitation typically begins after the 3-month post-operative visit. This assessment consists of responses to two questions: 1) How many sessions of PT/home exercise were prescribed for you in the past 7 days? and 2) How many sessions of PT/home exercise did you attend in the past 7 days? An overall average attendance was computed for the 6 weeks.
Engagement in PT is based on physical-therapist-reported assessments using the Hopkins Rehabilitation Engagement Rating Scale and is assessed at the 6-month post-operative visit [42]. This instrument is a five-item Likert scale for rating behavioral observations of a patient during PT. It has been used for individuals with spinal cord injuries, stroke, amputations, and hip or knee replacement and has been established as a consistent (Cronbach’s alpha >0.90) and reliable (test–retest 0.73) measure of engagement in rehabilitative therapy [42]. Evidence for its validity has been established through correlation with key clinical indicators. It is important to note that the physical therapist is unaware as to which treatment group the patient belongs and that because The Johns Hopkins Spine Center does not have an integrated rehabilitation unit, each patient can select his or her own therapist, thereby eliminating potential bias.
Secondary outcomes consist of pain intensity, health status, and disability and are assessed longitudinally at the pre-operative and 3-, 6-, 12-, and 24-month post-operative visits. Pain intensity is measured using the Numeric Rating Scale [43], with respondents reporting pain intensity on an 11-point scale (0 [no pain] to 10 [severe pain]). The Numeric Rating Scale has proven reliable (Pearson’s r > 0.80) and valid (highly correlated with the visual analog scale) in older as well as younger adults and is simple to administer and easy to score [44,45]. More specifically, it has been shown to be free of the response error associated with other pain intensity scales when measuring pain among individuals aged 65 years old or more [46,47].
Health status is measured using SF-12v2 [48], a standard patient-centered measure. It is an abbreviated version of the 36-item SF-36 that includes the physical and emotional limitations placed on work and social activities. The SF-12v2 provides a measure of how an individual values his or her current health state and has been shown to be a reliable (Pearson’s r > 0.70), valid (highly correlated with SF-36), and responsive measure of health status in many patient populations [49,50].
Disability is measured using the Oswestry Disability Index (ODI) [51]. The ODI is a disease-specific instrument that assesses the impact of spinal disorders on 10 aspects of daily living. These aspects address basic functions such as walking or climbing stairs and participation such as sex-life and social activities. The ODI has been shown to have excellent re-test reliability (Pearson’s r N 0.80) and validity (moderately high correlations with McGill Pain Questionnaire and visual analog scale for pain) [51]. An expert panel convened by the journal Spine recommended use of this instrument because it has shown good psychometric properties [51].
Correlates are assessed at the pre-operative visit and include: 1) socio-demographic characteristics, 2) presence of co-morbid conditions, 3) depressive symptoms, 4) education and economic resources, and 5) health habits. Socio-demographic characteristics of the patient, including age, gender, race, ethnicity, and primary language are assessed through self-report using a standard instrument. Education/economic resources, including highest grade/degree attained, and household income are assessed through self-report. Co-morbidities are assessed using self-report presence of other medical conditions via the Elixhauser Comorbidity measure [52]. Depressive symptoms are measured with the Patient Health Questionnaire-9 [53,54], a brief screening tool designed to identify the presence of depressive symptoms. Health habits are assessed through self-report. Two health habits measured are cigarette smoking and obesity. Additional variables that will be included in the analysis include:1) surgical procedure (e.g. fusion, laminectomy, etc.) and number of levels involved and 2) rehabilitation therapy (e.g. scheduled and attended).
3. Statistical methods and power analysis
3.1. Measurement parameters and group size
Patient activation will be assessed for all participants using the PAM pre-operatively (enrollment) and at the 3-month post-operative visit. Our previous work in this field has shown good test–retest reliability for this measure [20,41,55]; therefore, any changes in observed PAM score are attributed to changes in underlying patient activation. PAM scores are translated to measures of patient activation ranging from 0 (no activation) to 100 (perfect activation). The change in patient activation from baseline to 6 months after surgery will be computed for each individual. A paired t-test will test the null hypothesis of no difference in change from baseline between the two study groups. Based on our previous research examining the relationship between patient activation and adherence to PT [20], it was determined that a clinically meaningful difference in PAM score of 10 points led to increased adherence to PT. We also determined that it would be necessary to enroll and follow 108 participants (54 per group) to yield >80% power to reject the null hypothesis (assuming a 10% loss to follow-up, disenrollment, or failure to collect both administrations of the PAM).
Evidence for the impact of the intervention will be assessed through comparison of the primary outcome measures between the control (standard care) and intervention (HBCC intervention) groups. An analysis of variance will be used to estimate mean differences in participation and engagement between the control and intervention groups. Our experience with adherence to post-operative PT among individuals undergoing elective lumbar spine surgery provides us with reasonable estimates of clinically meaningful differences and standard deviations (SD) [20]. With respect to participation, our previous study showed that individuals in the lower two quartiles of patient activation reported attending 58% of their PT sessions, whereas individuals in the upper two quartiles of patient activation reported attending 88% of their PT sessions [20]. To show effectiveness of the intervention, we have estimated that individuals in the intervention group would approximate the health behavior of those in the upper two quartiles of patient activation. Therefore, to detect as significant the difference between 58% and 88% (SD 28%), it would be necessary to enroll 51 individuals per group (assuming type I error of 2.5%, power of 80%, and 10% loss to follow-up, disenrollment, or failure to collect participation measures). With respect to engagement, our previous study showed that individuals in the lower two quartiles of participation scored 18.68 (SD 5.18), whereas individuals in the upper two quartiles scored 24.39 (SD 3.86) [20]. Following the same logic as our comparison of participation, we would need to enroll 27 individuals per group (assuming type I error of 2.5%, power of 80%, and 20% loss to follow-up, disenrollment, or failure to collect engagement measures).
The secondary outcomes are assessed longitudinally and will be compared between the two groups. This comparison will be conducted using a repeated measures mixed models analysis to allow fixed effects for the intervention and random effects for each participant’s pre-operative measure. Assuming a clinically meaningful difference in the change scores for the pain intensity of two Likert points, it would be necessary to enroll 51 individuals each in the control and intervention groups (assuming type I error of 1.7%, power of 80%, and 10% loss to follow-up, disenrollment, or failure to collect pain intensity measure). Assuming a clinically meaningful difference in the change scores for the component scores of the SF-12v2 [54] of 10 points, it would be necessary to enroll 54 individuals each in the control and intervention groups (assuming type I error of 1.7%, power of 80%, and 10% loss to follow-up, disenrollment, or failure to collect functional measure). A similar analytic strategy was used to test the significance of observed differences in the pre- and post-operative administrations of the ODI [53]; 48 individuals were required in each group (assuming type I error of 1.7%, power of 80%, and 10% loss to follow-up, disenrollment, or failure to collect functional measure).
In addition to an examination of long-term functional recovery, it is useful to examine recovery at intermediate time points. For this purpose, the assessment schedule included collection of pain intensity and functional status at 3, 6, 12, and 24 months after surgery. Using repeated measures analysis of variance, within-subjects and between-subjects factors were examined. Equivalence of functional recovery at 24 months between the control and intervention groups would indicate earlier recovery, translating to early improvements in quality of life, among those receiving the intervention. This finding would be deemed a success for the intervention.
Based on these sample size estimates, we enrolled 120 participants.
3.2. Baseline demographic characteristics
The control and intervention groups were not statistically different in terms of socio-demographic characteristics (Table 6). The mean age was 59 ± 13.3 years. The participants were predominantly female (63.1%), married or living with spouse or partner (73.8%), white (77.1%), and non-Hispanic (95.1%). Most individuals reported a household income of more than $50,000 annually (63.9%) and an educational attainment of less than college (57.4%).
Table 6.
Baseline characteristics of participants enrolled in the FRiLSS HBCC study.
| Characteristic | Overall (n = 122) | Control (standard care) group (n = 59) | Intervention (HBCC) group (n = 63) | P valuea |
|---|---|---|---|---|
| Mean age (yr ± SD) | 59.0 ±13.3 | 58.1 ± 13.5 | 59.9 ±13.2 | .449 |
| Female gender (%) | 77 (63.1) | 39 (66.1) | 38 (60.3) | .508 |
| Marital status | ||||
| Married/living with spouse (%) | 87 (71.3) | 44 (74.6) | 43 (68.3) | .731 |
| Living with partner (%) | 3 (2.5) | 1 (1.7) | 2 (3.2) | |
| Separated, divorced, or widowed (%) | 24 (19.7) | 10 (17.0) | 14 (22.2) | |
| Never married (%) | 8 (6.6) | 4 (6.8) | 4 (6.4) | |
| Race | ||||
| White (%) | 94 (77.1) | 45 (76.3) | 49 (77.8) | .328 |
| Black (%) | 23 (18.9) | 13 (22.0) | 10 (15.9) | |
| Other (%) | 5(4.1) | 1 (1.7) | 4 (6.4) | |
| Ethnicity | ||||
| Hispanic (%) | 6 (4.9) | 1 (1.7) | 5 (7.9) | .111 |
| Non-Hispanic (%) | 116 (95.1) | 58 (98.3) | 58 (92.1) | |
| Household income | ||||
| <$30,000 (%) | 19 (15.7) | 9 (15.3) | 10 (15.9) | .384 |
| $30,000-$50,000 (%) | 13 (10.7) | 7(11.9) | 6 (9.5) | |
| >$50,000 (%) | 78 (63.9) | 40 (67.8) | 38 (60.3) | |
| Not reported (%) | 12 (9.8) | 3(5.1) | 9 (14.3) | |
| Education | ||||
| <College (%) | 70 (57.4) | 33 (55.9) | 37 (58.7) | .141 |
| College Degree (%) | 23 (18.9) | 8 (13.6) | 15 (23.8) | |
| Post-graduate Degree/Study (%) | 29 (23.8) | 18 (30.5) | 11 (17.5) | |
FRiLSS = Functional recovery in lumbar spine surgery; HBCC = Health behavior change counseling; SD = Standard deviation.
Comparison of standard care and HBCC intervention groups.
We are in the process of following participants according to our schedule of assessments. Once data collection is complete, a two-sample comparison will be used to statistically evaluate the effectiveness of the brief HBCC intervention in increasing patient activation and impacting post-operative health behavior and recovery.
4. Discussion
Recent evidence has suggested that patient activation may have an influence on post-operative health behavior [20] and functional recovery [21] after lumbar spine surgery. The current FRiLSS study will allow for a comparison of an intervention designed to increase patient activation with standard care.
The implementation of the FRiLSS study was accomplished in partnership with the Spine Outcomes Research Center and surgeons and other health care providers in the Departments of Neurosurgery and Orthopaedic Surgery at The Johns Hopkins University School of Medicine. Given the nature of the HBCC intervention, namely to increase an individual’s engagement in his or her own health and recovery, a traditional randomized clinical trial would not provide an adequate protection against interventionist contamination. The proposed brief HBCC intervention seeks to change the way that the health care providers interact with the patient. Once providers integrate MI-based HBCC into their practice it would not be feasible to expect them to not use these interaction strategies in other patients. Thus, in the design and implementation of this study, it was necessary to use a lagged-control design.
The conceptual model that forms the foundation for the FRiLSS study incorporates patient activation as an intermediary factor in the influence of biologic, psychosocial, and demographic factors on health behavior and recovery after lumbar spine surgery. The FRiLSS study will collect data on intermediate and final endpoints. By collecting information on health behavior and functional recovery, we will be able to determine early and long-term influences of the HBCC intervention. In the case that long-term differences are not seen, we may still be able to detect differences in postoperative health behavior. This finding could lead to additional work to augment the PT or HEP that patients are prescribed. In addition, the collection of correlated information, such as demographic and socio-economic characteristics, will allow us to examine the relationship between the intervention and patient activation in the setting of these potentially confounding variables. This will be important as the analysis may identify groups of patients for whom the intervention is more effective.
5. Conclusion
There is considerable research documenting the importance of an individual’s participating in and taking responsibility for his or her health and recovery [21]. Patient activation has been defined as an individual’s propensity to engage in adaptive health behaviors that may lead to improved health outcomes. Patient activation has been identified as a mediator between psychologic factors and personal competencies and health behavior and functional recovery [5].
Promoting patient activation is central to effective patient-centered care. The Institute of Medicine issued a challenge to the American health care system to improve the quality of care by addressing six major areas: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity [56]. The FRiLSS study incorporates this recommendation to focus on positive psychologic characteristics and personal competencies of individuals undergoing lumbar spine surgery to increase the likelihood of positive health outcomes.
Acknowledgments
Role of funding source
The FRiLSS HBCC trial is supported by a grant from the Agency for Healthcare Research and Quality (1 R01 HS 017990).
Abbreviations:
- FRiLSS
Functional Recovery in Lumbar Spine Surgery
- HBCC
Health Behavior Change Counseling
- HEP
home exercise programs
- MI
motivational interviewing
- ODI
Oswestry Disability Index
- PAM
patient activation measures
- PT
physical therapy
- SD
standard deviation
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
References
- [1].Davis H Increasing rates of cervical and lumbar spine surgery in the United States, 1979–1990. Spine (Phila Pa 1976) 1994;19(10):1117–23. [DOI] [PubMed] [Google Scholar]
- [2].Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30(12):1441–5. [DOI] [PubMed] [Google Scholar]
- [3].Junge A, Frohlich M, Ahrens S, Hasenbring M, Sandler AJ, Grob D, et al. Predictors of bad and good outcome of lumbar spine surgery: a prospective clinical study with 2 years’ follow-up. Spine (Phila Pa 1976) 1996;21(9):1056–64. [DOI] [PubMed] [Google Scholar]
- [4].Dvorak J, Gauchat MH, Valach L. The outcome of surgery for lumbar disc herniation. I. A 4–17 years’ follow-up with emphasis on somatic aspects. Spine (Phila Pa 1976) 1988;13(12):1418–22. [DOI] [PubMed] [Google Scholar]
- [5].Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39(4): 1005–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med 2012;27(5):520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].North American Spine Society Task Force on Clinical Guidelines. Phase III Clinical Guidelines for Multidisciplinary Spine Care Specialists. Spondylosis, Lytic Spondylolisthesis, and Degenerative Spondyloslisthesis (SLD). LaGrange (IL). North American Spine Society; 2000. [Google Scholar]
- [8].Verbunt JA, Seelen HA, Vlaeyen JW, van der Heijden GJ, Knottnerus JA. Fear of injury and physical deconditioning in patients with chronic low back pain. Arch Phys Med Rehabil 2003;84(8):1227–32. [DOI] [PubMed] [Google Scholar]
- [9].Greiner-Perth R, Bohm H, Allam Y. A new technique for the treatment of lumbar far lateral disc herniation: technical note and preliminary results. Eur Spine J 2003;12(3):320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996;74(4):511–44. [PubMed] [Google Scholar]
- [11].Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann Intern Med 1997;127(12):1097–102. [DOI] [PubMed] [Google Scholar]
- [12].Stewart M Towards a global definition of patient centred care. BMJ 2001;322(7284):444–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wagner EH, Bennett SM, Austin BT, Greene SM, Schaefer JK, Vonkorff M. Finding common ground: patient-centeredness and evidence-based chronic illness care. J Altern Complement Med 2005;11(Suppl. 1):S7–S15. [DOI] [PubMed] [Google Scholar]
- [14].Michie S, Miles J, Weinman J. Patient-centredness in chronic illness: what is it and does it matter? Patient Educ Couns 2003;51(3):197–206. [DOI] [PubMed] [Google Scholar]
- [15].Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother 2009;37(2):129–40. [DOI] [PubMed] [Google Scholar]
- [16].Miller WR, Rollnick S. Motivational interviewing. Preparing people for change. New York: The Guilford Press; 2002. [Google Scholar]
- [17].Carels RA, Darby L, Cacciapaglia HM, Konrad K, Coit C, Harper J, et al. Using motivational interviewing as a supplement to obesity treatment: a stepped-care approach. Health Psychol 2007;26(3):369–74. [DOI] [PubMed] [Google Scholar]
- [18].Samet JH, Rollnick S, Barnes H. Beyond CAGE. A brief clinical approach after detection of substance abuse. Arch Intern Med 1996;156(20): 2287–93. [DOI] [PubMed] [Google Scholar]
- [19].Golin CE, Earp J, Tien HC, Stewart P, Porter C, Howie L. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr 2006;42(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Skolasky RL, MacKenzie EJ, Wegener ST, Riley III LH. Patient activation and adherence to physical therapy in persons undergoing spine surgery. Spine (Phila Pa 1976) 2008;33(21):E784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Skolasky RL, Mackenzie EJ, Wegener ST, Riley III LH. Patient activation and functional recovery in persons undergoing spine surgery. J Bone Joint Surg Am 2011;93(18):1665–71. [DOI] [PubMed] [Google Scholar]
- [22].Wegener ST, Mackenzie EJ, Ephraim P, Ehde D, Williams R. Self-management improves outcomes in persons with limb loss. Arch Phys Med Rehabil 2009;90(3):373–80. [DOI] [PubMed] [Google Scholar]
- [23].Williams GC, McGregor H, Zeldman A, Freedman ZR, Deci EL, Elder D. Promoting glycemic control through diabetes self-management: evaluating a patient activation intervention. Patient Educ Couns 2005;56(1): 28–34. [DOI] [PubMed] [Google Scholar]
- [24].Hibbard JH, Greene J, Tusler M. Improving the outcomes of disease management by tailoring care to the patient’s level of activation. Am J Manag Care 2009;15(6):353–60. [PubMed] [Google Scholar]
- [25].Linden A, Butterworth SW, Prochaska JO. Motivational interviewing-based health coaching as a chronic care intervention. J Eval Clin Pract 2010;16(1):166–74. [DOI] [PubMed] [Google Scholar]
- [26].Bombardier CH, Cunniffe M, Wadhwani R, Gibbons LE, Blake KD, Kraft GH. The efficacy of telephone counseling for health promotion in people with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil 2008;89(10):1849–56. [DOI] [PubMed] [Google Scholar]
- [27].Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72(6):1050–62. [DOI] [PubMed] [Google Scholar]
- [28].Miller WR, Hedrick KE, Orlofsky DR. The Helpful Responses Questionnaire: a procedure for measuring therapeutic empathy. J Clin Psychol 1991;47(3): 444–8. [DOI] [PubMed] [Google Scholar]
- [29].Eichholz KM, Ryken TC. Complications of revision spinal surgery. Neurosurg Focus 2003;15(3):E1–4. [DOI] [PubMed] [Google Scholar]
- [30].Moyers TB, Martin T, Manuel JK, Miller WR, Ernst D. Revised global scales: motivational interviewing treatment integrity 3.1.1 (MITI 3.1.1). Available at http://casaa.unm.edu/download/MITI3_1.pdf. [Accessed on February 7, 2013].
- [31].Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the Patient Activation Measure. Health Serv Res 2005;40(6):1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Skolasky RL Jr. Functional recovery in lumbar spine surgery: understanding patient activation. Doctor of Science Dissertation, Bloomberg School of Public Health. Baltimore, MD: The Johns Hopkins University; 2007. [Google Scholar]
- [33].Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum 1989;32(1):37–44. [DOI] [PubMed] [Google Scholar]
- [34].Buckelew SP, Huyser B, Hewett JE, Parker JC, Johnson JC, Conway R, et al. Self-efficacy predicting outcome among fibromyalgia subjects. Arthritis Care Res 1996;9(2):97–104. [DOI] [PubMed] [Google Scholar]
- [35].Colon RM, Wiatrek DE, Evans RI. The relationship between psychosocial factors and condom use among African-American adolescents. Adolescence 2000;35(139):559–69. [PubMed] [Google Scholar]
- [36].Fontaine KR, Cheskin LJ. Self-efficacy, attendance, and weight loss in obesity treatment. Addict Behav 1997;22(4):567–70. [DOI] [PubMed] [Google Scholar]
- [37].Fortenberry JD, Brizendine EJ, Katz BP, Orr DP. The role of self-efficacy and relationship quality in partner notification by adolescents with sexually transmitted infections. Arch Pediatr Adolesc Med 2002;156(11):1133–7. [DOI] [PubMed] [Google Scholar]
- [38].Sitharthan T, Kavanagh DJ. Role of self-efficacy in predicting outcomes from a programme for controlled drinking. Drug Alcohol Depend 1990;27(1): 87–94. [DOI] [PubMed] [Google Scholar]
- [39].Strecher VJ, DeVellis BM, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Health Educ Q 1986;13(1):73–92. [DOI] [PubMed] [Google Scholar]
- [40].Zebracki K, Drotar D. Outcome expectancy and self-efficacy in adolescent asthma self-management. Child Health Care 2004;33(2): 133–49. [Google Scholar]
- [41].Skolasky RL, Mackenzie EJ, Riley III LH, Wegener ST. Psychometric properties of the Patient Activation Measure among individuals presenting for elective lumbar spine surgery. Qual Life Res 2009;18(10): 1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kortte KB, Falk LD, Castillo RC, Johnson-Greene D, Wegener ST. The Hopkins Rehabilitation Engagement Rating Scale: development and psychometric properties. Arch Phys Med Rehabil 2007;88(7):877–84. [DOI] [PubMed] [Google Scholar]
- [43].Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis 1978;37(4):378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Breivik EK, Bjornsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain 2000;16(1):22–8. [DOI] [PubMed] [Google Scholar]
- [45].Flaherty SA. Pain measurement tools for clinical practice and research. AANA J 1996;64(2):133–40. [PubMed] [Google Scholar]
- [46].Delmas PD. Clinical effects of strontium ranelate in women with postmenopausal osteoporosis. Osteoporos Int 2005;16(Suppl. 1):S16–9. [DOI] [PubMed] [Google Scholar]
- [47].Gagliese L, Weizblit N, Ellis W, Chan VWS. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain 2005;117(3):412–20. [DOI] [PubMed] [Google Scholar]
- [48].Hurst NP, Ruta DA, Kind P. Comparison of the MOS short form-12 (SF12) health status questionnaire with the SF36 in patients with rheumatoid arthritis. Br J Rheumatol 1998;37(8):862–9. [DOI] [PubMed] [Google Scholar]
- [49].Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- [50].Luo X, George ML, Kakouras I, Edwards CL, Pietrobon R, Richardson W, et al. Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine (Phila Pa 1976) 2003;28(15): 1739–45. [DOI] [PubMed] [Google Scholar]
- [51].Fairbank J Use of Oswestry Disability Index (ODI) [letter]. Spine (Phila Pa 1976) 1995;20(13):1535–6. [PubMed] [Google Scholar]
- [52].Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- [53].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Spitzer RL, Williams JBW, Kroenke K, Linzer M, de Gruy III FV, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272(22):1749–56. [PubMed] [Google Scholar]
- [55].Skolasky RL, Riley III LH, Albert TJ. Psychometric properties of the Cervical Spine Outcomes Questionnaire and its relationship to standard assessment tools used in spine research. Spine J 2007;7(2): 174–9. [DOI] [PubMed] [Google Scholar]
- [56].Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, D.C: National Academy Press; 2001. [Google Scholar]


