Abstract
Background and Aims
Currently, functional–structural plant models (FSPMs) mostly resort to static descriptions of leaf spectral characteristics, which disregard the influence of leaf physiological changes over time. In many crop species, including soybean, these time-dependent physiological changes are of particular importance as leaf chlorophyll content changes with leaf age and vegetative nitrogen is remobilized to the developing fruit during pod filling.
Methods
PROSPECT, a model developed to estimate leaf biochemical composition from remote sensing data, is well suited to allow a dynamic approximation of leaf spectral characteristics in terms of leaf composition. In this study, measurements of the chlorophyll content index (CCI) were linked to leaf spectral characteristics within the 400–800 nm range by integrating the PROSPECT model into a soybean FSPM alongside a wavelength-specific light model.
Key Results
Straightforward links between the CCI and the parameters of the PROSPECT model allowed us to estimate leaf spectral characteristics with high accuracy using only the CCI as an input. After integration with an FSPM, this allowed digital reconstruction of leaf spectral characteristics on the scale of both individual leaves and the whole canopy. As a result, accurate simulations of light conditions within the canopy were obtained.
Conclusions
The proposed approach resulted in a very accurate representation of leaf spectral properties, based on fast and simple measurements of the CCI. Integration of accurate leaf spectral characteristics into a soybean FSPM leads to a better, dynamic understanding of the actual perceived light within the canopy in terms of both light quantity and quality.
Keywords: PROSPECT, GroIMP, light modelling, phylloclimate, canopy, Glycine max (L.) Merill, leaf senescence
INTRODUCTION
In crops, light is a major determinant of plant growth, architecture, phenology and, ultimately, yield and quality of the primary product. For instance, the quantity of intercepted light has a direct effect on photosynthesis and consequently on the amount of available assimilates. Moreover, at low light intensities and a low red:far-red ratio (R:FR), a shade avoidance response is triggered in many plant species, leading to an increase in internode and petiole elongation often at the cost of reduced leaf area and storage organ development (Franklin and Whitelam, 2005). Additionally, it can lead to reduced branching in dicots and inhibit tillering in grasses (Casal et al., 1986), and can accelerate the induction of flowering (Halliday et al., 1994). These are all important determinants of both productivity and product quality.
Light conditions within a crop canopy are affected by the external light conditions together with the canopy architecture and composition. The crop architecture is largely determined by the spatial arrangement, size, shape and orientation of the leaves, which define the amount of incident light on the crop canopy. However, the interaction of the canopy with the incident light is largely dependent on the spectral characteristics of the plant leaves, as these affect the light perceived by the leaf (through absorption) as well as the light conditions within the canopy (through reflection and transmission). Therefore, knowledge of leaf spectral characteristics is critical in studying plant–light interactions. However, leaf spectral characteristics are not homogeneous in a crop canopy and change throughout the growing season due to leaf senescence (Wu et al., 2012). In soybean [Glycine max (L.) Merill] specifically, leaf chlorophyll content changes with leaf age (Fritschi and Ray, 2007). Additionally, during the generative phase of development, nitrogen, an important component of chlorophyll, is remobilized from vegetative plant parts for pod filling (Lugg and Sinclair, 1981). These biochemical changes heavily impact the leaf spectral characteristics, resulting in a dynamic and heterogeneous crop canopy and finally affecting crop growth and development.
Functional–structural plant models (FSPMs), which combine the architecture of individual plants with ecophysiological aspects of plant growth, are ideal tools to study the interactions between plant structure, plant physiology and the environment (Vos et al., 2010). Given the relevance of light for plant growth and development, some FSPMs already incorporate an architectural plant response to the light phylloclimate [i.e. the light actually perceived by the plant organs (Chelle, 2005)], e.g. tillering of spring wheat (Triticum aestivum) in response to the local R:FR ratio (Evers et al. 2007), tillering and internode elongation of barley (Hordeum vulgare L.) as a mechanical response to R:FR (Buck-Sorlin et al., 2008), and internode elongation in cucumber (Cucumis sativus) considering variations in both photosynthetically active radiation (PAR) and R:FR (Kahlen and Stützel, 2011). However, in FSP modelling, a focus on the quantitative plant response to light (i.e. photosynthesis) is still prevalent. Therefore, a functional approach for capturing and incorporating the dynamics of leaf spectral characteristics would aid in evaluating light interception and light phylloclimate. This requires knowledge of leaf biochemistry over time and a model to translate this knowledge into leaf spectral characteristics.
Translation of leaf biochemistry to leaf spectral characteristics can be done using the PROSPECT radiative transfer model (Jacquemoud and Baret, 1990; Féret et al., 2008), which simulates absorbance, reflectance and transmittance as a function of leaf composition on the individual leaf level. It has been successfully applied to various monocot and dicot species, including senescent leaves (Jacquemoud et al., 2009). Therefore, combining knowledge of leaf biochemistry with the PROSPECT model allows a dynamic calculation of leaf spectral characteristics, essential for studying light conditions within a dynamic, heterogeneous canopy. A necessary step for this is the acquisition of accurate, high-resolution measurements of leaf biochemistry for model parameterization. These data have been traditionally captured using destructive analysis of relevant leaf components (Jacquemoud and Baret, 1990). As an alternative, several optical methods have been developed for estimating these components non-destructively [e.g. chlorophyll (Parry et al., 2014), carotenoid (Fassnacht et al., 2015) and leaf water content (Baret and Fourty, 1997; Ceccato et al., 2001)]. However, when spatio-temporal patterns within a crop canopy must be monitored, such measurements can be costly and time consuming. Furthermore, if the underlying dynamics of all the biochemical components required for a full parameterization of the PROSPECT model are not known, it becomes difficult to integrate their behaviour in a dynamically developing FSPM. A reduction in complexity of the PROSPECT model may therefore aid in both the monitoring and modelling aspects of leaf spectral characteristics without heavily impacting the spectral range of interest within FSPMs.
The present study aimed to (1) integrate the PROSPECT radiative transfer model into an FSPM; (2) evaluate the accuracy of estimations of leaf spectral characteristics through the PROSPECT model using only non-destructive, optical measurements of the chlorophyll content index (CCI); and (3) determine the applicability of this approach on the whole-canopy scale by evaluating light conditions within a soybean FSPM. We demonstrate that this approach results in an elegant description of leaf transmission, absorption and reflection in FSPMs, allowing improvement of simulation of light interception and light phylloclimate within a complex canopy, while requiring only simple and fast measurements for model parameterization.
MATERIALS AND METHODS
PROSPECT model description
The PROSPECT radiative transfer model simulates directional–hemispherical reflectance and transmittance (Schaepman-Strub et al., 2006) over the spectral range of 400–2500 nm on the individual leaf level. Currently, the model is primarily used in its inverse form, as a means to estimate leaf biochemistry from reflectance data in the field of remote sensing (Jacquemoud et al., 1995, 1996, 2009; Le Maire et al., 2004). The model calculates the combined effect of several photosynthetic pigments and other leaf constituents in a number of elemental layers within the leaf. The latest adaptation of PROSPECT, namely PROSPECT-D (Féret et al., 2017), is built on a limited number of input parameters (seven) to describe both the leaf structure and its composition: a parameter representing the amount of photosynthetically active ‘layers’ within the leaf [N, 1 < N < 3 (dimensionless)], the leaf chlorophyll content [Cab (μg cm–2)], the leaf carotenoid content [Cxc (μg cm–2)], the anthocyanin content [Cant (μg cm–2), the relative amount of brown pigment content [Cb, 0 < Cb < 1 (dimensionless)], the equivalent water thickness [EWT (cm)] and the dry leaf mass per area [LMA (g cm–2)].
In a first step, the PROSPECT model was implemented by adapting the freely available MATLAB code for PROSPECT-D (Féret et al., 2017) to RStudio (R Core Team, 2016) (for model calibration and validation on individual leaves) and Java (for model validation on the canopy scale in GroIMP). A Java implementation of the exponential integral, required for the PROSPECT calculations, was used (taken from Lau, 2003), which is integrated in MATLAB with no readily available alternatives for R or Java. It was chosen to focus on the spectral range of 400–800 nm as this includes the PAR and far-red, both of which are relevant towards evaluating light quality and quantity within a canopy. This allowed us to set the PROSPECT parameters for EWT and LMA as constants (EWT set to 0.015 cm; LMA set to 0.007 g cm–2), as these do not significantly influence the wavelength range of interest in comparison with the other parameters (Féret et al., 2008).
Model calibration
For model calibration, a soybean [Glycine max (L.) Merill of cultivar ‘Adsoy’] field experiment was conducted at the ILVO site (50°58ʹ26.7ʺN, 3°46ʹ45.1ʺE). A field of 5 m2 was sown on 13 May 2017 with a row distance of 0.25 m and planting density of 67 plants m–2. On 29 August, nearing the end of the growing season, 15 leaves were selected, ensuring coverage of a wide range of leaf chlorophyll content (Fig. 1), as measured with a CCI meter (CCM-200, Opti-Sciences, Hudson, NH, USA). The transmission spectrum of these leaves was then measured using a spectrometer (Jaz Spectrometer, Ocean Optics, Dunedin, FL, USA, responsive between 200 and 800 nm) under clear sky conditions. The transmission spectrum was obtained by comparing measurements of the sky conditions with the spectrum acquired by the same set-up while stretching each individual leaf over the spectrometer sensor. Special care was taken to take the CCI measurements on the same position as the spectrometer measurements, and three measurements were taken on different positions of each leaf. Finally, a total of 45 CCI point measurements were obtained within a CCI range of 1.1–97.7, along with their respective transmission spectra.
Fig. 1.
Visual difference in leaf spectral characteristics in soybean [Glycine max (L.) Merill ‘Adsoy’]. These leaves were harvested on plants of the same age, illustrating the heterogeneity of leaves within a soybean canopy.
The RStudio implementation of the PROSPECT-D model was combined with a genetic algorithm (package ‘genalg’, Willighagen, 2014) to compare PROSPECT-D simulations with random parameter sets of N, Cab, Cxc, Cant and Cb with the measured transmission spectra for a wavelength range of 400–800 nm. The goal function was set as the residual sum of squares (RSS) at a resolution of 1 nm. As a result, for each measurement, the corresponding PROSPECT biochemical leaf parameters were obtained. The resulting data set allowed us to relate the measured CCI to the obtained PROSPECT parameters N, Cab, Cxc, Cant and Cb.
Model validation
The modelling approach was evaluated at two scales: the individual leaf and the whole canopy. For model validation at the leaf level, two smaller data sets of the same soybean cultivar were obtained using the method described above. Validation data set 1 included measurements at the same site with plants sown on 13 June 2017, exactly 1 month later than the calibration set. The inter-row distance was also 0.25 cm, but in this case the average plant density was 32 plants m–2. Data were recorded on 20 leaves on 16 October, resulting in 60 measurements over a CCI range of 1.2–98.8. The second validation data set concerns plants sown on 1 July 2016 in 4 L containers filled with potting soil and grown for 2 months in a growth chamber (day-length of 12 h, night temperature of 15 °C, day temperature of 25 °C, daytime light intensity of 800 μmol PAR m–2 s–1 and relative humidity ranging between 35 and 80 %). This set comprises measurements of nine leaves carried out on 1 September 2016 for a total of 27 measurements over a CCI range of 1.1–113.5. Measurements of the transmission spectrum of these leaves were conducted under a constant white light source (emission between 350 and 715 nm) which did not cover the whole range of 400–800 nm. Nonetheless, this data set allowed us to verify whether the model calibration holds under growth chamber conditions as well, at least for the PAR range.
Checking if the model performs well for estimating leaf spectral characteristics in complex canopy architectures requires a metric that can be measured objectively in the field as well as recreated in a virtual canopy, preferably with low variability. Therefore, it was chosen to measure and compare whole-canopy light transmission on a soybean canopy that had achieved both a high degree of heterogeneity in terms of leaf spectral characteristics as well as a high degree of canopy closure. The former is important for inclusiveness of a large range of different leaves contributing to whole-canopy light interception. The latter is important to reduce horizontal variability of whole-canopy light transmission due to the absence of sun flecks. Model validation was then performed by directly comparing below canopy light measurements from a field trial with a virtual recreation of the same trial, generated using a soybean FSPM integrated with the PROSPECT model.
The field experiment was conducted at the ILVO site (50°59’33.3’’N, 3°47’04.9’’E), and consisted of one square plot of 4 m2 comprising rows separated by 0.25 m and sown on 11 May 2016 using seeds of cultivar ‘Adsoy’. On 1 June 2016, the rows were thinned to eight plants m–1, resulting in final plant density of 32 plants m–2. On 23 August, a date on which the canopy was substantially heterogeneous in terms of leaf colour, five light measurements were conducted above the canopy and six below the canopy using a spectrometer (Jaz Spectrometer, responsive between 200 and 800 nm) between 12.45 and 12.48 h under clear sky conditions. Because of the dense canopy, in situ capturing of plant architecture was impossible. Therefore, the four plants closest to the positions where spectrometer measurements were taken were harvested to determine plant architecture and dimensions. From these four plants, the dimensions of each internode, petiole and leaf were captured. The average shape of the leaves was determined from a large set of leaves from the same soybean cultivar (>100) through image analysis (Coussement et al., 2018), which allowed direct estimation of the leaf area index which was calculated to be 3.8. The CCI was measured on three different positions on each leaf of each measured plant, and averaged to deal with horizontal variability of CCI within the same leaf. At the time of measurement, the canopy was relatively planophile and, as in situ measurements of leaf angles was difficult due to the dense canopy, a visual estimation of the mean branching and leaf angles was conducted. Actual in situ rotation of the stem, and thus phyllotaxis, could not be taken into account.
A static soybean canopy of 4 m2 was simulated by integrating the measured plant dimensions and leaf CCI in a soybean FSPM in the GroIMP modelling platform (Hemmerling et al., 2008; Kniemeyer et al., 2008). The virtual field (Fig. 2) contained eight rows of 16 plants with the same planting alignment as in the field (four rows m–1, eight plants per row m–1). The four plants at the centre were set to correspond to their real-life counterparts, meaning their organ dimensions and leaf CCI were directly taken from the measured data. The other plants in the canopy were randomly chosen from the set of four measured plants. Internodes and petioles were modelled as green cylinders. Leaves were modelled as meshes with a fixed shape, which was obtained from calculating the average shape. Leaf spectral characteristics were implemented using the relationships between the measured CCI and the PROSPECT parameters, obtained as described above. GroIMP implementation of the PROSPECT model allowed direct translation of measured CCI to leaf absorption, transmission and reflection. These values were calculated for each 5 nm interval between 400 and 800 nm and integrated in a spectral leaf shader in the FSPM.
Fig. 2.
Rendered image of the virtual 4 m2 soybean canopy on 23 August 2016. The virtual canopy contains 128 soybean plants consisting of approx. 25 000 plant organs, including almost 7000 individual leaves.
The modelling of light within the canopy was done by using a GPU-based Monte Carlo ray tracing implementation within GroIMP (Henke and Buck-Sorlin, 2017), which allows the simulation of light over the full spectrum down to a resolution of 1 nm. Combining this algorithm with the information integrated in the spectral leaf shaders (Phong shader, including reflection, transmission, absorption of the whole spectrum) allowed detailed evaluation of the light conditions within the canopy. For simplification, a 5 nm resolution within the 400–800 nm range was chosen. The light sources were positioned to enable combination of direct and diffuse radiation. The diffuse light sources were approximated using a dome of directional light sources (Evers et al., 2007; Buck-Sorlin et al., 2011), while the direct light source was set as a fixed point corresponding to the time and location of the field measurement (adapted from Evers et al., 2010). The same models use a theoretical calculation to account for the relative contribution of diffuse and direct radiation, respectively, depending on the time and location (Spitters et al., 1986). To minimize the variation caused by the stochastic approach of Monte Carlo ray tracing, the light within each of the 50 random canopy set-ups was calculated five times with different random seeds for the light model. Each light calculation was done with 2 × 109 rays spread across the wavelength spectrum and a recursion depth (i.e. the maximum amount of reflections) of 20, which is enough to ensure light absorption by either soil or canopy, or reflection back to the atmosphere, as the chance of a ray reflecting >20 times within the canopy before being absorbed is very slim.
The model was validated by adding a virtual light sensor above and below the centre of the virtual canopy. Light sensors in GroIMP are virtual spheres designed to measure passing rays without interacting (i.e. reflecting or absorbing) with them. To recreate the shape of the real spectrometer sensor, the virtual sensors were contained in a surrounding, black cylinder with an open top, only allowing light from above to pass through the sensor. The measured spectrum at the moment of measurement in the field was compared with the virtual light measurement in the top sensor and used to calibrate the light model in GroIMP. As a result, the virtual light measurement of the sensor below the virtual canopy is directly comparable with the real spectral measurement in the field. This approach depends strongly on the occurrence of gaps within the canopy, as the quantity of transmitted light is substantially higher in potential sunflecks. In the experimental field, a complete absence of canopy gaps was observed visually and confirmed by the low spatial variation in the below-canopy sensor data. Complete absence of canopy gaps was not always true for the recreated virtual scene, due to the absence of data on the in situ phyllotaxis. To cope with uncertainty on the in situ phyllotaxis in the virtual canopy, the canopy simulations were repeated 25 times by randomly rotating the plants. To evaluate the degree of canopy closure in each random field set-up, a second light simulation was conducted on each set-up in which the recursion depth of the light model had been reduced to one. As a result, only light which travelled directly from the light source to the sensor is sensed, as each ray in the model is only simulated until the first object (i.e. the ground, the plants in the canopy or the sensor) is hit. A virtual plant set-up was then selected as ‘sufficiently closed’ if the sum of transmitted light through canopy gaps was below an arbitrary selection boundary, which was chosen as 0.5 %.
RESULTS
Model calibration
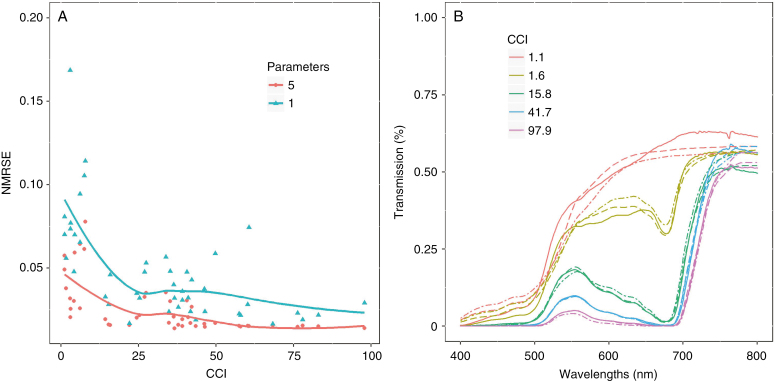
The measured CCI of a leaf is strongly related to the transmission spectrum of that leaf, where leaves with lower CCI values transmit more light (Fig. 3). The optimized transmission spectra from the PROSPECT model accurately capture the measured spectra, allowing comparisons between the optimized PROSPECT parameters and the measured CCI values. A first calibration round was conducted allowing free variations of the five retained PROSPECT parameters (N, Cb, Cant, Cab and Cxc). The PROSPECT model managed to fit to the measured transmission spectra with high accuracy, though with slightly lower accuracy at very low CCI values [Fig. 3, dashed line, mean normalized root-mean-square error (NRMSE) 0.026]. Inspection of the parameter trends as a function of the measured CCI revealed that parameter N (mean 1.08; s.d. 0.12; data not shown) was nearly constant. Additionally, Cant (mean 2.16; s.d. 1.26; data not shown) and Cb (mean 0.04; s.d. 0.06; data not shown) displayed no significant trend as a function of the CCI and were found to be consistently close to their relative lower parameter boundaries. This indicated that the presence of brown pigments and anthocyanin in the sampled leaves was very low. Therefore, a second optimization round was done where N, Cb and Cant were fixed to their mean values, effectively reducing the PROSPECT model complexity to two parameters, Cab and Cxc. This still allowed a very good estimation of the measured spectra (mean NMRSE 0.039).
Fig. 3.
Comparison of model accuracy before and after reducing the amount of parameters in the PROSPECT-D model. (A) Normalized mean-root-square error (NMRSE) between the measured and simulated transmission spectrum with the PROSPECT-D model for each sample in the calibration set. The red line represents the model error after calibration of all five selected PROSPECT-D parameters, and the blue line represents the model error after reducing the model to a single input parameter (i.e. the CCI). (B) Visual representation of the measured transmission spectra of five point measurements (solid lines) with a vastly different chlorophyll content index (CCI), their fitted transmission spectrum after optimization with five PROSPECT-D parameters (dashed lines) and after prediction using only the CCI (dot-dashed lines).
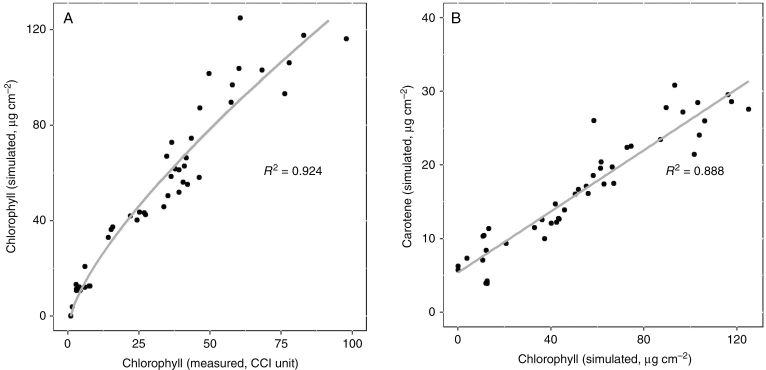
As expected, PROSPECT parameter Cab was highly correlated to the measured CCI. The lowest measured value for CCI was 1.1, which corresponded to a transmission spectrum completely devoid of the diagnostic absorption peak (and consequently a dip in the transmission spectrum) of chlorophyll at 675 nm (Fig. 3). At slightly higher CCI values (e.g. 1.6), this dip in the transmission spectrum is already clearly visible (Fig. 3). The relationship between chlorophyll concentration and CCI is often expressed as a logarithmic relationship (Parry et al., 2014). To ensure a chlorophyll content of 0 at CCI 1.1, the following relationship was fitted to express Cab in terms of the CCI (Fig. 4A, R2 = 0.924):
Fig. 4.
Correlations of the measured chlorophyll content index (CCI) with parameters resulting from an optimization of the PROSPECT-D model on the measured leaf transmission spectra. (A) A logarithmic model was fitted to express the relationship between the CCI and chlorophyll content (Cab). (B) A linear relationship was found between Cab and the carotene content (Cxc).
| (1) |
Furthermore, Cxc showed a linear relationship to Cab (Fig. 4B, R2 = 0.888):
| (2) |
This leads to a reduction of the PROSPECT model to a single parameter for estimating the transmission spectrum of each leaf with only a slight reduction in accuracy (Fig. 3, dot-dashed line, mean NMRSE 0.047).
Model validation
At the individual leaf level, the relationships described in eqns (1) and (2) were used to convert measured CCI values into PROSPECT parameters Cab and Cxc, with N, Cb and Cant set as constants (as well as EWT and LMA which were set as constants earlier). The predicted transmission spectra were very close to the measured spectra in the field (validation data set 1, mean NMRSE 0.046) and in the growth chamber (validation data set 2, mean NMRSE 0.11).
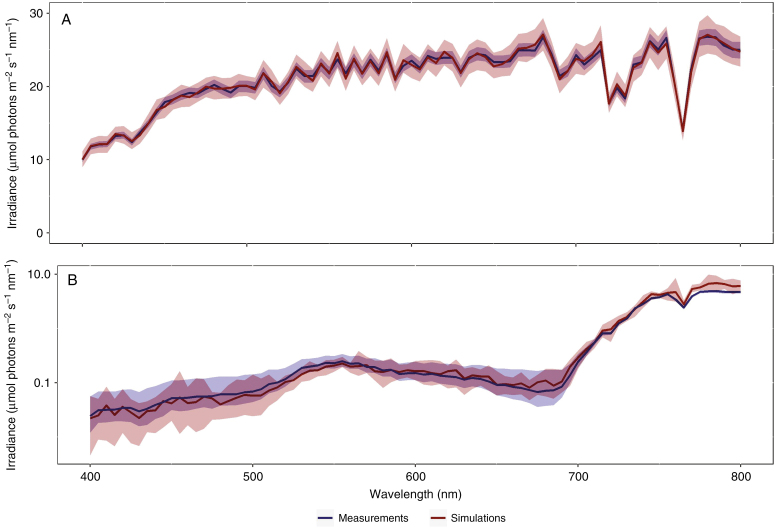
At the full canopy scale, the light model in the virtual scene was calibrated using spectral measurements obtained above the canopy in the field. As a result, the average simulated spectrum closely matched the measured spectrum and intensity, albeit with slightly more variation due to the stochastic nature of the Monte Carlo ray tracing (Fig. 5A). The spectrum of the bottom sensor in the virtual scene exhibited a strong dependence upon the occurrence of canopy gaps with strong variations between different set-ups. Out of the original 25 random virtual field set-ups, only seven were evaluated as sufficiently closed based on the criterion that <0.5 % of the light underneath the canopy originated from gaps. These canopy set-ups allowed a direct comparison between the simulated light in the virtual canopy and the measured light below the canopy.
Fig. 5.
Illustration of the measured and simulated spectra of a soybean canopy at the ILVO site (12.45–12.48 h local time, 23 August 2016, 50°59’33.3’’N, 3°47’04.9’’E). (A) The measured and simulated spectrum above the canopy, with the solid blue line representing the mean of five measurements and the surrounding area the minimal and maximal measured values. The mean measured spectrum was used to calibrate the light model in the GroIMP simulation and resulted in a very good recreation of the light distribution in the simulation, albeit with slightly larger variation due to the stochastic nature of the Monte Carlo approach on which the light model is based. (B) Direct comparison of the measured spectrum (blue) of intensity at different wavelengths below the canopy with simulated measurement (red) in randomized virtual recreations of the canopy (Fig. 2) in GroIMP. Spectral values are displayed on a logarithmic scale to highlight differences in both the lower transmission and higher transmission areas; the blue area encompasses the minimal and maximal values from six measurements below the canopy, while the blue line represents the mean. The red area encompasses the minimum and maximum values from seven simulations below a virtual canopy with sufficient canopy closure. The solid red line represents the mean.
Total simulated PAR, calculated by integrating the measured values over the range of 400–700 nm, above the virtual canopy averaged 1259.20 μmol photons m–2 s–1, which is a near perfect recreation of the corresponding field measurements (1259.85 μmol photons m–2 s–1; Fig. 5A). The measured PAR below the canopy averaged 6.69 μmol photons m–2 s–1 (ranging from a minimum of 4.78 to a maximum of 11.34 μmol photons m–2 s–1), which represents a total PAR transmission of 0.53 % within the canopy. Simulated PAR within the closed architectural field simulations (Fig. 5B) averaged 6.63 μmol photons m–2 s–1 (ranging from 5.34 to 8.97 μmol photons m–2 s–1). The R:FR ratio was calculated by dividing the total intensity of the wavelength range 655–665 (i.e. R) by that of the wavelength range 725–735 (i.e. FR). The measured R:FR ratio averaged 0.051 (with a minimum of 0.032 and a maximum of 0.101), while the simulated R:FR ratio of the best two canopy set-ups averaged 0.052 (with a minimum of 0.038 and maximum of 0.069).
DISCUSSION
The original version of the PROSPECT-D model takes seven parameters (as described in the Materials and Methods) to describe the relationship between leaf spectral characteristics and leaf biochemistry in detail. As our main goal was to perform an accurate evaluation of light conditions within a canopy for FSPM research, such a high degree of complexity regarding leaf biochemistry was not necessary. Therefore, one of the goals was to find a way to estimate the leaf spectral characteristics by quantifying an easy to measure variable such as the leaf CCI. While this reduction in model complexity comes at a small cost in accuracy, it allows straightforward mapping of variability of leaf spectral characteristics within the canopy with an easily comparable variable. Light modelling within FSPMs is typically done in the spectral range of 400–700 nm, sometimes extended to 800 nm when FR light is also considered. Within this range the effect of the two PROSPECT parameters equivalent water thickness (EWT) and leaf mass per area (LMA) is negligible when compared with the influence of Cab and Cxc, and these were therefore assigned a fixed value (Jacquemoud et al., 2009). Additionally, no clear trend was found in the parameters N, anthocyanin content (Cant) and brown pigment content (Cb), which were therefore also set as constants. This led to a significant reduction of the PROSPECT model complexity, with only two parameters left to be estimated from the CCI: the chlorophyll content (Cab) and the carotene content (Cxc).
A very high, non-linear correlation was found between measured CCI and corresponding Cab from the transmission spectra (Fig. 3A). CCI values were expected to be related to the chlorophyll content as a logarithmic function, as CCI is an index based on a difference in transmission between near infra-red (NIR) and red light, and transmission of radiation is logarithmically related to the amount of absorbing compound in the tissue (Parry et al., 2014). Therefore, a logarithmic function was fitted to calculate Cab from the CCI (Fig. 3A). The good fit of this function confirmed the physiological interpretation of Cab as a direct expression of chlorophyll content. As Cxc related very well to Cab, eqns (1) and (2) allowed estimation of the leaf spectral characteristics based solely on CCI measurements. Since this is a non-destructive and very fast measurement (typically 2–3 s per data point), this approach has great potential for model parameterization.
Combining the PROSPECT model with a soybean FSPM leads to accurate approximations of the light distribution and intensity below the canopy (Fig. 5B). However, the occurrence of canopy gaps within the virtual canopy makes it difficult to compare simulations and measurements directly in the field. As the influence of canopy gaps on light transmission is significant, the measurements were specifically conducted in a field set-up with a high degree of heterogeneity concerning leaf spectral characteristics and a high homogeneity with respect to canopy closure. This resulted in measurements below the canopy with low variability and good comparability. Dealing with canopy gaps within a virtual canopy proved more difficult as, to the best of our knowledge, a modelling approach for incorporating canopy closure in FSPMs has not yet been developed. Therefore, it was opted to conduct a large number of random canopy simulations and to use a boundary to select only those simulations that had achieved a high degree of canopy closure, as a means to evaluate our approach of incorporating PROSPECT into an FSPM. While this selection seems arbitrary, it is worth noting that canopy gaps are the result of an imperfect virtual recreation of the crop canopy in terms of actual phyllotaxis, rather than the modelling of the spectral characteristics themselves. On the other hand, our experiment indicated that soybean plasticity in phyllotaxis can be quite effective in terms of optimizing light harvesting. The importance of this plasticity should therefore be further investigated using FSP modelling.
While the approach presented herein was applied to a static canopy architecture for model validation, the methodology is also applicable to a dynamic model in which the chlorophyll content depends on leaf age and senescence. Leaf senescence plays an important role in determining the photosynthetic potential of a canopy. Incorporation of leaf senescence into crop models is therefore not new (e.g. Lizaso et al., 2003; Müller et al., 2007; Evers et al., 2010; Casadebaig et al., 2011; Kang et al., 2014). However, a mechanistic approach to model leaf senescence is, to date, not available, as key issues regarding the onset of senescence due to environmental or internal factors have not been elucidated (Thomas and Stoddart, 2007). For that reason, modelling leaf senescence is mostly done as a function of developmental age, as leaf development progresses (e.g. Lizaso et al., 2003; Casadebaig et al., 2011; Kang et al., 2014) or the timing of leaf senescence can be taken straight from empirical data (e.g. Evers et al., 2010). However, for the establishment of a mechanistic link between leaf senescence and light interception in FSPMs, modelling of leaf spectral properties is required. Therefore, spatiotemporal mapping of CCI is a quick and easy way to capture the dynamics of both leaf senescence and photosynthetic potential.
Overall, the PROSPECT model is a good tool to estimate leaf spectral characteristics from the CCI, and integration into an FSPM can lead to multiple advantages. First, as leaf chlorophyll content is strongly linked to leaf photosynthetic capacity, it is often already considered in FSPMs (e.g. Müller et al., 2007; Wernecke et al., 2007; Evers et al., 2010). Therefore, our approach allows extraction of further information, namely the leaf spectral characteristics, from an already important variable, i.e. the CCI, while also expressing them in a functional way (i.e. in terms of leaf biochemistry). Secondly, our approach allows good estimation of leaf spectral characteristics with quick, non-destructive measurements of the CCI, using basic measurement tools (e.g. a hand-held leaf photospectrometer). Thirdly, the model is not computationally heavy, especially when compared with other steps in an FSPM calculation (e.g. calculation of the light model). Lastly, it can lead to a better approximation of light quality and quantity within the canopy, which, in turn, can lead to better evaluations of photosynthetically absorbed light or photomorphogenic responses to light quality. Both of these are crucial in accurately describing growth, architecture development and competition effects in FSPMs.
ACKNOWLEDGEMENTS
We would like to thank Katleen Sucaet, Thomas Vanderstocken and Luc Van Gyseghem for their extensive help with the field trials and the growth chamber experiment, Jonas Aper for his expertise regarding soybean, and Johan Snoeck for his help in setting up the field trial. This work was supported by a personal PhD grant issued by the Agency for Innovation by Science & Technology (IWT) to J.C.
LITERATURE CITED
- Baret F, Fourty T. 1997. Estimation of leaf water content and specific leaf weight from reflectance and transmittance measurements. Agronomie, EDP Sciences 17: 455–464. [Google Scholar]
- Buck-Sorlin GH, Hemmerling R, Kniemeyer O, Burema B, Kurth W. 2008. A rule-based model of barley morphogenesis, with special respect to shading and gibberellic acid signal transduction. Annals of Botany 101: 1109–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck-Sorlin GH, de Visser PHB, Henke M, et al. 2011. Towards a functional–structural plant model of cut-rose: simulation of light environment, light absorption, photosynthesis and interference with the plant structure. Annals of Botany 108: 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadebaig P, Guilioni L, Lecoeur J, Christophe A, Champolivier L, Debaeke P. 2011. SUNFLO, a model to simulate genotype-specific performance of the sunflower crop in contrasting environments. Agricultural and Forest Meteorology 151: 163–178. [Google Scholar]
- Casal J, Sanchez R, Deregibus V. 1986. The effect of plant density on tillering: the involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environmental and Experimental Botany 26: 365–371. [Google Scholar]
- Ceccato P, Flasse S, Tarantola S, Jacquemoud S, Grégoire JM. 2001. Detecting vegetation leaf water content using reflectance in the optical domain. Remote Sensing of Environment 77: 22–33. [Google Scholar]
- Chelle M. 2005. Phylloclimate or the climate perceived by individual plant organs: what is it? How to model it? What for?New Phytologist 166: 781–790. [DOI] [PubMed] [Google Scholar]
- Coussement JR, Steppe K, Lootens P, Roldán-ruiz I, De Swaef T. 2018. A flexible geometric model for leaf shape descriptions with high accuracy. Silva Fennica 51: 1–14. [Google Scholar]
- Evers JB, Vos J, Chelle M, Andrieu B, Fournier C, Struik PC. 2007. Simulating the effects of localized red:far-red ratio on tillering in spring wheat (Triticum aestivum) using a three-dimensional virtual plant model. New Phytologist 176: 325–336. [DOI] [PubMed] [Google Scholar]
- Evers JB, Vos J, Yin X, Romero P, van der Putten PEL, Struik PC. 2010. Simulation of wheat growth and development based on organ-level photosynthesis and assimilate allocation. Journal of Experimental Botany 61: 2203–2216. [DOI] [PubMed] [Google Scholar]
- Fassnacht FE, Stenzel S, Gitelson AA. 2015. Non-destructive estimation of foliar carotenoid content of tree species using merged vegetation indices. Journal of Plant Physiology 176: 210–217. [DOI] [PubMed] [Google Scholar]
- Féret JB, François C, Asner GP, et al. 2008. PROSPECT-4 and 5: advances in the leaf optical properties model separating photosynthetic pigments. Remote Sensing of Environment 112: 3030–3043. [Google Scholar]
- Féret JB, Gitelson AA, Noble SD, Jacquemoud S. 2017. PROSPECT-D: towards modeling leaf optical properties through a complete lifecycle. Remote Sensing of Environment 193: 204–215. [Google Scholar]
- Franklin KA, Whitelam GC. 2005. Phytochromes and shade-avoidance responses in plants. Annals of Botany 96: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi FB, Ray JD. 2007. Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 45: 92–98. [Google Scholar]
- Halliday KJ, Koornneef M, Whitelam GC. 1994. Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana to low red/far-red ratio. Plant Physiology 104: 1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerling R, Kniemeyer O, Lanwert D, Kurth W, Buck-Sorlin G. 2008. The rule-based language XL and the modelling environment GroIMP illustrated with simulated tree competition. Functional Plant Biology 35: 739–750. [DOI] [PubMed] [Google Scholar]
- Henke M, Buck-Sorlin GH. 2017. Using a full spectral raytracer for calculating light microclimate in functional–structural light modelling. Computing and Informatics 36: 1492–1522. [Google Scholar]
- Jacquemoud S, Baret F. 1990. PROSPECT : a model of leaf optical properties spectra. Remote Sensing of Environment 34: 75–91. [Google Scholar]
- Jacquemoud S, Baret F, Andrieu B, Danson FM, Jaggard K. 1995. Extraction of vegetation biophysical parameters by inversion of the PROSPECT + SAIL models on sugar beet canopy reflectance data. Application to TM and AVIRIS sensors. Remote Sensing of Environment 52: 163–172. [Google Scholar]
- Jacquemoud S, Ustin SL, Verdebout J, Schmuck G, Andreoli G, Hosgood B. 1996. Estimating leaf biochemistry using the PROSPECT leaf optical properties model. Remote Sensing of Environment 56: 194–202. [Google Scholar]
- Jacquemoud S, Verhoef W, Baret F, et al. 2009. PROSPECT + SAIL models: a review of use for vegetation characterization. Remote Sensing of Environment 113: S56–S66. [Google Scholar]
- Kahlen K, Stützel H. 2011. Modelling photo-modulated internode elongation in growing glasshouse cucumber canopies. New Phytologist 190: 697–708. [DOI] [PubMed] [Google Scholar]
- Kang F, Cournède P-H, Lecoeur J, Letort V. 2014. SUNLAB: a functional–structural model for genotypic and phenotypic characterization of the sunflower crop. Ecological Modelling 290: 21–33. [Google Scholar]
- Kniemeyer O, Barzik G, Hemmerling R, Kurth W. 2008. Relational growth grammars – a parallel graph transformation approach with applications in biology and architecture. In: Schürr A, Nagl M, Zündorf A, eds. Applications of graph transformations with industrial relevance. AGTIVE 2007. Lecture Notes in Computer Science, vol. 5088 Berlin, Heidelberg: Springer, 152–167. [Google Scholar]
- Lau HT. 2003. A numerical library in Java for scientists and engineers. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- Le Maire G, François C, Dufrêne E. 2004. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sensing of Environment 89: 1–28. [Google Scholar]
- Lizaso JI, Batchelor WD, Westgate ME. 2003. A leaf area model to simulate cultivar-specific expansion and senescence of maize leaves. Field Crops Research 80: 1–17. [Google Scholar]
- Lugg DG, Sinclair TR. 1981. Seasonal changes in photosynthesis of field-grown soybean leaflets. 2. Relation to nitrogen content. Photosynthetica 15: 138–144. [Google Scholar]
- Müller J, Wernecke P, Braune H, Diepenbrock W. 2007. Photosynthesis and carbon balance. In: Vos J, Marcelis LFM, Visser PHB, Struik PC, Evers JB, eds. Functional–structural plant modelling in crop production. Wageningen: Springer Netherlands, 91–101. [Google Scholar]
- Parry C, Blonquist JM, Bugbee B. 2014. In situ measurement of leaf chlorophyll concentration: analysis of the optical/absolute relationship. Plant, Cell and Environment 37: 2508–2520. [DOI] [PubMed] [Google Scholar]
- R Core Team 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Schaepman-Strub G, Schaepman ME, Painter TH, Dangel S, Martonchik JV. 2006. Reflectance quantities in optical remote sensing – definitions and case studies. Remote Sensing of Environment 103: 27–42. [Google Scholar]
- Spitters CJT, Toussaint HAJM, Goudriaan J. 1986. Separating the diffuse and direct component of global radiation and its implications for modeling canopy photosynthesis Part I. Components of incoming radiation. Agricultural and Forest Meteorology 38: 217–229. [Google Scholar]
- Thomas H, Stoddart JL. 2007. Leaf senescence. Annual Review of Plant Physiology and Plant Molecular Biology 58: 115–136. [DOI] [PubMed] [Google Scholar]
- Vos J, Evers JB, Buck-Sorlin GH, Andrieu B, Chelle M, de Visser PHB. 2010. Functional–structural plant modelling: a new versatile tool in crop science. Journal of Experimental Botany 61: 2101–2115. [DOI] [PubMed] [Google Scholar]
- Wernecke P, Müller J, Dornbusch T, Wernecke A, Diepenbrock W. 2007. The virtual crop-modelling system ‘Vica’ specified for barley. In: Vos J, Marcelis LFM, Visser PHB, Struik PC, Evers JB, eds. Functional–structural plant modelling in crop production. Wageningen: Springer Netherlands, 53–64. [Google Scholar]
- Willighagen E. 2014. genalg: R based genetic algorithm. R package version 0.1.1.1 http://CRAN.R-project.org/package=genalg.
- Wu X-Y, Kuai B-K, Jia J-Z, Jing H-C. 2012. Regulation of leaf senescence and crop genetic improvement. Journal of Integrative Plant Biology 54: 936–952. [DOI] [PubMed] [Google Scholar]