Abstract
Background and Aims
The cuticular waxes sealing plant surfaces against excessive water loss are complex mixtures of very-long-chain aliphatics, with compositions that vary widely between plant species. To help fill the gap in our knowledge about waxes of non-flowering plant taxa, and thus about the cuticle of ancestral land plants, this study provides comprehensive analyses of waxes on temperate fern species from five different families.
Methods
The wax mixtures on fronds of Pteridium aquilinum, Cryptogramma crispa, Polypodium glycyrrhiza, Polystichum munitum and Gymnocarpium dryopteris were analysed using gas chromatography–mass spectrometry for identification, and gas chromatography–flame ionization detection for quantification.
Key Results
The wax mixtures from all five fern species contained large amounts of C36–C54 alkyl esters, with species-specific homologue distributions. They were accompanied by minor amounts of fatty acids, primary alcohols, aldehydes and/or alkanes, whose chain length profiles also varied widely between species. In the frond wax of G. dryopteris, C27–C33 secondary alcohols and C27–C35 ketones with functional groups exclusively on even-numbered carbons (C-10 to C-16) were identified; these are characteristic structures similar to secondary alcohols and ketones in moss, gymnosperm and basal angiosperm waxes. The ferns had total wax amounts varying from 3.9 μg cm–2 on P. glycyrrhiza to 16.9 μg cm–2 on G. dryopteris, thus spanning a range comparable with that on leaves of flowering plants.
Conclusions
The characteristic compound class compositions indicate that all five fern species contain the full complement of wax biosynthesis enzymes previously described for the angiosperm arabidopsis. Based on the isomer profiles, we predict that each fern species, in contrast to arabidopsis, has multiple ester synthase enzymes, each with unique substrate specificities.
Keywords: Chain length distribution, cuticular waxes, GC-MS, ketones, polypod ferns, positional isomers, secondary alcohols, seedless plants, wax esters
INTRODUCTION
Plant colonization of terrestrial habitats relied heavily on the evolution of lipid coatings that seal organ surfaces against excessive water loss (Yeats and Rose, 2013). The resulting extracellular structure covering the epidermal cells of all land plants, called the plant cuticle, consists of the insoluble fatty acid polyester cutin and of waxes embedded in and deposited on top of the cutin framework (Jeffree, 2006). The waxes help protect plants against UV radiation (Gordon et al., 1998), insects and pathogens (Uppalapati et al., 2012), and their primary function is to seal the vast surfaces of above-ground organs against water loss (Schönherr, 1976). Even though the cuticles of diverse plant species serve the same principal functions, they can have greatly varying transpiration barrier qualities and impose resistances on water vapour diffusion that differ by up to two orders of magnitude (Schreiber and Riederer, 1996).
It is important to understand the differences in ecophysiological performance between different plant species and stress adaptation strategies based on corresponding differences in the amounts and compositions of respective wax mixtures. Even though the wax mixtures of most species comprise the same classes of compounds, they vary greatly in terms of total wax amounts per surface area (wax coverage) and the exact amounts of individual compounds (wax composition) (Jetter et al., 2006; Busta et al., 2017). Hence, a major goal is to establish structure–function relationships linking biological properties to various chemical constituents of cuticular waxes. Two prerequisites for the successful realization of this long-term goal are a working knowledge of (1) the diversity of plant waxes across the plant kingdom and (2) the mechanisms by which wax constituents are biosynthesized.
Over decades, studies on diverse gymnosperms and angiosperms have shown that their leaves are all covered by wax mixtures comprising similar classes of lipids. In many species, cyclic compounds such as triterpenoids were found, often accumulating to fairly low percentages of the surface wax. The most abundant and widespread wax compounds have linear, saturated hydrocarbon chains with either an oxygen-containing functional group on one end (fatty acids, aldehydes and alcohols) or no functional group at all (alkanes) (Jetter et al., 2006). Multiple chain lengths of each of these compound classes are present, such that wax compositions are typically mixtures of multiple homologous series whose constituents range in length from C20 to almost C40. In addition to these monomeric chemicals, cuticular waxes may also include dimers of wax alcohols and fatty acids joined into alkyl esters, which can range in chain length from C36 to C60. In a few cases, more detailed mass spectrometric analyses have revealed that each ester homologue consists of various isomers resulting from different combinations of acids and alcohols with varying chain lengths (Gülz et al., 1993; Sümmchen et al., 1995). Based on such approaches, it seems that gymnosperm and angiosperm wax esters are typically composed of both long-chain (C16 and C18) and very-long-chain (VLC; C20–C38) primary alcohols and fatty acids.
The amounts and compositions of wax mixtures of the few plant species tested so far were fairly constant throughout organ development and further into maturity, with a few notable exceptions where either additional compounds appeared (Jetter and Schäffer, 2001) or chain length profiles shifted (Busta et al., 2017). There is also circumstantial evidence that wax mixtures may be regenerated after disturbance on mature tissues (Neinhuis et al., 2001), and many recent reports show that wax amounts and/or compositions may change in response to physiological stress either during or after organ growth (Bi et al., 2017; Kim et al., 2017).
The mechanisms leading to the accumulation of the ubiquitous wax constituents on gymnosperm and angiosperm surfaces are fairly well understood, in particular for the model species Arabidopsis thaliana (Samuels et al., 2008). There, wax biosynthesis begins with the elongation of long-chain fatty acyl precursors by fatty acyl elongase (FAE) complexes to VLC fatty acyl-CoAs. FAEs catalyse cycles of four enzymatic reactions that, together, extend an aliphatic chain by two carbons, and repeated elongation rounds generate higher homologues of acyl-CoAs with even total carbon numbers (TCNs). Several different FAE complexes are likely to be involved in the different rounds, each with substrate and product specificities mostly imposed by the enzyme catalysing the first step of the cycle, a ketoacyl-CoA synthase (KCS) (Millar and Kunst, 1997; Joubès et al., 2008). It has recently been shown that further proteins, CER2-LIKE members of the BAHD family of proteins, can confer further chain length specificity to the FAE complex (Haslam et al., 2012; Pascal et al., 2013). The resulting acyl-CoA products are then converted into various derivatives with characteristic chain length profiles, involving (1) hydrolysis to fatty acids (no enzyme characterized); (2) reduction to primary alcohols (by a fatty acyl-CoA reductase, FAR) and their esterification with acids (by a wax ester synthase, WS or WSD); or (3) reduction to aldehydes (by the reductase CER3 in arabidopsis) and decarbonylation (by the decarbonylase CER1 in arabidopsis) to alkanes with one carbon less (Samuels et al., 2008). Wax biosynthesis is accomplished exclusively within the endoplasmic reticulum of epidermal cells, and from there wax compounds are exported to the plant surface via ATP-binding cassette (ABC) transporters and glycosylphosphatidylinositol (GPI)-anchored lipid transfer proteins (LTPs) (Samuels et al., 2008).
Compounds with in-chain functional groups (secondary alcohols, ketones, alkanediols, ketols and β-diketones) and branched hydrocarbon skeletons have also been identified in many plant species. In some cases, these specialty compounds constitute the majority of a species’ cuticular wax, as do 16-hentriacontanone on Annona, Aristolochia and Allium species (Maier and Post-Beittenmiller, 1998; Meusel et al., 1999; Shanker et al., 2007), 10-nonacosanol on diverse gymnosperms and angiosperms (Jetter and Riederer, 1996; Nikolic et al., 2013), and β-diketones on many Poaceae (Tulloch et al., 1980; Racovita et al., 2016). However, our understanding of wax diversity and biosynthesis in those taxa other than arabidopsis is relatively limited. For example, β-diketones (not observed in arabidopsis) have long been recognized as polyketides, and it is well established that they are formed on dedicated pathways involving polyketide synthase(s) (von Wettstein-Knowles, 1993). Since then, these pathways have not been elucidated in detail, and the first genes involved in the process were only recently identified and partially characterized in barley and wheat (Hen-Avivi et al., 2016; Schneider et al., 2016). Even less is known about the biosynthesis of the other specialty wax compounds with in-chain functional groups.
So far, the cuticles of only a few seedless plants have been studied. For example, the cuticular waxes of the moss Funaria hygrometrica contain mainly alkyl esters together with diol esters and β-hydroxy-fatty acid alkyl esters (Busta et al., 2016), while Polytrichales mosses have waxes comprising relatively large amounts of secondary alcohols (Neinhuis and Jetter, 1995). Thus, these few studies indicate that there is substantial unexplored wax diversity outside of the frequently studied seed plant taxa. However, it is still difficult to envisage cuticle evolution during the colonization of land, in part because relatively little is known about the composition of the water barrier-forming waxes in plant taxa other than the gymnosperms and angiosperms.
Ferns are also of special interest, as an early-diverging lineage of seedless plants that possess internal water transport and, therefore, require mechanisms restricting water evaporation from above-ground organs. However, despite the widespread occurrence and phylogenetic diversity of extant ferns, little is known about their cuticular waxes. An early survey of 21 fern species belonging to 13 families identified n-alkanes and the triterpene fernene (Bottari et al., 1972), and fernene was also reported for the waxes of Polypodium glaucinum and Plagiogyra formosana (Wollenweber et al., 1981). The frond waxes of silver fern (Cyathea dealbata) and bracken fern (Pteridium aquilinum) bear wax esters (Franich et al., 1985a; Baker and Gaskin, 1987), while 10-nonacosanone predominates on surfaces of royal fern (Osmunda regalis) (Jetter and Riederer, 1999). These reports, especially in combination with observations that ferns have relatively recently undergone substantial diversification (Schneider et al., 2004), indicate that ferns probably have diverse cuticular wax chemistry and wax biosynthetic strategies that have yet to be explored.
To fill the gap in our understanding of early-diverging land plant cuticle chemistry, the current study aims to provide comprehensive wax analyses of diverse fern species. We selected five species of polypod ferns native to British Columbia, Canada, belonging to five major families of extant temperate ferns with drastically differing life history strategies: Pteridium aquilinum (Bracken fern; Dennstaedtiaceae) is a deciduous species growing in sunny habitats such as open forests or grasslands (Nishida and Hanba, 2017), Cryptogramma crispa (Parsley fern; Pteridaceae) is a deciduous species found in alpine rock crevices, Polypodium glycyrrhiza (Licorice fern; Polypodiaceae) is a summer-deciduous fern prevailing in areas with cool and moist summers and warm and wet winters, Polystichum munitum (Sword fern; Dryopteridaceae) is an evergreen growing in the understorey of moist coniferous forests at low elevations, and Gymnocarpium dryopteris (Oak fern; Cystopteridaceae) is a deciduous species widely distributed across North America and Eurasia from mesic to wet sites in mixed conifer and hardwood stands. Here, we aimed to identify and quantify compound classes, homologues and isomers in the wax mixtures of these fern species, to enable comparisons between the fern waxes with previous reports on gymnosperm and angiosperm waxes and future investigations into the wax biosynthesis machineries in ferns.
MATERIALS AND METHODS
Plant material and wax sampling
Fronds of Pteridium aquilinum, Cryptogramma crispa, Polypodium glycyrrhiza, Polystichum munitum and Gymnocarpium dryopteris were collected from wild specimens growing outdoors in Vancouver, British Columbia. For C. crispa, the tips of ten fronds comprising rachis and 4–10 leaflets were randomly selected and harvested for each independent sample. Similarly, 20–30 leaflets from 4–5 Pteridium aquilinum fronds, 15 leaflets from one Polystichum munitum frond, 36 leaflets from one Polypodium glycyrrhiza frond and 16 leaflets from three G. dryopteris fronds were used per sample (all without rachis). A photograph was taken of all the material used for each sample alongside a ruler, and surface areas were determined by pixel counting with Image J software. The average areas of frond materials used per parallel for each species were 19.0 ± 2.7, 68.9 ± 7.3, 101.5 ± 6.8, 105.2 ± 21.2 and 70.8 ± 7.7 cm2 for G. dryopteris, C. crispa, Pteridium aquilinum, Polystichum munitum and Polypodium glycyrrhiza, respectively (n = 5 independent parallels, ± s.e.).
For wax extraction, samples were immersed twice for 30 s in chloroform. The two wax solutions were combined and spiked with a known amount of n-tetracosane internal standard, and then filtered through glass wool. Next, the solvent was removed under nitrogen gas, and the wax mixture reacted with BSTFA (20 μL) and pyridine (20 μL) at 70 °C for 45 min. After derivatization, excess reagents were evaporated under N2, and chloroform (50 μL) was added. For each fern species, five independent samples were extracted.
Wax analysis
Wax compounds were identified with gas chromatography–mass spectrometry (GC-MS). Samples were injected with a cool on-column injector at 54 °C into a flow of helium gas (1.4 mL min–1). Wax compounds were separated with a HP1 capillary GC column in an Agilent 6890N gas chromatograph (Agilent, Avondale, PA, USA), with the oven programmed to hold at 50 °C for the first 2 min, then to increase by 40 °C min–1 to 200 °C and hold for 2 min, to increase again at 3 °C min–1 until 320 °C and then to hold for 30 min. The MS detector (5973N, Agilent) employed electron impact ionization (70 eV), a quadrupole mass analyser and electron multiplier detection. Chemical structures were assigned to GC peaks according to their MS fragmentation characteristics in comparison with spectra of authentic standards or MS spectral data from the NIST 2002 library.
Wax compounds were quantified using GC-FID employing the same gas chromatography system described above for GC-MS, except that hydrogen gas (2 mL min–1) was used as the mobile phase. Eluting compounds were detected by a flame ionization detector (FID) set at 250 °C. Total wax amounts were determined by comparing the sum areas of GC–FID peaks against the area of the internal standard peak. Each wax compound was quantified by peak area integration with respect to the known amount of tetracosane internal standard. Compound quantities were calculated as averages of the five independent samples for each species ± standard errors.
Under the GC conditions used here, ester homologues,but not ester isomers, could be separated. Therefore, the distributions of fatty acids and alcohols within each ester homologue could not be determined by GC-FID, and instead all samples were re-analysed with GC-MS. Different ester isomers within each ester homologue yielded different protonated acid ions, RCOOH2+, indicative of acyl moiety chain lengths, and quantities of these characteristic fragments were used to determine the percentage of each isomer within each homologue. Multiplication of isomer percentages within each homologue by the homologue abundances and addition across all homologues led to overall chain length profiles of esterified acids and alcohols.
RESULTS
This study aimed to identify and quantify the components of cuticular wax mixtures on diverse fern species. Accordingly, waxes were extracted from fronds with chloroform, spiked with an internal standard, TMS-derivatized and analysed with GC-MS and GC-FID. The resulting chromatographic data were used to determine total wax coverages and relative amounts of compound classes within each mixture, as well as the homologue and isomer distributions within compound classes.
Wax coverage and compound class composition
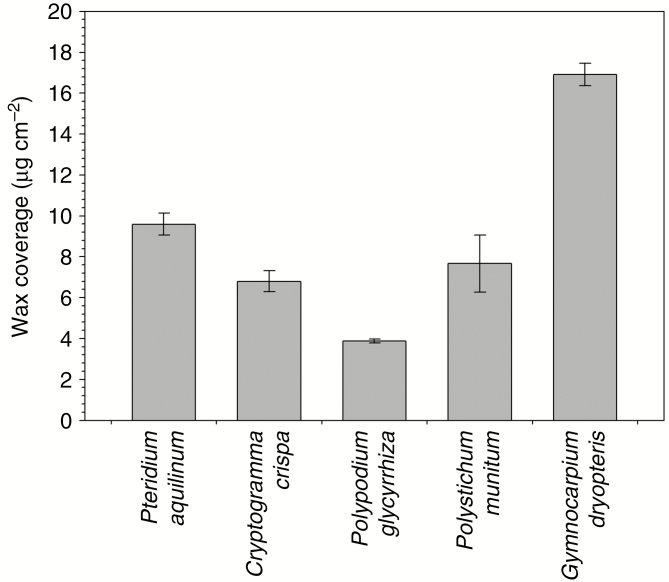
Wax coverages on fronds of the five fern species varied from 3.9 μg cm–2 on P. glycyrrhiza to 6.8 μg cm–2 on C. crispa, 7.7 μg cm–2 on P. munitum, 9.6 μg cm–2 on P. aquilinum and 16.9 μg cm–2 on G. dryopteris (Fig. 1).
Fig. 1.
Total wax coverage on fronds of five fern species. Wax coverages are given in µg per square centimetre extracted (averages ± s.e., n = 5).
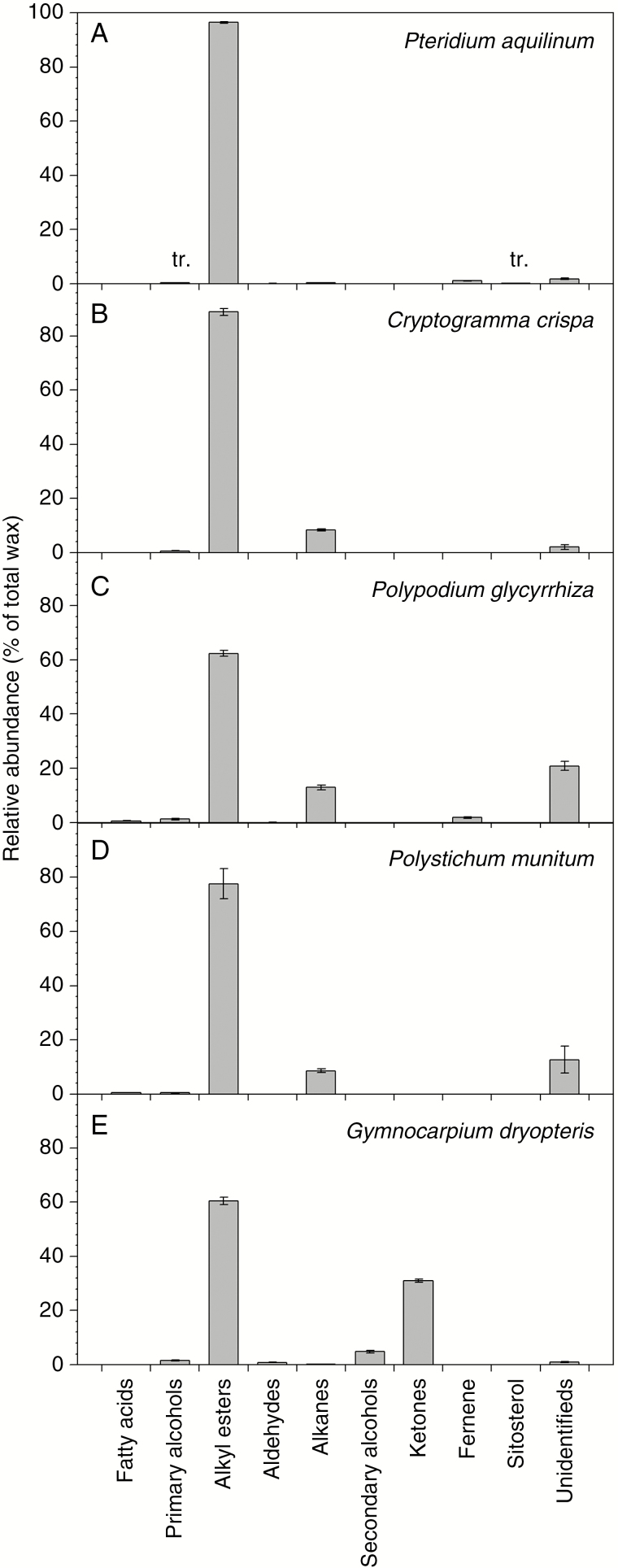
In the wax mixtures of all five species, alkyl esters were the most abundant compound class, comprising between 60 and 95 % of the total wax (Fig. 2). On C. crispa, P. glycyrrhiza and P. munitum, alkanes accumulated to 8–13 % of the total wax, while G. dryopteris wax contained approx. 30 and 5 % of ketones and secondary alcohols, respectively. Several other wax compound classes were identified in small amounts (e.g. <2 %), including primary alcohols on all five species, fatty acids on P. glycyrrhiza and P. munitum, and aldehydes on P. glycyrrhiza, P. aquilinum and G. dryopteris. Fernene was found in the waxes of P. glycyrrhiza and P. aquilinum, and trace amounts of β-sitosterol on P.aquilinum. Relatively small portions of the wax mixtures remained unidentified, ranging from 1 % for G. dryopteris wax to 21 % for P. glycyrrhiza. In summary, the wax mixtures of all five fern species had high amounts of alkyl esters, but were differentiated by the relative amounts of other components.
Fig. 2.
Relative abundances of wax compound classes on fronds of five fern species. Relative abundance of each compound class expressed as a percentage within the total wax mixture for: (A) Pteridium aquilinum; (B) Cryptogramma crispa; (C) Polypodium glycyrrhiza; (D) Polystichum munitum; and (E) Gymnocarpium dryopteris (averages ± s.e., n = 5). tr., trace.
Homologue distributions of fatty acids, alcohols, aldehydes and alkanes
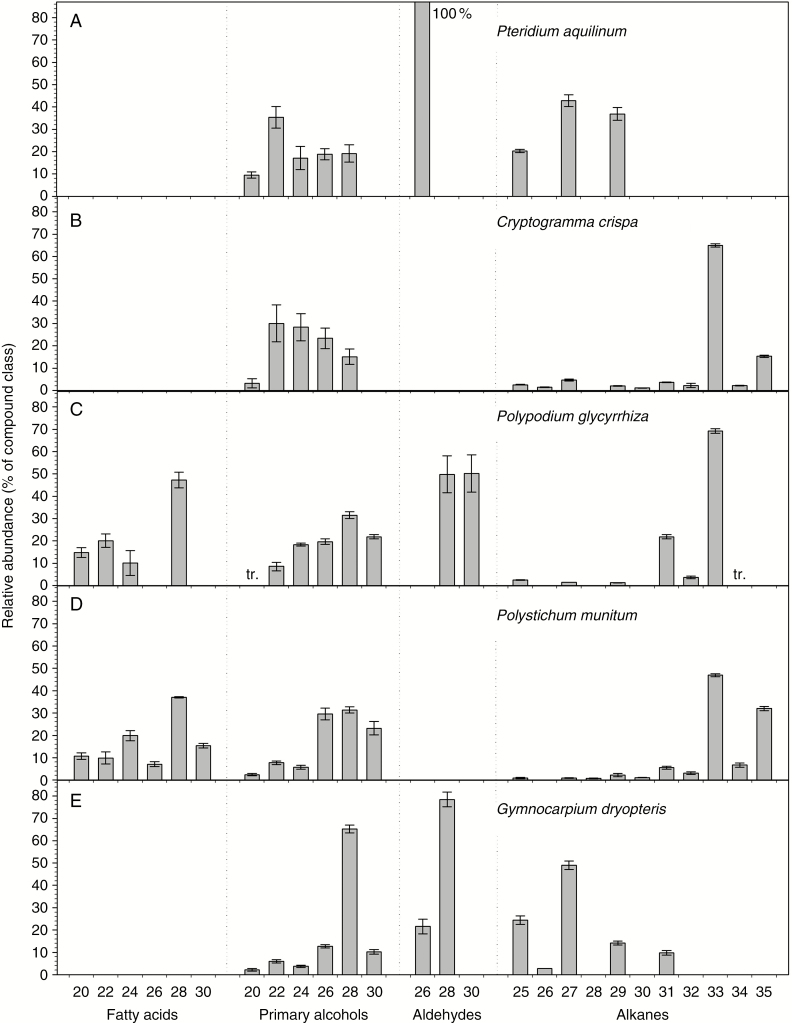
The free fatty acids, primary alcohols, aldehydes and alkanes were present as homologous series with characteristic chain distributions on one or more fern species. Homologues with even TCNs predominated among the fatty acids, primary alcohols and aldehydes. Fatty acids were only observed in the waxes of P. glycyrrhiza and P. munitum, with chain lengths ranging from C20 to C28 and C20 to C30, respectively, and C28 fatty acid predominating in both species (Fig. 3). The primary alcohols ranged from C20 to C28 or C30, depending on the species, peaking at C22 in P. aquilinum and C. crispa, and at C28 in P. glycyrrhiza, P. munitum and G. dryopteris. Pteridium aquilinum bore solely C26 aldehyde, P. glycyrrhiza equal amounts of C28 and C30, and G. dryopteris C26 and (mostly) C28.
Fig. 3.
Chain length distributions within single-isomer compound classes in the cuticular wax from five fern species. Relative abundances of each homologue expressed as a percentage within its compound class for: (A) Pteridium aquilinum; (B) Cryptogramma crispa; (C) Polypodium glycyrrhiza; (D) Polystichum munitum; and (E) Gymnocarpium dryopteris (averages ± s.e., n = 5). The total carbon number (chain length) for each homologue in the respective compound classes is noted on the x-axis. tr., trace.
Alkanes were present as unbranched homologues with mainly odd TCNs, ranging from C25 to C35. Small amounts of alkanes with even TCNs were detected on all species except P. aquilinum. Alkane chain lengths culminated at C33 and C35 in P. munitum (47 and 32 %, respectively) and C. crispa (65 and 15 %), C33 and C31 in P. glycyrrhiza (69 and 21 %), C27 and C29 in P. aquilinum (43 and 37 %), and C27 and C25 in G. dryopteris (49 and 24 %). Thus, the predominant chain lengths of alkanes were longer than those of fatty acids, primary alcohols and aldehydes in most fern species.
Homologue and isomer distributions of secondary alcohols and ketones
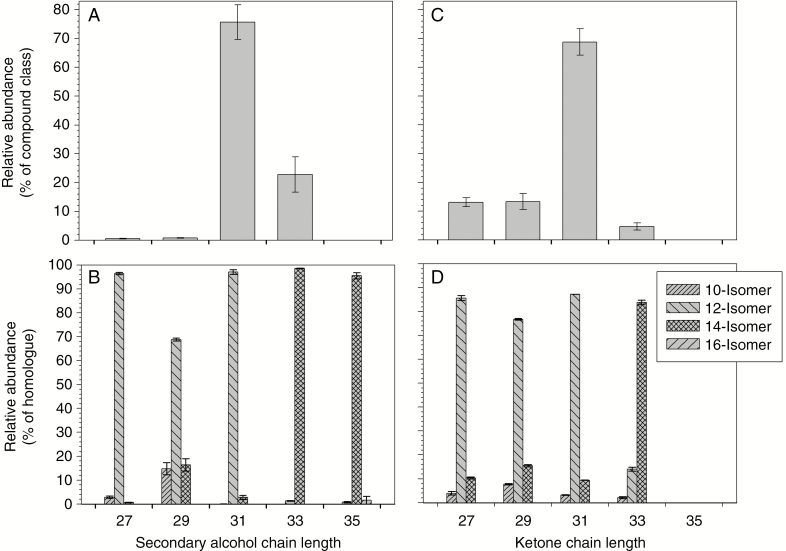
The secondary alcohols and ketones on G. dryopteris were present as a homologous series (Supplementary Data Fig. S1A), with each homologue comprising multiple isomers. The homologues could be resolved by GC and therefore quantified by FID, revealing that the secondary alcohols had odd TCNs ranging from C27 to C35 and peaking at C31 (Fig. 4). The location of the secondary oxygen functional group was determined using diagnostic α-fragments in the mass spectrum of each homologue (Supplementary Data Fig. S1B, C), and respective fragment abundances showed that the C27, C29 and C31 secondary alcohols had functional groups predominantly on C-12, with minor amounts of isomers carrying functional groups on C-14 or C-10. In contrast, the C33 and C35 secondary alcohols had functional groups predominantly on C-14, with minor amounts of the C-16 isomer in the C35 homologue.
Fig. 4.
Homologue and isomer distributions within the secondary alcohols and ketones from Gymnocarpium dryopteris fronds. Relative abundances of secondary alcohols expressed as percentages: (A) of homologues within the compound class, and (B) of isomers within each homologue; relative abundances of secondary alcohols are expressed as percentages (C) of homologues within the compound class, and (D) of isomers within each homologue. Bar heights and error bars represent the averages and standard errors of five independent replicates, respectively.
The G. dryopteris wax ketones had odd TCNs ranging from C27 to C33 (Supplementary Data Fig. S2A), with C31 being the most abundant (Fig. 4). Functional group positions were again determined using diagnostic MS fragments (Supplementary Data Fig. S2B, C), revealing that the C27, C29 and C31 ketones had carbonyl groups predominantly on C-12, accompanied by minor amounts of C-14 and C-10 isomers, and the C-14 isomer comprised the majority of the C33 homologue (Fig. 4).
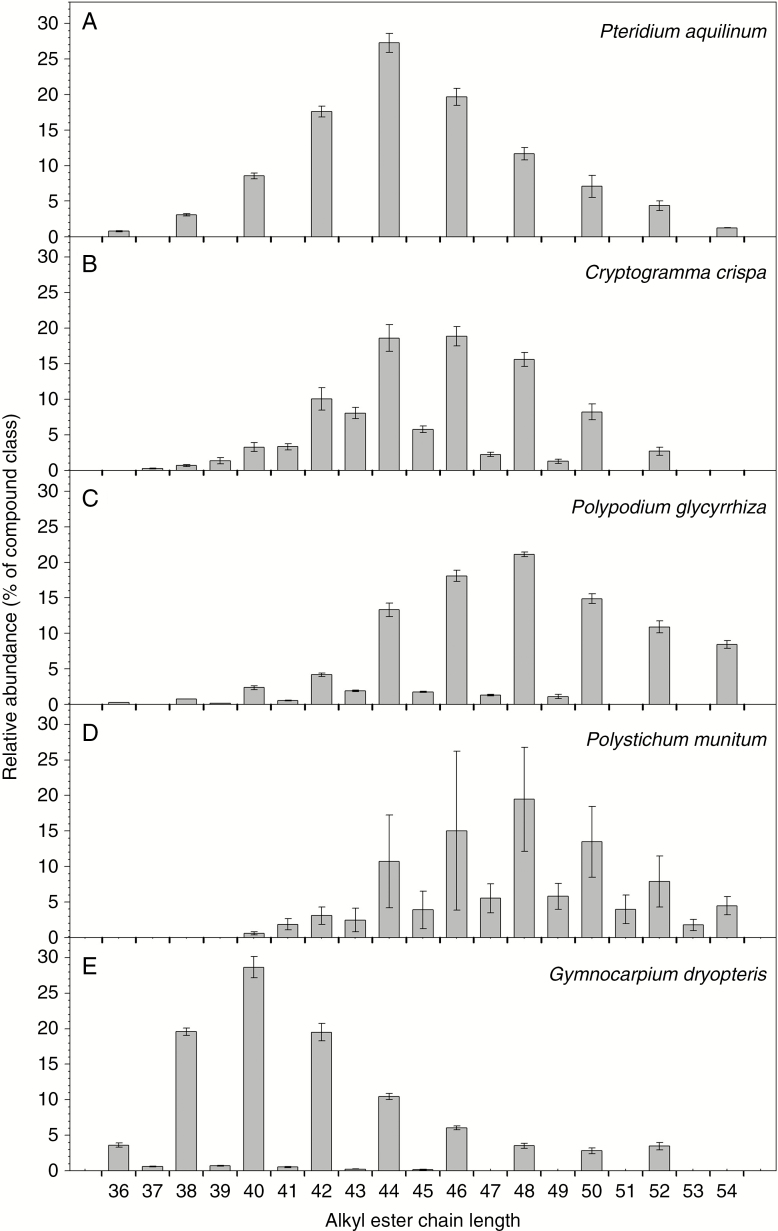
Homologue and isomer distributions of alkyl esters
Alkyl esters are dimeric wax compounds formed from long-chain or VLC alcohol and acid moieties, and thus have much larger TCNs. On all species tested here, ester chain lengths ranged from C38 or C40 to C52 or C54 (Fig. 5). On P. aquilinum and C. crispa, ester homologue distributions peaked at C44, while P. glycyrrhiza and P. munitum wax ester chain lengths culminated at C48 and G. dryopteris had mainly C40 ester. Esters with even TCNs were much more abundant than those with odd TCNs, with even-over-odd ratios of 3.5, 13.9, 3.0 and 42.5 for C. crispa, P. glycyrrhiza, P. munitum and G. dryopetris, respectively (no esters with odd TCNs were detected on P. aquilinum).
Fig. 5.
Chain length distributions of alkyl esters in the cuticular waxes on fronds of five fern species. Relative abundances of each ester homologue expressed as a percentage within the compound class for: (A) Pteridium aquilinum; (B) Cryptogramma crispa; (C) Polypodium glycyrrhiza; (D) Polystichum munitum; and (E) Gymnocarpium dryopteris. The total carbon number (chain length) for each homologue is noted on the x-axis. Bar heights and error bars represent the averages and standard errors of five independent replicates, respectively.
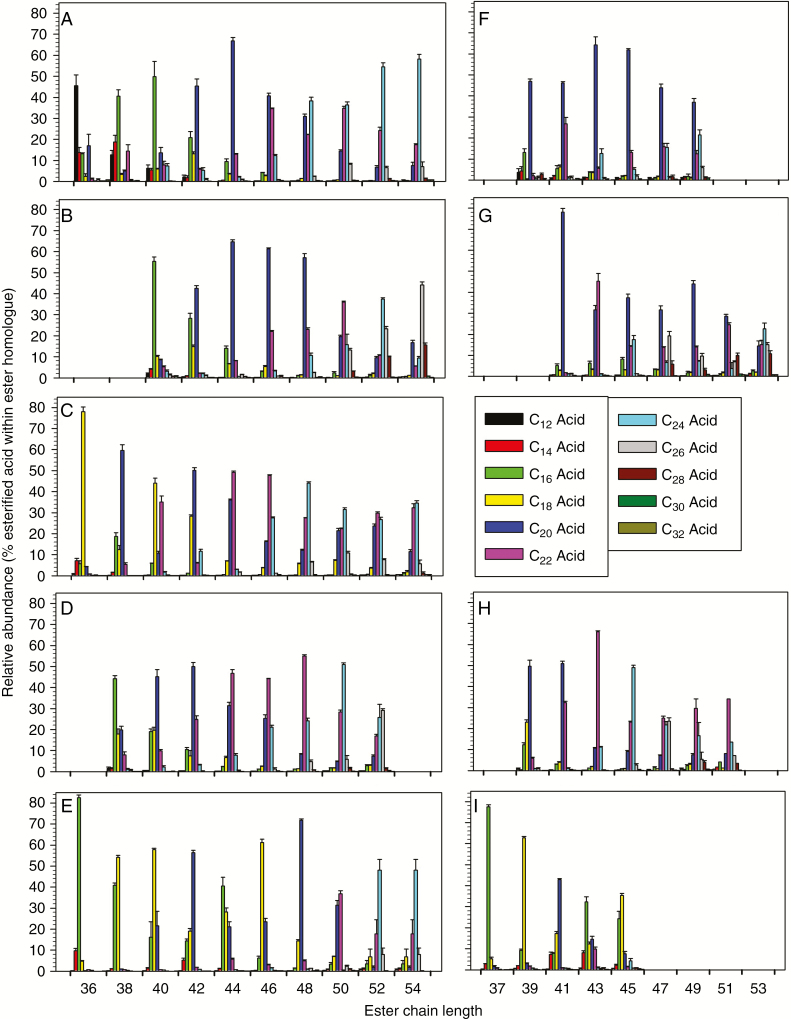
Each alkyl ester homologue may be composed of several metamers, a special type of isomer resulting from different combinations of alcohol and acid homologues with the same overall ester chain length. Accordingly, we next aimed to identify and quantify the acid and alcohol moieties within each ester homologue. Alkyl esters exhibit prominent MS fragments diagnostic of the fatty acid moieties within the ester, and thus respective acylium ions can be used to identify and quantify the metamers within each ester homologue. In all five fern species, even-numbered acid moieties dominated both the even-numbered and the odd-numbered ester homologues (Fig. 6). The even-numbered ester homologues were thus mainly due to combinations of two even-numbered moieties, and the odd-numbered ester homologues were due to combinations of odd-numbered alcohols with even-numbered esters (but not vice versa).
Fig. 6.
Distribution of even-numbered fatty acids within alkyl ester homologues on the fronds of five fern species. Each cluster of bars shows the percentage of esterified fatty acids within a particular ester homologue. Panels on the left show fatty acid distributions within esters with even total carbon numbers for: (A) Pteridium aquilinum; (B) Cryptogramma crispa; (C) Polypodium glycyrrhiza; (D) Polystichum munitum; and (E) Gymnocarpium dryopteris; panels on the right show fatty acid distributions within ester homologues with odd total carbon numbers for: (F) Pteridium aquilinum; (G) Cryptogramma crispa; (H) Polystichum munitum; and (I) Gymnocarpium dryopteris. Ester chain lengths are denoted on the x-axis. Bar heights and error bars represent the averages and standard errors of five independent replicates, respectively. No esters with odd total carbon numbers were detected on Polypodium glycyrrhiza.
On fronds of P. aquilinum, the ester homologue C36 was dominated by C12 acid, homologues C38 and C40 by C16 acid, homologues C42–C46 by C20 acid and homologues C48 and higher by C24 acid (Fig. 6A). The alkyl esters of C. crispa had fairly similar metamer distributions, also dictated by acids differing by four carbons, except that homologues C50, C52 and C54 were dominated by C22, C24 and C26 acids, respectively (Fig. 6B). In contrast, P. glycyrrhiza had C18 acid mainly incorporated into the C36 and C40/42 esters, and, accordingly, C20 acid into the C38 and C42/44 esters, C22 acid into the C40 and C44/46 esters and C24 acid into the C42 and C46–50 esters (Fig. 6C). Similarly, G. dryopteris esters also had bimodal acid distributions (C16 acid dominating in C36 and C44 esters, C18 acid in C38/40 and C46 esters, and C20 acid in C42 and C48 esters), along with C22 acid in C50 ester and C24 acid in higher homologues (Fig. 6E). Finally, P. munitum had various ester homologues dominated by incrementally increasing acid chain lengths (C38 ester by C16 acid, C40/42 esters by C18 acid, C44–48 esters by C20 acid and C50/52 esters by C24 acid; Fig. 6D). The odd-numbered ester homologues had acid moiety distributions similar to those of the even-numbered esters (Fig. 6F–I; Supplementary Data Fig. S3).
Based on the differences between ester TCNs and esterified acid TCNs, the relative abundances of alcohol homologues in each ester were calculated. Accordingly, ester homologues with even TCNs contained primarily even-numbered alcohols (Supplementary Data Fig. S4), and esters with odd TCNs had odd-numbered alcohols (Supplementary Data Fig. S5).
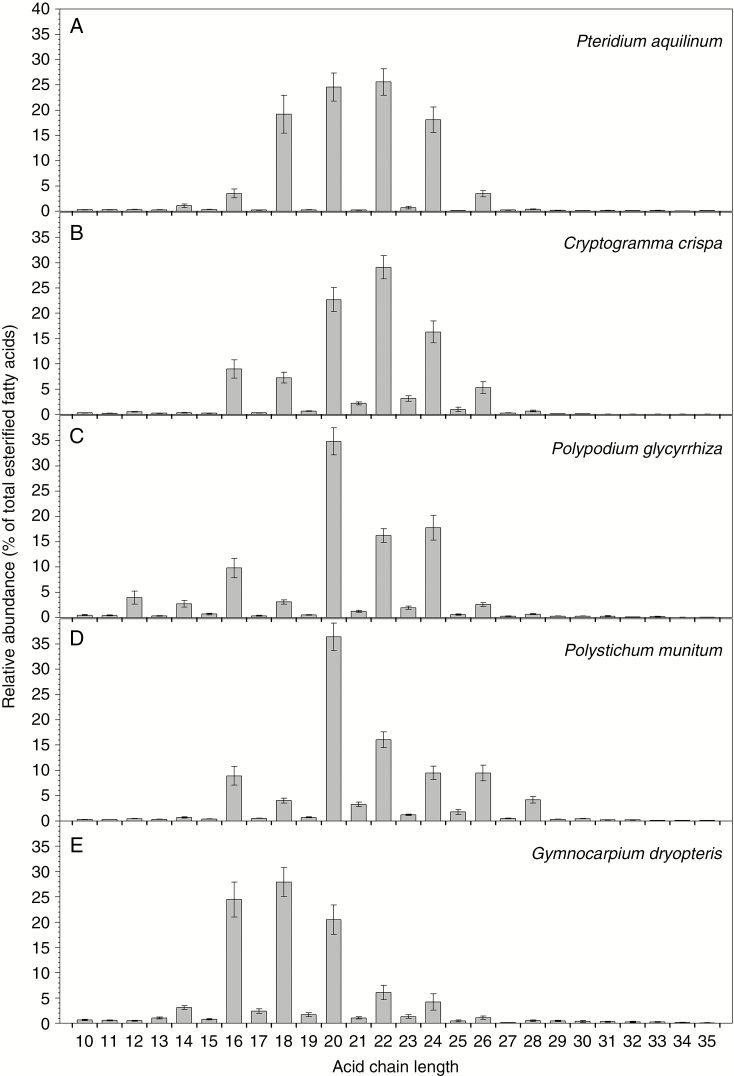
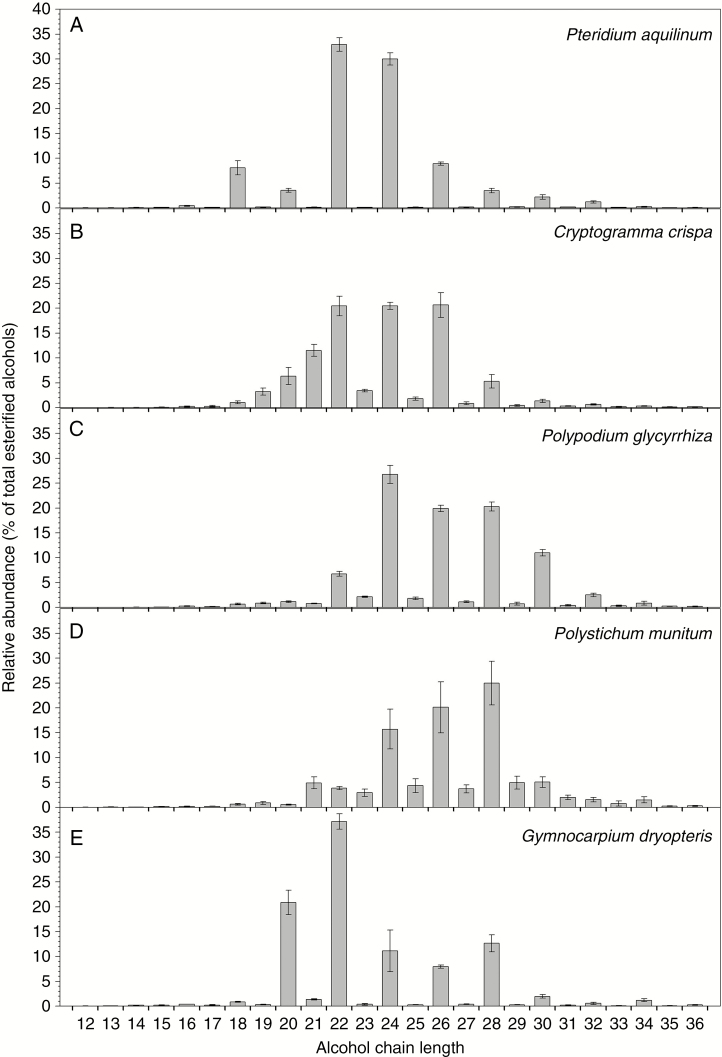
Finally, the relative abundances of fatty acid homologues across all ester chain lengths were summed to determine the overall homologue distribution of esterified acids. Esters from all five fern species comprised mainly acid moieties with chain lengths ranging from C14 to C28 (Fig. 7). Pteridium aquilinum and C. crispa had relatively broad, roughly bell-shaped distributions of esterified acids peaking around C20 and C22, while P. glycyrrhiza and P. munitum esters had bimodal distributions centred at C16 and C20 acids, and G. dryopteris had a broad array of esterified acids around C18 acid (Fig. 7). Conversely, all five species had distinct chain length patterns of esterified alcohols, centred around C22 in P. aquilinum and G. dryopteris, C22 and C24 in P. glycyrrhiza, C22, C24 and C26 in Polystichum munitum and C28 in C. crispa (Fig. 8).
Fig. 7.
Chain length distributions of all fatty acids in the alkyl esters from fronds of five fern species. Relative abundances of esterified fatty acids expressed as percentage of all acid chain lengths for: (A) Pteridium aquilinum; (B) Cryptogramma crispa; (C) Polypodium glycyrrhiza; (D) Polystichum munitum; and (E) Gymnocarpium dryopteris. Bar heights and error bars represent the averages and standard errors of five independent replicates, respectively.
Fig. 8.
Overall chain length distributions of alcohols in the alkyl ester fractions of the cuticular wax mixtures on the fronds of five fern species. Relative abundances of esterified alcohols expressed as a percentage of all alcohol chain lengths for: (A) Pteridium aquilinum; (B) Cryptogramma crispa; (C) Polypodium glycyrrhiza; (D) Polystichum munitum; and (E) Gymnocarpium dryopteris. Bar heights and error bars represent the averages and standard errors of five independent replicates, respectively.
DISCUSSION
This study details wax coverages and compositions on fronds of five diverse fern species. All the investigated fern wax mixtures were characterized by high concentrations of alkyl esters, and most of them contained compound classes derived from the alcohol- and alkane-forming wax biosynthesis pathways. The five fern species were distinguished from one another by qualitative wax traits, with distinct combinations of wax compound classes present on each. For example, two of the investigated fern species accumulated fernene in their frond waxes, while this triterpene could not be detected in the other three species. The five fern species were further differentiated by quantitative traits, including their total wax coverages and the chain length distributions within wax compound classes. These findings can now be compared with wax compositions of non-vascular and seed plants in the context of the biosynthesis pathways effecting the elongation of acyl chains, and the formation of alcohols and alkyl esters, alkanes and secondary alcohols and ketones.
Elongation profiles
All five fern species bore some compounds derived from acyl precursors with ≥30 carbons. This indicates that ferns must have molecular machinery capable of elongating acyl-CoA precursors to chain lengths similar to those in seed plants, implying that elongation beyond C26 in the ferns probably involves FAE complexes containing KCS enzymes, and probably an AtKCS6 orthologue (Joubès et al., 2008). It will be interesting to study whether such elongation also requires CER2-LIKE enzymes analogous to those recently characterized for flowering plants such as Arabidopsis thaliana and Oryza sativa (Haslam et al., 2015; Wang et al., 2017).
The predominant chain lengths within each compound class differed among fern species. For example, the alcohol fractions were dominated by the C22 homologue on P. aquilinum and C. crispa, and by C28 on the other three species, while the alkane profiles peaked at C27 on P. aquilinum and G. dryopteris, and at C33 on the other three species. This degree of variation between fern species of different families is considerable, comparable with the chain length diversities typically observed between angiosperm families (when comparing waxes on the same organs). For example, the wax alcohol fractions of monocot or dicot families tend to be dominated by homologues varying from C26 to C32, and corresponding alkane mixtures by homologues in the range C29–C33 (Jetter et al., 2006). There is substantial evidence that the chain length variation among seed plants is dictated by substrate and product specificities of the KCS(s) (Millar et al. 1999) and/or CER2-LIKE gene(s) in each species (Haslam et al., 2015), or else these enzymes may be differentially expressed, as has been recorded for arabidopsis organs (Joubès et al., 2008). It therefore seems plausible that the characteristic homologue profiles of the different fern species may also be determined by KCSs and possible orthologues of the AtCER2-LIKEs. Overall, the drastic differences between chain length profiles in the waxes of the ferns investigated here make them good targets for studies testing the biochemical characteristics of these enzymes.
Acyl reduction pathway
Alkyl esters were the most abundant compound class in the wax mixtures from all fern species investigated here. Other species from diverse lineages have been reported to have abundant alkyl esters as well, including the moss Funaria hygrometrica (Busta et al., 2016), the fronds of the tree fern Cyathea dealbata (Franich et al., 1985a) and two subspecies of P. aquilinum (Baker and Gaskin, 1987), the internodes and leaf sheaths of the horsetail Equisetum telmateia (Brune and Haas, 2011), leaves of the oak tree Quercus ilex (Martins et al., 1999), leaves of the oil seed crop Camelina sativa (Razeq et al., 2014) and leaves of the palm Copernicia cerifera (Lawrence et al., 1982). However, some ferns and fern allies have relatively low amounts of wax esters, including the fronds of Osmunda regalis (Jetter and Riederer, 2000) and several organs of various Equisetum species (Brune and Haas, 2011). Overall, these observations show that alkyl ester amounts vary in particularly wide ranges within and between plant taxa, from the species to the division level, and the fern wax esters are, therefore, of prime interest for future studies of biosynthesis and biological function.
In species with characterized wax biosynthesis pathways, alkyl esters are the final products of the two-step acyl reduction pathway, which first generates primary alcohols and then esterifies them with fatty acid moieties to produce alkyl esters. In this context, it is interesting to note that the fern species analysed here had high amounts of esters accompanied by relatively low amounts of primary alcohols, the acyl reduction pathway intermediates. This is in contrast to many other species, where high concentrations of primary alcohols are found together with relatively low amounts of alkyl esters, including, for example, Poa alpina and P. trivialis (Pilon et al., 1999), Medicago sativa (Zhang et al., 2005) and Solanum tuberosum (Szafranek and Synak, 2006). We conclude that the majority of the VLC alcohols formed by the acyl reduction pathways active in the ferns analysed here are utilized for ester synthesis, and that, therefore, the ester-forming enzymes in these species are expressed at high levels and/or are highly active. This finding has biotechnological relevance, as the fine-tuning of wax ester synthases is one important aspect of biodiesel production (Jetter and Kunst, 2008).
Our detailed analysis of the homologue and isomer compositions revealed that the wax ester mixture of each fern species could be described as sets of preferred combinations of fatty acids and alcohols. While some of the predominant ester isomers showed common alcohol chain lengths, others shared the same predominant fatty acid homologues instead, pointing to preferences of the ester-forming enzymes for certain alcohol or acid substrate chain lengths, respectively. Based on these findings, we hypothesize that each fern species has several ester synthase enzymes with different substrate specificities. Furthermore, the ester metamer profiles also differed between the ferns studied here, suggesting further differences in substrate specificities of the ester-forming enzymes between species. However, based on the chemical profiles alone, it cannot be ruled out that the species-specific metamer compositions are, at least in part, due to differential expression within sets of similar enzymes in the different fern species. Interestingly, the detailed metamer analyses of wax esters from the moss F. hygrometrica (Busta et al., 2016), the gymnosperm Pinus radiata (Franich et al., 1985b), the dicot Solanum tuberosum (Guo and Jetter, 2017) and the monocot Phyllostachys aurea (Racovita et al., 2016) revealed similar patterns that also suggested the presence of multiple wax ester synthase genes in that species. If true, this might imply that the fine-tuning of wax ester composition via control of wax ester synthase isoforms is a strategy employed by diverse moss, fern and gymnosperm and angiosperm species.
Esters with odd TCNs were found in minor quantities in most of the fern species. These odd-numbered esters contained mainly alcohol moieties with odd TCNs, as opposed to acids with odd TCNs. A similar bias was also observed in ester profiles of Picea abies (Sümmchen et al., 1995), and in Pinus radiata (Franich et al., 1985b). Our GC-MS analyses did detect trace amounts of odd alcohols, however, in concentrations too low for accurate quantification. In contrast, we could not detect free fatty acids with odd TCNs, suggesting that the observed predominance of esterified odd alcohols over esterified odd acids might merely reflect substrate pools.
Alkane pathway
In the waxes of all the ferns investigated here, alkanes were present at least in trace amounts, suggesting that each species possesses alkane pathway genes. Thus, these ferns all contained the full complement of chain length elongation and head group modification enzymes previously characterized in seed plants. However, the flux of wax precursors into the alcohol- and alkane-forming pathways is tightly controlled in all five fern species, probably through differential gene expression but possibly also through differences in enzyme activities.
Interestingly, the alkane chain length distributions varied greatly among fern species, suggesting either that fern CER1/CER3 orthologues may vary in their substrate chain length preferences, or that they have access to substrate pools with different chain length compositions. It should be noted that the predominant alkane chain lengths in most of the fern species were longer than in co-occurring fatty acids, primary alcohols and aldehydes. Thus, it seems probable that these alkanes are formed by enzymes with chain length specificities differing from those of the respective alcohol-forming pathway machinery, and/or that the alkane-forming enzymes are associated with FAE(s) different from the acyl reduction pathway.
Secondary alcohol and ketone pathway
In the wax mixture from G. dryopteris, several secondary alcohols and ketones were detected, with the respective C29 homologues predominating and functional groups exclusively on even-numbered (and thus alternating) carbons: C-10, C-12, C-14 and C-16. The chain length distributions of these compounds differed markedly from those of the accompanying primary alcohols, aldehydes and alkanes, which all had chain length profiles similar to each other. This suggests that the biosynthesis of G. dryopteris secondary alcohols and ketones diverges in a fairly early step from the pathways leading to the other wax compounds.
The biosynthesis of secondary alcohols and ketones on arabidopsis stems is well characterized; however, the structures of the G. dryopteris secondary alcohols and ketones also differ drastically from these. Arabidopsis secondary alcohols and ketones have functional groups located on even- and odd-numbered carbons, C-13, C-14 and C-15 (Wen and Jetter, 2009). In arabidopsis, this isomer distribution results from hydroxylation of the corresponding C29 alkane by the P450-dependent hydroxylase enzyme MAH1 (Greer et al., 2007), and secondary alcohol or ketone isomer mixtures with functional groups on adjacent carbons of the hydrocarbon backbone are thus a hallmark of the limited product specificity of the arabidopsis hydroxylase. Conversely, the very different isomer distribution of the fern secondary alcohols and ketones therefore makes it very unlikely that they are formed by hydroxylation of alkanes catalysed by a MAH1-like enzyme.
Other wax compounds with secondary functional groups also have the characteristic pattern exhibited by the G. dryopteris secondary alcohols and ketones (i.e. functional groups on alternating carbons). Chief among these are the β-diketones found widely in the Poaceae, and 10-nonacosanol occurring on the royal fern, Osmunda regalis (Jetter and Riederer, 2000), mosses (Neinhuis and Jetter, 1995), gymnosperms (Franich et al., 1978; Günthardt-Goerg, 1986) and some early-diverging angiosperms (Holloway et al., 1976; Jetter and Riederer, 1996). These compounds are thought to be generated using elongation intermediates as substrates, followed by elongation and head group modification along the alkane-forming pathway (von Wettstein-Knowles, 1993; Hen-Avivi et al., 2016). Based on the shared similar isomer patterns between these compounds and the G. dryopteris secondary alcohols and ketones described here, it seems plausible that the latter fern compounds are biosynthesized by a mechanism similar to β-diketones and/or 10-nonacosanol. Our current findings thus imply that pathways leading from elongation intermediates to secondary alcohols and ketones are likely to be employed by species from fairly diverse moss, fern, gymnosperm and angiosperm taxa.
Conclusions
In summary, the wax mixtures covering the five fern species investigated here had compositions similar to those of seed plants. The fern wax coverages ranged from 4 to 17 μg cm–2 and thus span a range similar to those of gymnosperm and angiosperm species. All the fern waxes were dominated by alkyl esters, accompanied by varying amounts of fatty acids, aldehydes, primary alcohols and alkanes, suggesting that they all had the full set of wax biosynthesis enzymes previously characterized in arabidopsis. The presence of several wax ester synthase enzymes in each of the fern species can be inferred from the ester metamer profiles, with significantly different substrate specificities between species. Most interestingly, secondary alcohol and ketones were found in the wax from G. dryopteris, with isomer distributions suggesting that the in-chain functionalities are introduced as a by-product of elongation. This characteristic feature thus distinguishes the fern wax biosynthesis machinery from that in A. thaliana (with its P450 enzyme MAH1). Most interestingly, both the absolute amounts and the relative compositions of the wax mixtures in all the fern species investigated here resembled those of vascular plants.
Our survey of diverse fern species belonging to major extant families showed that all their cuticular wax mixtures comprised FAE elongation products with head groups modified by both the alkane- and alcohol-forming pathways. We conclude that all these ferns probably harbour the full complement of wax biosynthesis enzymes and genes known from higher plants, which is thus probably a basal trait shared by all ferns and seed plants. This suggests that the cuticle of ancestral vascular plants already had the potential to contain all the wax compound classes and chain lengths found in extant lineages, with further evolutionary adaptations restricted to specific compound class and chain length profiles.
It is interesting to note that, despite their very different life history strategies, all the fern species investigated here shared certain wax chemical characteristics, including the overall amounts of waxes and the high concentration of alkyl esters. For example, it had been reported that one of the ferns investigated here, Polystichum munitum, may use foliar water uptake to relieve local water stress, and it had been speculated that water may be transported across the leaf cuticle in the process (Limm et al., 2009). Our chemical analyses indicate that the cuticular wax of P. munitum is very similar to those of other ferns and seed plants, suggesting that such foliar water uptake may not be enabled by special chemical characteristics of the cuticle, but may instead be facilitated by some other aspect of the cuticle, such as a unique arrangement of wax chemicals that create special physical structures on the surface.
All together, the wax compositions of the five investigated fern species reflect a common set of underlying biosynthetic machinery and regulation rather than fine-tuned adaptation to certain growth conditions, including abiotic parameters and biotic stress caused by pathogens or insects. Based on the large diversity present among extant ferns, however, further wax analyses of more species are needed to corroborate these conclusions further.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: identification of secondary alcohols in G. dryopteris wax. Figure S2: identification of ketones in G. dryopteris wax. Figure S3: distribution of odd-numbered fatty acids within the alkyl ester homologues in the cuticular wax mixtures on the fronds of five fern species. Figure S4: distribution of even-numbered alcohols within the alkyl ester homologues in the cuticular wax mixtures on the fronds of five fern species. Figure S5: distribution of odd-numbered alcohols within the alkyl ester homologues in the cuticular wax mixtures on the fronds of five fern species.
ACKNOWLEDGEMENTS
The authors are very grateful to Dr Jessica Budke, University of Tennessee, for thoughtful comments on an early version of this manuscript. This work was supported by the Natural Science and Engineering Research Council of Canada [Discovery grant #262461] and the National Natural Science Foundation of China [grant number 31670407, 2016]. Y.G. conducted this study as a visiting scholar with financial support from Chongqing Municipal Education Commission Fund.
LITERATURE CITED
- Baker EA, Gaskin RE. 1987. Composition of leaf epicuticular waxes of Pteridium subspecies. Phytochemistry 26: 2847–2848. [Google Scholar]
- Bi H, Kovalchuk N, Langridge P, Tricker PJ, Lopato S, Borisjuk N. 2017. The impact of drought on wheat leaf cuticle properties. BMC Plant Biology 17: 85. doi: 10.1186/s12870-017-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottari F, Marsili A, Morelli I, Pacchiani M. 1972. Aliphatic and triterpenoid hydrocarbons from ferns. Phytochemistry 11: 2519–2523. [Google Scholar]
- Brune T, Haas K. 2011. Equisetum species show uniform epicuticular wax structures but diverse composition patterns. AoB Plants 2011: plr009. doi: 10.1093/aobpla/plr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busta L, Budke JM, Jetter R. 2016. The moss Funaria hygrometrica has cuticular wax similar to vascular plants, with distinct composition on leafy gametophyte, calyptra and sporophyte capsule surfaces. Annals of Botany 118: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busta L, Hegebarth D, Kroc E, Jetter R. 2017. Changes in cuticular wax coverage and composition on developing Arabidopsis leaves are influenced by wax biosynthesis gene expression levels and trichome density. Planta 245: 297–311. [DOI] [PubMed] [Google Scholar]
- Franich RA, Wells LG, Holland PT. 1978. Epicuticular wax of Pinus radiata needles. Phytochemistry 17: 1617–1623. [Google Scholar]
- Franich RA, Goodin SJ, Hansen E. 1985a Wax esters of the new zealand silver fern, Cyathea dealbata. Phytochemistry 24: 1093–1095. [Google Scholar]
- Franich RA, Goodin SJ, Volkman JK. 1985b Alkyl esters from Pinus radiata foliage epicuticular wax. Phytochemistry 24: 2949–2952. [Google Scholar]
- Gordon DC, Percy KE, Riding RT. 1998. Effects of UV-B radiation on epicuticular wax production and chemical composition of four Picea species. New Phytologist 138: 441–449. [Google Scholar]
- Greer S, Wen M, Bird D, et al. 2007. The cytochrome P450 enzyme CYP96A15 is the midchain alkane hydroxylase responsible for formation of secondary alcohols and ketones in stem cuticular wax of Arabidopsis. Plant Physiology 145: 653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülz P-G, Markstädter C, Riederer M. 1993. Isomeric alkyl esters in Quercus robur leaf cuticular wax. Phytochemistry 35: 79–81. [Google Scholar]
- Günthardt-Goerg MS. 1986. Epicuticular wax of needles of Pinus cembra, Pinus sylvestris and Picea abies. European Journal of Forest Pathology 16: 400–408. [Google Scholar]
- Guo YJ, Jetter R. 2017. Comparative analyses of cuticular waxes on various organs of potato (Solanum tuberosum L.). Journal of Agricultural and Food Chemistry 65: 3926–3933. [DOI] [PubMed] [Google Scholar]
- Haslam TM, Mañas-Fernández A, Zhao L, Kunst L. 2012. Arabidopsis ECERIFERUM2 is a component of the fatty acid elongation machinery required for fatty acid extension to exceptional lengths. Plant Physiology 160: 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam TM, Haslam R, Thoraval D, et al. 2015. ECERIFERUM2-LIKE proteins have unique biochemical and physiological functions in very-long-chain fatty acid elongation. Plant Physiology 167: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen-Avivi S, Savin O, Racovita RC, et al. 2016. A metabolic gene cluster in the wheat W1 and the barley cer-cqu loci determines β-diketone biosynthesis and glaucousness. The Plant Cell 28: 1440–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway PJ, Jeffree CE, Baker EA. 1976. Structural determination of secondary alcohols from plant epicuticular waxes. Phytochemistry 15: 1768–1770. [Google Scholar]
- Jeffree C. 2006. The fine structure of the plant cuticle. In: Riederer M, Müller C, eds. Biology of the plant cuticle. Oxford: Blackwell Publishing Ltd, 11–144. [Google Scholar]
- Jetter R, Kunst L. 2008. Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. The Plant Journal 54: 670–683. [DOI] [PubMed] [Google Scholar]
- Jetter R, Riederer M. 1996. Cuticular waxes from the leaves and fruit capsules of eight Papaveraceae species. Canadian Journal of Botany 74: 419–430. [Google Scholar]
- Jetter R, Riederer M. 1999. Long-chain alkanediols, ketoaldehydes, ketoalcohols and ketoalkyl esters in the cuticular waxes of Osmunda regalis fronds. Phytochemistry 52: 907–915. [Google Scholar]
- Jetter R, Riederer M. 2000. Composition of cuticular waxes on Osmunda regalis fronds. Journal of Chemical Ecology, 26: 399–412. [Google Scholar]
- Jetter R, Schäffer S. 2001. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiology 126: 1725–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter R, Kunst L, Samuels AL. 2006. Composition of plant cuticular waxes. In: Riederer M, Müller C, eds. Biology of the plant cuticle. Oxford: Blackwell Publishing Ltd, 145–181. [Google Scholar]
- Joubès J, Raffaele S, Bourdenx B, et al. 2008. The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression profiling. Plant Molecular Biology 67: 547–566. [DOI] [PubMed] [Google Scholar]
- Kim H, Choi D, Suh MC. 2017. Cuticle ultrastructure, cuticular lipid composition, and gene expression in hypoxia-stressed Arabidopsis stems and leaves. Plant Cell Reports 36: 815–827. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Iyengar J, Page B, Conacher H. 1982. Characterization of commercial waxes by high-temperature gas chromatography. Journal of Chromatography 236: 403–419. [Google Scholar]
- Limm EB, Simonin KA, Bothman AG, Dawson TE. 2009. Foliar water uptake: a common water acquisition strategy for plants of the redwood forest. Oecologia 161: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CGA, Post-Beittenmiller D. 1998. Epicuticular wax on leek in vitro developmental stages and seedlings under varied growth conditions. Plant Science 134: 53–67. [Google Scholar]
- Martins CMC, Mesquita SMM, Vaz WLC. 1999. Cuticular waxes of the holm (Quercus ilex L. subsp. ballota (Desf.) Samp.) and cork (Q. suber L.) oaks. Phytochemical Analysis 10: 1–5. [Google Scholar]
- Meusel I, Markstädter C, Neinhuis C, Barthlott W. 1999. Ultrastructure, chemical composition, and recrystallization of epicuticular waxes: transversely ridged rodlets. Canadian Journal of Botany 77: 706–720. [Google Scholar]
- Millar AA, Kunst L. 1997. Very‐long‐chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. The Plant Journal 12: 121–131. [DOI] [PubMed] [Google Scholar]
- Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC, Kunst L. 1999. CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. The Plant Cell 11: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neinhuis C, Jetter R. 1995. Ultrastructure and chemistry of epicuticular wax crystals in Polytrichales sporophytes. Journal of Bryology 18: 399–406. [Google Scholar]
- Neinhuis C, Koch K, Barthlott W. 2001. Movement and regeneration of epicuticular waxes through plant cuticles. Planta 213: 427–434. [DOI] [PubMed] [Google Scholar]
- Nikolic B, Tesevic V, Bojovic S, Marin PD. 2013. Chemotaxonomic implications of the n-alkane composition and the nonacosan-10-ol content in Picea omorika, Pinus heldreichii, and Pinus peuce. Chemistry & Biodiversity 10: 677–686. [DOI] [PubMed] [Google Scholar]
- Nishida K, Hanba YT. 2017. Photosynthetic response of four fern species from different habitats to drought stress: relationship between morpho-anatomical and physiological traits. Photosynthetica 55: 689–697. [Google Scholar]
- Pascal S, Bernard A, Sorel M, et al. 2013. The Arabidopsis cer26 mutant, like the cer2 mutant, is specifically affected in the very long chain fatty acid elongation process. The Plant Journal 73: 733–746. [DOI] [PubMed] [Google Scholar]
- Pilon JJ, Lambers H, Baas W, Tosserams M, Rozema J, Atkin OK. 1999. Leaf waxes of slow-growing alpine and fast-growing lowland Poa species: inherent differences and responses to UV-B radiation. Phytochemistry 50: 571–580. [Google Scholar]
- Racovita RC, Hen-Avivi S, Fernandez-Moreno J-P, Granell A, Aharoni A, Jetter R. 2016. Composition of cuticular waxes coating flag leaf blades and peduncles of Triticum aestivum cv. Bethlehem. Phytochemistry 130: 182–192. [DOI] [PubMed] [Google Scholar]
- Razeq FM, Kosma DK, Rowland O, Molina I. 2014. Extracellular lipids of Camelina sativa: Characterization of chloroform-extractable waxes from aerial and subterranean surfaces. Phytochemistry 106: 188–196. [DOI] [PubMed] [Google Scholar]
- Samuels L, Kunst L, Jetter R. 2008. Sealing plant surfaces: cuticular wax formation by epidermal cells. Annual Review of Plant Biology 59: 683–707. [DOI] [PubMed] [Google Scholar]
- Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallon S, Lupia R. 2004. Ferns diversified in the shadow of angiosperms. Nature 428: 553–557. [DOI] [PubMed] [Google Scholar]
- Schneider L, Adamski NM, Christensen CE, et al. 2016. The Cer-cqu gene cluster determines three key players in a β-diketone synthase polyketide pathway synthesizing aliphatics in epicuticular waxes. Journal of Experimental Botany 67: 2715–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr J. 1976. Water permeability of isolated cuticular membranes – effect of cuticular waxes on diffusion of water. Planta 131: 159–164. [DOI] [PubMed] [Google Scholar]
- Schreiber L, Riederer M. 1996. Ecophysiology of cuticular transpiration: comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 107: 426–432. [DOI] [PubMed] [Google Scholar]
- Shanker KS, Kanjilal S, Rao BVSK, Kishore KH, Misra S, Prasad RBN. 2007. Isolation and antimicrobial evaluation of isomeric hydroxy ketones in leaf cuticular waxes of Annona squamosa.Phytochemical Analysis 18: 7–12. [DOI] [PubMed] [Google Scholar]
- Sümmchen P, Markstädter C, Wienhaus O. 1995. Composition of the epicuticular wax esters of Picea abies (L.) Karst. Zeitschrift für Naturforschung C 50: 11–14. [Google Scholar]
- Szafranek BM, Synak EE. 2006. Cuticular waxes from potato (Solanum tuberosum) leaves. Phytochemistry 67: 80–90. [DOI] [PubMed] [Google Scholar]
- Tulloch AP, Baum BR, Hoffman LL. 1980. A survey of epicuticular waxes among genera of Triticeae. 2. Chemistry. Canadian Journal of Botany 58: 2602–2615. [Google Scholar]
- Uppalapati SR, Ishiga Y, Doraiswamy V, et al. 2012. Loss of abaxial leaf epicuticular wax in Medicago truncatula irg1/palm1 mutants results in reduced spore differentiation of anthracnose and nonhost rust pathogens. The Plant Cell 24: 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guan Y, Zhang D, Dong X, Tian L, Qu LQ. 2017. A beta-ketoacyl-CoA synthase is involved in rice leaf cuticular wax synthesis and requires a CER2-LIKE protein as a cofactor. Plant Physiology 173: 944–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Jetter R. 2009. Composition of secondary alcohols, ketones, alkanediols, and ketols in Arabidopsis thaliana cuticular waxes. Journal of Experimental Botany 60: 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein-Knowles P. 1993. Waxes, cutin, and suberin. In: Moore T, ed. Lipid metabolism in plants. Boca Raton, FL: CRC Press, 128–166. [Google Scholar]
- Wollenweber E, Malterud KE, Gomez LD. 1981. 9(11)-Fernene and its 21-epimer as an epicuticular layer on ferns. Zeitschrift für Naturforschung 36c: 896–899. [Google Scholar]
- Yeats TH, Rose JKC. 2013. The formation and function of plant cuticles. Plant Physiology 163: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Broeckling CD, Blancaflor EB, Sledge MK, Sumner LW, Wang ZY. 2005. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). The Plant Journal 42: 689–707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.