Abstract
Objective:
To determine the effect of health behavior change counseling (HBCC) on patient activation and the influence of patient activation on rehabilitation engagement, and to identify common barriers to engagement among individuals undergoing surgery for degenerative lumbar spinal stenosis.
Design:
Prospective clinical trial.
Setting:
Academic medical center.
Participants:
Consecutive lumbar spine surgery patients (N=122) defined in our companion article (Part I) were assigned to a control group (did not receive HBCC, n=59) or HBCC group (received HBCC, n=63).
Intervention:
Brief motivational interviewing-based HBCC versus control (significance, P<.05).
Main Outcome Measures:
We assessed patient activation before and after intervention. Rehabilitation engagement was assessed using the physical therapist-reported Hopkins Rehabilitation Engagement Rating Scale and by a ratio of self-reported physical therapy and home exercise completion. Common barriers to rehabilitation engagement were identified through thematic analysis.
Results:
Patient activation predicted engagement (standardized regression weight, .682; P<.001). Postintervention patient activation was predicted by baseline patient activation (standardized regression weight, .808; P<.001) and receipt of HBCC (standardized regression weight, .444; P<.001). The effect of HBCC on rehabilitation engagement was mediated by patient activation (standardized regression weight, .079; P=.395). One-third of the HBCC group did not show improvement compared with the control group. Thematic analysis identified 3 common barriers to engagement: (1) low self-efficacy because of lack of knowledge and support (62%); (2) anxiety related to fear of movement (57%); and (3) concern about pain management (48%).
Conclusions:
The influence of HBCC on rehabilitation engagement was mediated by patient activation. Despite improvements in patient activation, one-third of patients reported low rehabilitation engagement. Addressing these barriers should lead to greater improvements in rehabilitation engagement.
Keywords: Laminectomy, Motivational interviewing, Patient participation, Rehabilitation, Spinal stenosis
Degenerative lumbar spine disorders are the leading cause of disability,1 comprising a proportion larger than that of all other musculoskeletal conditions combined.2 Surgery is indicated in the management of patients with severe neurogenic claudication caused by degenerative spinal stenosis when nonoperative treatment has failed.3 Annual Medicare spending on spine surgery (one of the fastest growing inpatient procedures, especially among those aged >50y4) has increased to more than $1 billion,5 similar to the per-patient costs associated with diabetes and cardiovascular disease.6 The surgical technique for lumbar degenerative conditions is well established, and the benefits of surgical care have been documented.7,8 The Spine Patient Outcomes Research Trial9 found that surgery led to significant reduction in pain and disability and improvement in health status at 2 and 4 years after surgery. However, even with appropriate patient selection, up to 40% of patients treated surgically report persistent pain, disability, and poor quality of life, and approximately 20% of patients require reoperation.10–13
Although there is strong evidence for the benefits of post-surgical intensive exercise programs on short-term functional status and earlier return to work (see Part I of our companion article), such rehabilitative exercise programs require active participation and engagement on the part of the patient. The importance of individuals actively participating in their own health and recovery has been highlighted by recent research on the role of patient activation.14–16 Patient activation measures an individual’s propensity to engage in positive health behavior. Promoting patient activation is a core component to patient-centered care.17 The goal of addressing patient activation and engagement is to overcome a major limitation in the treatment of persons undergoing lumbar spine surgery: the lack of effective methods to increase patient participation and responsibility in those who are at high risk for poor outcomes. It has been argued that empowering and engaging patients through increasing patient activation is critical to addressing this problem and is the central distinction between patient-centered care and other health care quality improvement initiatives.18,19 Previous work has shown the relationship between high patient activation and high rehabilitation engagement20 and better functional recovery.21 Patients with low activation are less likely to attend prescribed physical therapy (PT) sessions and are rated as less engaged by their therapists than individuals with high activation. In addition, patients with high activation experience a greater reduction in disability and a greater improvement in physical function after surgery compared with individuals with low activation.21
Health behavior change counseling (HBCC) is a brief telephone-administered intervention, based on the principals and strategies of motivational interviewing (MI), to improve rehabilitation engagement among individuals undergoing spine surgery.22 Interventions based on MI and delivered via telephone have been shown to lead to improved health and functional status among individuals with multiple sclerosis23,24 and moderate to severe traumatic brain injury. In our companion article (Part I), we showed that persons who received the HBCC intervention had better rehabilitation engagement after undergoing lumbar spine surgery compared with those who did not receive the HBCC intervention.
Our objectives for the current analysis were to (1) determine whether changes in patient activation mediate the relationship between HBCC and rehabilitation engagement, and (2) use qualitative methods to identify the barriers to rehabilitation engagement.
Methods
Our institutional review board approved this study. Informed consent was obtained from all participants. We conducted all research-related events in a private room to ensure confidentiality.
Study population
This study was conducted in 122 individuals undergoing lumbar spine surgery who were assigned to a control group (did not receive HBCC, n=59) or HBCC group (received HBCC, =Z63). For additional details on the study population, see our companion article (Part I).
Participant assessment
Patients were assessed with the Patient Activation Measure, the Hopkins Rehabilitation Engagement Rating Scale (HRERS), and self-report of PT/home exercise program (HEP) completion.
Demographic and social information
We used a patient-completed questionnaire to gather sociodemo-graphic information (eg, age, sex, race/ethnicity, education, household income; see Part I).
Patient activation
The Patient Activation Measure is a participant-completed 13-item questionnaire that addresses key psychological factors and personal competencies related to engagement in health behavior.25 The validity of the scale has been established through correlation with key clinical indicators, such as overall self-efficacy to participate in PT26 and self-management behaviors.25 The Patient Activation Measure has been shown to be a reliable and valid assessment of patient activation in a cohort of individuals about to undergo surgery for low back pain.26 In our study population, patient activation was assessed before and after the intervention.
Engagement in rehabilitation
Engagement in rehabilitation was measured with the physical therapist-reported HRERS27 and self-reported attendance in PT and HEP based on assessments after 6 weeks of PT (see Part I). The HRERS allows the physical therapist to rate a patient’s engagement across 5 items (attendance, need for additional prompts, positive attitude, acknowledged need for rehabilitative services, active participation) using a 6-point scale ranging from “never” to “always.”
Statistical analyses
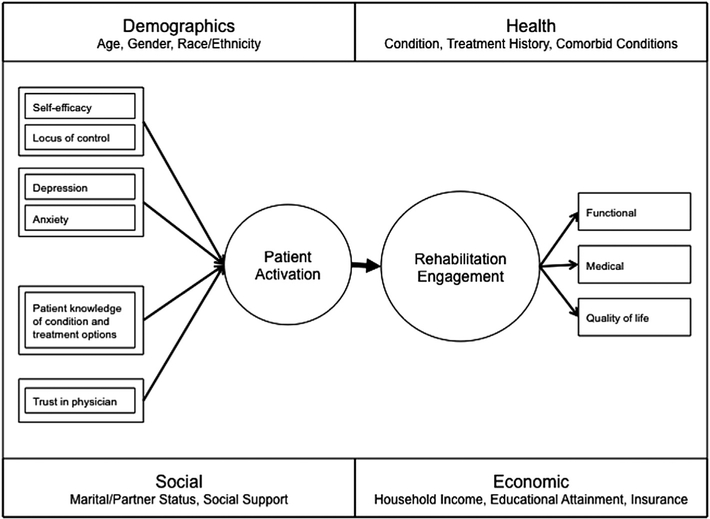
We based our analysis on our conceptual understanding of the role of patient activation in rehabilitation engagement and outcomes after spine surgery (fig 1). To estimate the mediation effect of patient activation on the relationship between HBCC and engagement in rehabilitation, we used structural equation modeling. Structural equation modeling provides estimates of the magnitude and significance of hypothesized connections between sets of variables. Compared with multiple regression, structural equation modeling has 2 major advantages: it allows (1) the specification and testing of multiple mediators in a single model, and (2) the use of multiple indicators to measure latent constructs—thereby increasing the reliability of parameter estimates by controlling for measurement error.28 The current sample size of 122 participants was deemed acceptable given the number of parameters in our structural equation modeling.29,30 Monte Carlo simulation showed that a sample size of 60 to 120 cases would be sufficient based on the number of parameters and the magnitude of factor loadings and path coefficients.31
Fig 1.
Development and maintenance of patient activation.
We hypothesized that the association between HBCC and rehabilitation engagement would be partially mediated by post-intervention patient activation, which would be influenced by HBCC and preintervention patient activation. We tested only these associations and no other possible pathways. Our expectation was that participants who benefited from the intervention would understand the importance of their role in health care, have the confidence to ask questions about their condition and treatment, and be able to adopt positive health behaviors (such as physical activity) and maintain these changes during times of stress. To capture the complexity of engagement in rehabilitation after lumbar spine surgery, we incorporated multiple measures of rehabilitation engagement: measures of participation in rehabilitation (self-reported attendance in PT and HEP) and physical therapist-reported engagement. These measures were used to construct the latent variable, rehabilitation engagement. Overall model fit was assessed using established fit indices (Tucker-Lewis index32 and comparative fit index33).
We used SAS, version 9.2,a and Stata SE, version 12,b for all analyses, with significance set to .05.
Qualitative analysis
This intervention study explored the barriers patients face as they attempt to engage in postsurgical rehabilitation. All participants (control and HBCC groups) engaged in pre- and postsurgical telephone interview sessions. During the telephone interview sessions, the research staff used a standard script to identify the participant’s current health state, expectations for surgery, confidence to perform PT and home exercise, and perceived barriers to and facilitators of postsurgical rehabilitation. All sessions with the participants were audio recorded. Qualitative analysis of the data was conducted using a standardized procedure: (1) review of the audio tapes; (2) review of the tape transcriptions; (3) discussions among the investigators regarding key elements of participants’ statements; (4) determination of conceptual themes; and (5) assignment of relevant responses to appropriate thematic constructs.34,35 All research team members had experience with the target population through clinical work and research efforts. We analyzed the prevalence of themes across participants in both groups.
Results
Changes in behavior
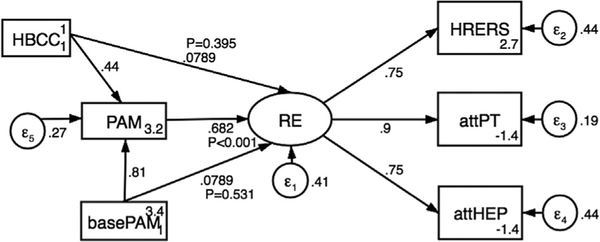
Self-reported measures of attendance to PT and HEPs were correlated (r=.778, P<.001). Physical therapist-rated engagement (using the HRERS) was significantly correlated with attendance to both PT (r=.634, P<.001) and HEPs (r=.537, P<.001). Patient activation had a mediating influence on the relationship between the HBCC intervention and engagement in rehabilitation (fig 2, table 1). Higher patient activation, measured after the intervention, predicted greater rehabilitation engagement on the basis of self-reported and physical therapist-reported measures (standardized regression weight, .682; P<.001). Postintervention patient activation was predicted by baseline patient activation (standardized regression weight, .808; P<.001) and receipt of the HBCC intervention (standardized regression weight, .444; P<.001). To determine whether baseline patient activation or receipt of the HBCC intervention had an independent influence on rehabilitation engagement, we included these pathways in the model; neither was significant (P=.531 and P=.395, respectively).
Fig 2.
Structural equation model. P values have been added to show the significance of direct and indirect effects on rehabilitation engagement. Abbreviations: attHEP, self-reported attendance in home exercise program; attPT, self-reported attendance in physical therapy; basePAM, Patient Activation Measure at baseline; HRERS, Hopkins Rehabilitation Engagement Rating Scale; PAM, Patient Activation Measure; RE, rehabilitation engagement.
Table 1.
Standardized regression weights for causal pathways included in the structural equation model (N=122)
| Pathway | Regression Weight (SE) | P |
|---|---|---|
| Patient activation measure → rehabilitation engagement | .682 (.127) | <.001 |
| Baseline patient activation measure → patient activation measure | .808 (.253) | <.001 |
| HBCC intervention → patient activation measure | .444 (.042) | <.001 |
| Baseline patient activation measure → rehabilitation engagement | .079 (.126) | .531 |
| HBCC intervention → rehabilitation engagement | .079 (.093) | .395 |
Goodness-of-fit measures for the model were the Tucker-Lewis index, .739; comparative fit index, .888; and root mean square error of approximation, .259 (90% confidence interval, .199– .324). These values indicate good approximate model fit.
Although most individuals in the HBCC group showed an improvement in rehabilitation engagement compared with those in the control group, approximately one-third of the HBCC group did not show improvement in either therapist-rated or self-reported engagement. This lack of improvement relative to the control group supports the need for investigation of potential barriers to rehabilitation engagement.
Barriers
On the basis of the qualitative data analyses, 3 barriers were identified that undermined rehabilitation engagement: low self-efficacy because of lack of knowledge and support, anxiety related to fear of movement, and concern about pain management.
Low self-efficacyd—lack of knowledge and support
Confidence in their ability to participate in PT and home exercise was a major barrier facing individuals undergoing lumbar spine surgery. Seventy-six participants (62%) identified lack of confidence (self-efficacy) as a major barrier. Specifically, participants expressed comments that reflected a lack of knowledge regarding the role of exercise in recovery and how to implement an exercise program, and lack of support for this activity. Participants expressed uncertainty about being able to obtain family support of travel to appointments and about the ability to perform exercises after surgery. Sample comments included, “I’ve never exercised before in my life; I am not sure that I will know how to do the right exercises”; “It is going to be hard getting to and from physical therapy; I don’t think that I can get my family to help me”; and “I’m sure that the therapist will be helpful, but I’m not sure I will be able to do work on my own.”
Fear of movement
Patients expressed the belief that after spine surgery, the movements required by PT and home exercise might lead to an exacerbation of their condition and greater back or leg pain, or both. Seventy participants (57%) identified fear of movement as a major barrier. Specific comments included, “Exercise isn’t supposed to hurt, but I worry that with my surgery I could injure myself”; “My doctor told me that physical therapy was important, but I think that I should take it easy so as not to hurt my back”; and “I’ve hurt my back exercising and working around the house; how is this going to be different?”
Concern about pain management
Fifty-nine participants (48%) reported concern about pain management as a major barrier to engagement in rehabilitation. Specific comments included, “My main concern is dealing with the pain after my surgery; how will that be handled?”; “PT is all well and good, but will it cause extra pain?”; and “My husband told me that doctors like to wean you off of pain medication; is that going to happen to me?” Poor management of postoperative pain, therefore, was seen as a major barrier to engagement in rehabilitation.
Additional barriers
Additional barriers identified included issues related to payment and returning to work. Fewer than 15% of participants reported these as barriers to rehabilitation engagement.
Discussion
This study found that the influence of HBCC on rehabilitation engagement was mediated by postintervention patient activation. Patients who had high activation after HBCC were assessed as more engaged in their rehabilitation. Qualitative analysis identified 3 common barriers to engagement: (1) low self-efficacy to participate in PT and home exercise; (2) anxiety related to fear of movement; and (3) concern about pain management.
Early research to examine how patient activation influences health outcomes and behavior has focused on chronic conditions, such as hypertension and diabetes. Hibbard et al36 reported that individuals who were characterized as having high patient activation were more likely to perform regular exercise and follow a low-fat diet compared with individuals who were characterized as having low patient activation. In addition, individuals with high patient activation reported fewer unmet medical needs and greater support from health care providers for self-management of chronic conditions.37 A study38 of community-dwelling members of a large health plan reported a positive relationship between patient activation and use of services to promote self-management and encourage adherence to medication among individuals living with chronic conditions. Among individuals undergoing surgery of the lumbar spine, we showed that high activation was associated with increased rehabilitation engagement, as measured by participation (self-reported) and the HRERS (therapist reported).20 This study extends these findings to show that changes in patient activation mediate the impact of the MI-based intervention on patient engagement.
Despite efforts to increase patient activation, one-third of participants in the HBCC group did not show improved rehabilitation engagement compared with those in the control group. Qualitative analysis of interview data revealed 3 common barriers: low self-efficacy because of a lack of knowledge of the role of exercise in recovery and a lack of supports to perform exercises; fear that an increase in activity and exercise would increase pain; and concerns regarding pain management. HBCC was specifically designed to improve motivation to participate in rehabilitation. Using principles of MI, the study therapist identified and resolved ambivalence between participants’ rating of rehabilitation importance and their commitment to engage in rehabilitation exercise. HBCC was not, however, designed to address the concerns regarding fear of movement or lack of knowledge guiding implementation of exercise programs.
Participants voiced concern that performing PT and home exercise would lead to injury and increased pain after surgery. Fear of movement has been shown to be a significant predictor of pain-related outcomes, including disability, in patients with chronic low back pain.39,40 This fear, often present before surgery, lasts beyond the normal healing process after surgery and can lead to decreased physical activity among patients. After surgery, pain-related fear of movement has been associated with greater pain (at 6wk) and disability (at 6mo).41 Concerns related to pain caused by movement after surgery have been shown to be significantly associated with greater pain and disability.42 There is strong empirical evidence to support interventions based on cognitive-behavioral therapy to address self-efficacy and fear of movement among patients with chronic pain.43,44 Self-management programs based on cognitive-behavioral principles have been shown to improve patient outcomes and to increase physical activity.45,46
Lack of knowledge of rehabilitation exercises and adequate supports to perform them were also concerns among participants in this intervention trial. These findings are consistent with recent evidence supporting the role that lack of knowledge and insufficient support during rehabilitation play in poor engagement and health outcomes after lumbar spine surgery. Interventions aimed at improving physical activity, self-management, and knowledge about health conditions among older adults have demonstrated the effectiveness of web-based components. Recent systematic reviews47,48 have reported on the effectiveness of web-based interventions to promote physical activity in patients with type 2 diabetes. In a randomized controlled trial49 designed to increase physical activity among sedentary older adults, participants receiving a web-based intervention had significant improvements in general health (assessed using the Medical Outcomes Study 12-Item Short-Form Health Survey), behavioral intention and motivation to exercise, and self-reported physical activity (cardiovascular, stretching, and strengthening exercises) compared with the group that received usual care.
A strength of our study was the application of a structural equation modeling approach as a formal mediation analysis that accounts for interrelationships among variables of interest, adjusts for important confounders, and captures several aspects of engagement relevant for rehabilitation after lumbar spine surgery by combining multiple measures into a single latent variable.
Study limitations
Some limitations need to be considered in the interpretation of these study results. First, the study was conducted in the setting of an HBCC intervention trial. We used a lagged-control study design to examine the influence of the HBCC intervention on rehabilitation engagement. An alternative design in which individuals would be randomly assigned contemporaneously to a control or an HBCC group would limit the threat of history to the internal validity of the study. However, this would have introduced the threat of contamination, because providers would alternately be encountering patients who had undergone either usual care or the MI intervention. It is possible, in the design of future interventions, to make use of randomization when one can be assured that participants do not discuss treatment assignment with raters (ie, surgeons and physical therapists) and when adequacy of blinding can be tested. Second, our sample consisted of patients from a single academic medical center. These individuals may not represent all patients, especially those who present to community hospitals. We believe that this limitation is ameliorated by the fact that we enrolled participants from 2 hospitals (one a tertiary care center and the other a community hospital). Roughly 33% of patients were enrolled from the community hospital. Third, the use of self-reporting to measure attendance in PT and HEPs may introduce bias. In their review, Prince et al50 found that self-reports overestimated physical activity as the intensity of physical activity increased. In addition, population subgroups may have been differentially affected by this bias, although the authors did not discover any clear patterns of over- or underreporting. This bias may lead to spurious associations between intervention and self-reported attendance. Self-reporting bias may be mitigated by the inclusion of the physical therapist-rated measure of engagement in PT. That our findings were similar for both measures lends confidence to the self-reported measures of attendance in PT and HEPs.
The HBCC intervention was developed following the principles of MI, an evidence-based style of health behavior change consultation developed to address poor treatment outcomes among chronic drinkers.51 MI is a collaborative, person-centered form of guidance to elicit and strengthen motivation for change. Within an MI framework, motivation to engage in a new behavior, or to change an established one, is viewed as an alterable condition that can be increased via interpersonal, supportive, patient-centered counseling. Key principles of MI include (1) engaging the individual in a collaborative relationship; (2) focusing the conversation on the target of change; (3) evoking the individual’s reasons for change or engagement; and (4) planning for behavior change.52 MI has been shown to be effective in dozens of clinical trials published in diverse areas of health care behavior, including smoking cessation,53 increasing exercise,54 and improving adherence to diabetes management programs.55
The use of strategies to increase patient activation to support changes in health behavior have focused on ways that patients can be empowered to take an active role in their health care,56 ways that providers can educate patients on the importance of health behavior changes,57 and ways that the health care system can incorporate self-management practices in chronic conditions.58,59 Interventions with these foci have shown that improvements in patient activation are associated with the adoption of positive health behaviors that may lead to improvements in health outcomes. This is highlighted by a recent study14 of those living with a variety of chronic conditions. In that study, the authors reported that increased activation led to positive improvements in self-management. These findings, taken together with the data on MI-based interventions, suggest that HBCC may be effective in increasing patient activation and participation in targeted health behaviors.
Conclusions
This study supports the benefits associated with this brief HBCC intervention, which consists of contact via telephone between the patient and a trained interventionist to increase patient activation and improve rehabilitation engagement among those undergoing lumbar spine surgery. These results are consistent with those of similar studies of persons with multiple sclerosis24 and moderate to severe traumatic brain injury.23 Furthermore, there is strong empirical evidence showing that MI counseling techniques can be taught to a wide variety of individuals in the health care field, regardless of their level of training, through workshops lasting 1 or 2 days.60 Patients who undergo surgery for degenerative lumbar spine disease typically have engaged in nonoperative care, including PT or home exercise, without resolution of their symptoms. It is critical that these individuals recognize the importance of, and have the support to engage in, rehabilitation after surgery. These data suggest that incorporation of interventions to increase engagement, particularly for these individuals and those having low levels of patient activation, may positively mediate patient behaviors and, ultimately, long-term outcomes. Practitioners should consider assessing patient activation and barriers to engagement as part of their initial assessment and rehabilitation planning. Early identification of those at risk allows clinicians to reduce barriers through education regarding the role of safe exercise in recovery, problem solving to ensure adequate support for exercise program initiation, and provision of adequate pain management.
Acknowledgments
The Functional Recovery in Lumbar Spine Surgery Health Behavior Change Counseling intervention trial is supported by the Agency for Healthcare Research and Quality (grant no. 1 R01 HS 017990).
List of abbreviations:
- HBCC
health behavior change counseling
- HEP
home exercise program
- HRERS
Hopkins Rehabilitation Engagement Rating Scale
- MI
motivational interviewing
- PT
physical therapy
Footnotes
SAS, version 9.2; SAS Institute, Inc.
Stata SE, version 12; Stata Corp.
Disclosures: none.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990e2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990e2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costandi S, Chopko B, Mekhail M, et al. Lumbar spinal stenosis: therapeutic options review. Pain Pract 2014;15:68–81. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441–5. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein JN, Lurie JD, Olson PR, et al. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine (Phila Pa 1976) 2006;31:2707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen E, Tong KB, Laouri M. Surgical treatment patterns among Medicare beneficiaries newly diagnosed with lumbar spinal stenosis. Spine J 2010;10:588–94. [DOI] [PubMed] [Google Scholar]
- 7.Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev 2005;(4):CD001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreiner DS, Shaffer WO, Baisden JL, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J 2013;13:734–43. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 2010; 35:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagg O, Fritzell P, Ekselius L, et al. Predictors of outcome in fusion surgery for chronic low back pain. A report from the Swedish Lumbar Spine Study. Eur Spine J 2003;12:22–33. [DOI] [PubMed] [Google Scholar]
- 11.Junge A, Frohlich M, Ahrens S, et al. Predictors of bad and good outcome of lumbar spine surgery: a prospective clinical study with 2 years’ follow-up. Spine (Phila Pa 1976) 1996;21: 1056–64. [DOI] [PubMed] [Google Scholar]
- 12.Mannion AF, Denzler R, Dvorak J, et al. Five-year outcome of surgical decompression of the lumbar spine without fusion. Eur Spine J 2010;19:1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976) 2007;32:382–7. [DOI] [PubMed] [Google Scholar]
- 14.Hibbard JH, Tusler M. Assessing activation stage and employing a “next steps” approach to supporting patient self-management. J Ambul Care Manage 2007;30:2–8. [DOI] [PubMed] [Google Scholar]
- 15.Thiboutot J, Sciamanna CN, Falkner B, et al. Effects of a web-based patient activation intervention to overcome clinical inertia on blood pressure control: cluster randomized controlled trial. J Med Internet Res 2013;15:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkes MS, Day FC, Srinivasan M, et al. Pairing physician education with patient activation to improve shared decisions in prostate cancer screening: a cluster randomized controlled trial. Ann Fam Med 2013; 11:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington (DC): National Academy Pr; 2001. [Google Scholar]
- 18.Stewart M Towards a global definition of patient centred care. BMJ 2001;322:444–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner EH, Bennett SM, Austin BT, et al. Finding common ground: patient-centeredness and evidence-based chronic illness care. J Altern Complement Med 2005;11:S7–15. [DOI] [PubMed] [Google Scholar]
- 20.Skolasky RL, Mackenzie EJ, Wegener ST, et al. Patient activation and adherence to physical therapy in persons undergoing spine surgery. Spine (Phila Pa 1976) 2008;33:E784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skolasky RL, Mackenzie EJ, Wegener ST, et al. Patient activation and functional recovery in persons undergoing spine surgery. J Bone Joint Surg Am 2011;93:1665–71. [DOI] [PubMed] [Google Scholar]
- 22.Skolasky RL, Riley LH III, Maggard AM, et al. Functional recovery in lumbar spine surgery: a controlled trial of health behavior change counseling to improve outcomes. Contemp Clin Trials 2013;36: 207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell KR, Temkin NR, Esselman PC, et al. The effect of a scheduled telephone intervention on outcome after moderate to severe traumatic brain injury: a randomized trial. Arch Phys Med Rehabil 2005;86: 851–6. [DOI] [PubMed] [Google Scholar]
- 24.Bombardier CH, Cunniffe M, Wadhwani R, et al. The efficacy of telephone counseling for health promotion in people with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil 2008;89:1849–56. [DOI] [PubMed] [Google Scholar]
- 25.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the Patient Activation Measure. Health Serv Res 2005;40:1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skolasky RL, Mackenzie EJ, Riley LH III, et al. Psychometric properties of the Patient Activation Measure among individuals presenting for elective lumbar spine surgery. Qual Life Res 2009;18: 1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kortte KB, Falk LD, Castillo RC, et al. The Hopkins Rehabilitation Engagement Rating Scale: development and psychometric properties. Arch Phys Med Rehabil 2007;88:877–84. [DOI] [PubMed] [Google Scholar]
- 28.Li SD. Testing mediation using multiple regression and structural equation modeling analyses in secondary data. Eval Rev 2011;35: 240–68. [DOI] [PubMed] [Google Scholar]
- 29.Bentler PM, Chou CP. Practical issues in structural modeling. Sociol Methods Res 1987;16:78–117. [Google Scholar]
- 30.Stevens J Applied multivariate statistics for the social sciences. Hillsdale: Lawrence Erlbaum Associates; 1986. [Google Scholar]
- 31.Wolf EJ, Harrington KM, Clark SL, et al. Sample size requirements for structural equation models: an evaluation of power, bias, and solution propriety. Educ Psychol Meas 2013;73:913–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychol Bull 1980;88:588–606. [Google Scholar]
- 33.Bentler PM. Comparative fit indexes in structural models. Psychol Bull 1990;107:238–46. [DOI] [PubMed] [Google Scholar]
- 34.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. [Google Scholar]
- 35.Green J, Thorogood N. Analysing qualitative data In: Qualitative methods for health research. London: Sage Publications; 2006. p 173–200. [Google Scholar]
- 36.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39: 1005–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibbard JH, Cunningham PJ. How engaged are consumers in their health and health care, and why does it matter? Res Brief 2008;8: 1–9. [PubMed] [Google Scholar]
- 38.Mosen DM, Schmittdiel J, Hibbard J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage 2007;30:21–9. [DOI] [PubMed] [Google Scholar]
- 39.Crombez G, Vlaeyen JW, Heuts PH, et al. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain 1999;80:329–39. [DOI] [PubMed] [Google Scholar]
- 40.Verbunt JA, Seelen HA, Vlaeyen JW, et al. Fear of injury and physical deconditioning in patients with chronic low back pain. Arch Phys Med Rehabil 2003;84:1227–32. [DOI] [PubMed] [Google Scholar]
- 41.den Boer JJ, Oostendorp RA, Beems T, et al. Continued disability and pain after lumbar disc surgery: the role of cognitive-behavioral factors. Pain 2006;123:45–52. [DOI] [PubMed] [Google Scholar]
- 42.Archer KR, Wegener ST, Seebach C, et al. The effect of fear of movement beliefs on pain and disability after surgery for lumbar and cervical degenerative conditions. Spine (Phila Pa 1976) 2011;36: 1554–62. [DOI] [PubMed] [Google Scholar]
- 43.Butler AC, Chapman JE, Forman EM, et al. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev 2006;26:17–31. [DOI] [PubMed] [Google Scholar]
- 44.van Tulder MW, Ostelo R, Vlaeyen JW, et al. Behavioral treatment for chronic low back pain: a systematic review within the framework of the Cochrane Back Review Group. Spine (Phila Pa 1976) 2000;25: 2688–99. [DOI] [PubMed] [Google Scholar]
- 45.Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469–75. [DOI] [PubMed] [Google Scholar]
- 46.Nicholas MK, Asghari A, Corbett M, et al. Is adherence to pain self-management strategies associated with improved pain, depression and disability in those with disabling chronic pain? Eur J Pain 2012; 16:93–104. [DOI] [PubMed] [Google Scholar]
- 47.Bossen D, Veenhof C, Dekker J, et al. The effectiveness of self-guided web-based physical activity interventions among patients with a chronic disease: a systematic review. J Phys Act Health 2014; 11:665–77. [DOI] [PubMed] [Google Scholar]
- 48.Connelly J, Kirk A, Masthoff J, et al. The use of technology to promote physical activity in type 2 diabetes management: a systematic review. Diabet Med 2013;30:1420–32. [DOI] [PubMed] [Google Scholar]
- 49.Irvine AB, Gelatt VA, Seeley JR, et al. Web-based intervention to promote physical activity by sedentary older adults: randomized controlled trial. J Med Internet Res 2013;15:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prince SA, Adamo KB, Hamel ME, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller WR, Rollnick S. Motivational interviewing. Preparing people for change. 2nd ed New York: Guilford Pr; 2002. [Google Scholar]
- 52.Miller WR, Rollnick S. Motivational interviewing. Helping people change. 3rd ed New York: Guilford Pr; 2013. [Google Scholar]
- 53.Rollnick S, Butler CC, Stott N. Helping smokers make decisions: the enhancement of brief intervention for general medical practice. Patient Educ Couns 1997;31:191–203. [DOI] [PubMed] [Google Scholar]
- 54.Harland J, White M, Drinkwater C, et al. The Newcastle exercise project: a randomised controlled trial of methods to promote physical activity in primary care. BMJ 1999;319:828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith DE, Heckemeyer CM, Kratt PP, et al. Motivational interviewing to improve adherence to a behavioral weight-control program for older obese women with NIDDM. A pilot study. Diabetes Care 1997;20:52–4. [DOI] [PubMed] [Google Scholar]
- 56.Alegria M, Polo A, Gao S, et al. Evaluation of a patient activation and empowerment intervention in mental health care. Med Care 2008;46:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morisky DE, Bowler MH, Finlay JS. An educational and behavioral approach toward increasing patient activation in hypertension management. J Community Health 1982;7:171–82. [DOI] [PubMed] [Google Scholar]
- 58.Wegener ST, Mackenzie EJ, Ephraim P, et al. Self-management improves outcomes in persons with limb loss. Arch Phys Med Rehabil 2009;90:373–80. [DOI] [PubMed] [Google Scholar]
- 59.Williams GC, McGregor H, Zeldman A, et al. Promoting glycemic control through diabetes self-management: evaluating a patient activation intervention. Patient Educ Couns 2005;56:28–34. [DOI] [PubMed] [Google Scholar]
- 60.Miller WR, Yahne CE, Moyers TB, et al. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72:1050–62. [DOI] [PubMed] [Google Scholar]