Abstract
Tumour heterogeneity is a phenomenon where each cell that makes up a tumour, contains mutations that differ from that of other cells in the tumour. The clonal evolution and cancer stem cell theories of cancer formation, have been used to explain tumour heterogeneity. The theories both point to the existence of cells within a tumour that are capable of initiating the tumour in a different location. While the clonal evolution theory argues that all cells within a tumour possess this ability, the cancer stem cell theory argues that only a few cells (cancer stem cells or CSCs) within the tumour possess this ability to seed the tumour in a different location. Data supporting the cancer stem cell theory is accumulating. Researchers have targeted these CSCs therapeutically, hypothesizing that since these CSCs are the ‘drivers’ of tumour progression, their death may inhibit tumour progression. This was foiled by tumour cell plasticity, a phenomenon whereby a non-CSC spontaneously de-differentiates into a CSC. Researchers are now working on combinations that kill both CSCs and non-CSCs as well as drugs that prevent non-CSC-to-CSC transition. This review concisely describes CSCs and how they contribute to the difficulty in treating cancer.
Keywords: Cancer stem cells, Chemotherapy, De-differentiation, Metastasis, Tumour heterogeneity, Tumour cell plasticity
Introduction
Cancer is a name used to describe a large and diverse group of diseases most notably characterized by a rapid increase in the growth and proliferation of certain cells (that have acquired genomic mutations) and a resultant tumour mass. This malignant tumour mass is distinguished from benign tumours because of the ability to metastasize, i.e. spread to other distant organs.
How cancer originates
Cancer originates from a single mutation within the genome of a cell. Subsequent accumulation of more mutations can turn a normal cell into an aberrant cell.1 A mutation in which a tumour suppressor such as p53, Rb or p16INK4a/p14ARF is knocked down, or an oncogene such as Ras become constitutively active, leads to excessive proliferation of the cell,2, 3, 4 and with each cell division, the chances of cells acquiring more mutations increases. Eventually, cells accumulate enough mutations to trigger endless growth and tumour formation.3
The difficulty in treating cancer
Generally, cancer is not noticed by patients until it has gotten to a late stage – metastasis. At this stage, there is not much doctors can do. Doctors may try to remove the tumour mass surgically and administer chemotherapy and radiation, but apart from the side effects these techniques have on normal cells, chemotherapy is not very effective in completely wiping out cancer cells, because in a given tumour, there are different types of cancer cells with different kinds of mutations. Indeed, cancer tumours do not contain homogenous cancer populations, rather they contain heterogeneous cancer populations.5 This is one reason for the difficulty in treating cancer, as it is not easy to administer a drug combination that targets each of the cancer cells in a tumour, due to their genomic diversity. This concept is known as tumour heterogeneity (diversity in the cancer cells that make up a tumour). Based on literature review, tumour heterogeneity seems to be the main cause of cancer relapse and resistance to chemotherapy – the biggest challenges in cancer treatment.
Origin of tumour heterogeneity
Tumour heterogeneity is as a result of both intrinsic and extrinsic factors. Intrinsic factors include genetic and epigenetic mutations that contribute to tumorigenicity while extrinsic factors include the microenvironment around the tumour that interacts with the tumour to aid its progression.6
Theories of tumour heterogeneity
Researchers have put forward theories to explain the origin of tumour heterogeneity from the intrinsic point of view. In this review, I will discuss the two prominent theories: the clonal evolution theory and the cancer stem cell theory. Both theories have data supporting them, but not without inconsistencies.
Clonal evolution theory
This theory is based on Darwin's theory of evolution. According to this theory, an initial mutation in a cell is passed on to each of the daughter cells and with each subsequent division, these daughter cells acquire and accumulate more and more transforming mutations. Over time, the cells with the most mutations that cause a growth and proliferation advantage are clonally expanded, leading to the formation of a tumour mass with clones of different aberrant cells.7 The evidence for this theory came from observations of different tumours. It was found that all the cancer cells in a given tumour had one or few founder mutations in common, while some mutations were specifically found in individual cancer cells. This implies that the cancer cells evolved from one original mutated cancer cell.7
Cancer stem cell theory
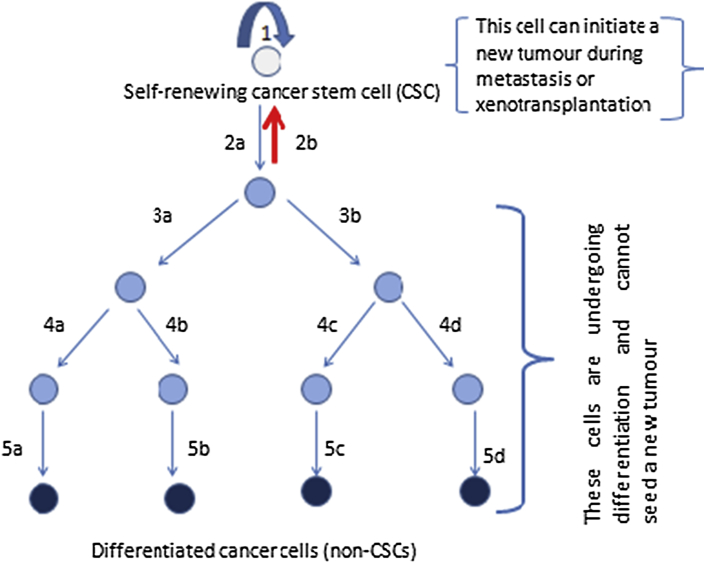
This is a more recent theory based on the observation that there are a few cells within many, if not all tumour masses that display stem cell-like properties namely: the ability to self-renew, as well as the ability to give rise to all the different types of cancer cells within that tumour.5 According to this theory, these cancer stem cells (CSCs) can be placed at the apex of a hierarchy, and can undergo either symmetric or asymmetric division (Fig. 1). When they undergo symmetric division, they either produce two identical daughter cells that are replicas of them (CSCs) – this is known as ‘self-renewal’; or two identical daughter cells that are progenitors (non-CSCs) and can subsequently differentiate to form any of the types of cancer cells within the tumour. There is also asymmetric division in which the stem cell divides to produce two non-identical daughter cells. One is a replica of the stem cell (CSC), while the other is a progenitor cell (non-CSC) which can go on to differentiate into any cancer cell type within the tumour (with contribution from stimuli produced by the microenvironment).5
Fig. 1.
Cancer stem cell hierarchy. According to the cancer stem cell theory, a cancer stem cell is at the top of a hierarchy. It has the ability to self-renew (arrow 1), i.e. give rise to a daughter cell that is an exact replica of itself. It also has the ability to give rise to any of the cancer cell types within a tumour. It does this by dividing to form a daughter cell (non-CSC) that can subsequently divide severally before differentiating into diverse cancer cells (arrows 2a–5d, excluding arrow 2b). Tumour cell plasticity also occurs, in which a progenitor cell (non-CSC) spontaneously de-differentiates into a CSC (arrow 2b). It is thus able to initiate a new tumour.
It is very likely that the two theories of tumour heterogeneity may apply to human cancers of different types, and in some tumours, both models may apply.
Evidence for the existence of cancer stem cells
In 1997, Bonnet and Dick8 showed that a specific group of leukaemic cells expressing CD34 on their cell surface and lacking CD38 were capable of initiating new tumours when injected into immunosuppressed NOD/SCID mice. Cancer cells with this type of ability have also been demonstrated to be present in solid human tumours.9 Importantly, these CSCs capable of initiating new tumours, have been shown to be relatively few within the tumour and this has raised the possibility that the current drugs used in managing cancer, destroy other cells within the tumour but not the CSCs (because they are relatively few), hence these CSCs regenerate the tumour once the therapy is discontinued, leading to relapse as well as resistance to the therapeutics previously used.5
This concept is an important difference between the clonal evolution and the CSC theories, i.e according to the CSC theory, only a subpopulation of the cancer cells within the tumour, are cancer stem cells and are capable of initiating a tumour in a different location (e.g during metastasis or xenotransplantation). Therefore, the CSCs are said to be the drivers of tumour progression, even though they are just a minority. The clonal evolution theory however, claims that all cancer cells within a tumour, have the ability to form a new tumour mass in a different location and each tumour mass consists of numerous clones of different cancer cells harbouring different genetic mutations; hence, the heterogeneous nature of malignant tumours.5
Challenges of the cancer stem cell theory
The cancer stem cell theory, like many other scientific theories has been subjected to critique. One major drawback of the CSC model is the lack of definitive surface markers. The markers seen on CSCs tend to be similar to those on normal adult stem cells, normal cancer cells or normal tissues10, 11, 12, 13 and also, the markers seen on different CSCs tend to differ from one another.13 Researchers have argued that there should be specific surface markers on CSCs that can be targeted specifically without the risk of damaging normal adult cells; and also, certain CSC markers should be expressed on many types of CSCs.13 In reality, it is not unrealistic to find CSCs sharing surface markers with normal adult stem cells, since it has been proposed that CSCs originate from mutations in normal stem cells.13 Also, tumour heterogeneity is useful in explaining the reason why CSCs of a particular organ express different markers in different locations (for instance in different human hosts).
Effect of selective targeting of cancer stem cells
Currently, researchers are developing drugs that take tumour heterogeneity into account, as this is obviously a key reason for the mass failure in cancer therapy that has been recorded over the years. Based on the cancer stem cell model, a scan of 16,000 drugs was carried out, and one of them (Salinomycin) was found to be highly effective in killing breast CSCs, while sparing non-CSCs.14 The idea behind this was that, since it is CSCs that drive tumour progression, then wiping out CSCs will lead to shrinkage of the tumour mass. Indeed, the results were very close to expectations, but not without problems. It has been found that under conditions of genetic manipulation, mammary non-CSCs are able to utilize the mechanism of epithelial-to-mesenchymal transition (EMT) in order to undergo de-differentiation and become mammary CSCs once again.5, 15, 16 This is known as tumour cell plasticity. Another mechanism by which mammary non-CSCs can spontaneously de-differentiate into mammary CSCs in vivo without any genetic manipulation, has subsequently been described17 – See Fig. 1. Furthermore, tumour cell plasticity has been demonstrated in JARID1B-negative melanoma cells which spontaneously revert into a JARID1B-positive state, thereby giving rise to an increase in tumour growth.18 Hence, administering drugs like Salinomycin and Abamectin, (which specifically destroy CSCs, while sparing non-CSCs) might lead to tumour shrinkage initially, but with time the tumour will relapse if one or more of the non-CSCs is able to de-differentiate into a CSC, and worse still, the resulting tumour will most likely be resistant to the chemotherapy previously used.5
Future strategies
Future research should be aimed at providing new drug combinations that kill both CSCs and non-CSCs or at least drugs that prevent non-CSC-to-CSC transition can be combined with drugs that selectively destroy CSCs. This may hopefully be more effective in the long run.
Conclusion
Cancer is extremely difficult to treat successfully, because there are too many things to be targeted therapeutically at the same time. Targeting one thing while leaving out the other, could lead to an initial tumour shrinkage, with an eventual relapse of the cancer and consequent drug resistance. The cancer stem cell theory is helping to advance the state of research, but not without its inherent challenges and limitations. Currently, research is on-going to develop combination therapies that will destroy cancer cells, prevent relapse, as well as have minimal side effects. This may seem to be ambitious, considering the complex nature of cancer, but I believe that, the remedy for individual cancers is nearer to us now than it ever was before!
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Sherr C.J. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg R.A. 2nd ed. Garland Science, Taylor and Francis Group LLC; New York, USA: 2013. The Biology of Cancer. [Google Scholar]
- 5.Marjanovic N.D., Weinberg R.A., Chaffer C.L. Cell plasticity and heterogeneity in cancer. Clin Chem. 2013;59:168–179. doi: 10.1373/clinchem.2012.184655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albini A., Bruno A., Gallo C., Pajardi G., Noonan D.M., Dallaglio K. Cancer stem cells and the tumour microenvironment: interplay in tumour heterogeneity. Connect Tissue Res. 2015;56:414–425. doi: 10.3109/03008207.2015.1066780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner N.C., Reis-Filho J.S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Visvader J.E., Lindeman G.J. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Medema J.P. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 11.Karsten U., Goletz S. What makes cancer stem cell markers different? SpringerPlus. 2013;2:301. doi: 10.1186/2193-1801-2-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Nenutil R., Appleyard M.V. Lack of correlation of stem cell markers in breast cancer stem cells. Br J Cancer. 2014;110:2063–2071. doi: 10.1038/bjc.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T., Shigdar S., Gantier M.P. Cancer stem cell targeted therapy: progress amid controversies. Oncotarget. 2015;6:44191–44206. doi: 10.18632/oncotarget.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta P.B., Chaffer C.L., Weinberg R.A. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 15.Mani S.A., Guo W., Liao M.J. The epithelial-to-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morel A.P., Lievre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaffer C.L., Brueckmann I., Scheel C. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. PNAS. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roesch A., Fukunaga-Kalabis M., Schmidt E.C. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]