Abstract
Background and Aims
Since pollen flow or seed dispersal can contribute to transgene persistence in the environment, the sympatric presence of transgenic crops with their wild relatives is an ecological concern. In this study, we tested the hypothesis that proximate growth of a herbivore-resistant Bt crop and wild relatives coupled with the presence of herbivores can increase relative frequency of crop-to-wild transgene flow persistence outside of cultivation.
Methods
We conducted a field experiment using insect enclosures with and without herbivores with cultivated Bt-transgenic Brassica napus (Bt OSR) and wild brown mustard (Brassica juncea) in pure and mixed stands. Low-density diamondback moth (Plutella xylostella) caterpillar infestation treatments were applied and transgene flow and reproductive organs were measured.
Key Results
Bt-transgenic B. napus produced more ovules and pollen than wild mustard, but the pollen to ovule (P/O) ratio in the two species was not significantly different. Low-level herbivory had no effects on fitness parameters of Bt OSR or wild brown mustard or on the transgene flow frequency. All progeny from wild brown mustard containing the Bt transgene came from mixed stands, with a gene flow frequency of 0.66 %. In mixed stands, wild brown mustard produced less pollen and more ovules than in pure stands of brown mustard. This indicates a decreased P/O ratio in a mixed population scenario.
Conclusions
Since a lower P/O ratio indicates a shift in sex allocation towards relatively greater female investment and a higher pollen transfer efficiency, the presence of transgenic plants in wild populations may further increase the potential transgene flow by altering reproductive allocation of wild species.
Keywords: Fitness parameters, P/O ratio, Plutella xylostella, sex allocation, sympatric presence, transgene flow, wild relatives
INTRODUCTION
One of the ecological concerns about genetically modified (GM) plants is the adventitious presence of transgenic crops and transgenes outside of cultivation and that outside their intended hosts transgenes may persist in the environment in unexpected ways (Darmency, 1994; Schafer et al., 2011; Marquardt et al., 2012; Ellstrand et al., 2013; Liu et al., 2013b; Santa-Maria et al., 2014). Some crop–transgene combinations could persist inside or outside of agronomic environments as volunteers through the movement of seeds during the harvest, transport and processing of transgenic crops (D’Hertefeldt et al., 2008; Reuter et al., 2008; Schafer et al., 2011; van Heerwaarden et al., 2012; Liu et al., 2013b). Moreover, transgene flow from some GM plants, for example Brassica napus (oilseed rape; OSR), to wild Brassica relatives via pollen dispersal and hybridization is often observed (Stewart et al., 2003; Warwick et al., 2008; Ellstrand et al., 2013; Liu et al., 2013b). Introgression involves several hybrid and backcross generations, which could occur in certain crop–wild relative cohorts existing simultaneously in unmanaged agroecosystems and field edges (Stewart et al., 2003).
Wild brown mustard (Brassica juncea), a widespread weedy plant in Chinese agricultural systems, can easily hybridize with OSR and produces viable progeny (Jørgensen et al., 1998; Song et al., 2010). Brassica juncea is the second-most compatible hybridization partner with OSR (after Brassica rapa) out of the 17 cross-compatible relatives that can form hybrid progeny (Scheffler and Dale, 1994; Ford et al., 2006). This relatively high number of compatible wild relatives might facilitate the introgression of transgenes in their progenies, and indeed there is evidence of this occurring. For example, the Bt Cry1Ac gene has been shown to be introgressed into wild brown mustard from Bt OSR with the expected Mendelian ratio (Cao et al., 2014). In addition, the Bt toxin in backcrossed generations was sufficient to kill a Bt-susceptible insect species, Plutella xylostella (diamondback moth) in a greenhouse assay (Cao et al., 2014). In another example, Warwick et al. (2008) found stable introgression of a glyphosate-resistant transgene into a B. rapa-like plant from 2001 to 2005 at two sites in Quebec, Canada.
When Bt-transgenic insect-resistant plants are sympatric with non-transgenic plants, Bt plants might outcompete native plants and interbreed with sexually compatible non-transgenic plants, including wild relatives of the Bt crop (Ellstrand et al., 2013). In general, Bt-transgenic plants have a competitive advantage, and the resistant Bt plants could suppress the growth of susceptible conspecifics or congeners when they coexist in the same region, particularly under high target-herbivore pressures (Ramachandran et al., 2000; Vacher et al., 2004; Liu et al., 2013a).
Together, the combination of Bt crops, wild relatives and target herbivores could influence the fate of transgenes in natural ecosystems. At the primary stage of transgenic plants entering a wild population, the presence of Bt-transgenic OSR had few impacts on wild mustard plants in the greenhouse. However, when the two types of plants were grown together in the field, there was decreased growth and reproduction of both Bt OSR and wild brown mustard (Liu et al., 2015b). In an advanced backcross generation (BC2) in the B. juncea background, Bt plants did not have a competitive advantage in mixed stands of insect-resistant and sensitive plants in the absence of insects. However, Bt BC2 plants were more competitive than their non-transgenic counterparts in the presence of herbivorous insects (Liu et al., 2015a). In addition, herbivore infestation delayed the flowering time, and decreased flower and seed production of wild brown mustard in the greenhouse (Liu et al., 2015b).
The aim of the present study was to test whether the sympatric presence of transgenic crop and wild relatives coupled with the presence of herbivores increased transgene flow from the transgenic crop to wild species via pollen flow by altering reproductive traits. A number of studies have focused on the factors that affect transgene flow from crops to their wild relatives (FitzJohn et al., 2007; Warwick et al., 2008; Ellstrand et al., 2013), but few studies have considered the effects of plant reproductive strategy (sex allocation) on gene flow. Such a fundamental factor in plant reproduction could have important ramifications for risk assessment. Furthermore, the dynamic evolution of plant reproduction could conceivably be affected by the ratio of pollen number to ovule number (P/O) per flower. Changes in P/O would be of interest as a metric of the evolution of resource allocation in potential changes of reproductive strategies (Galloni et al., 2007; Burd, 2011). As an evolutionary driver, sympatric pollination efficiency may decrease the allocation of resources to pollen production and thus decrease the P/O ratio; i.e. a higher pollen transfer efficiency can lead to a decreased pollen production requirement (Cruden, 1977; Gong and Huang, 2014). It follows, therefore, that interspecific pollen transfer would be of interest to risk assessors and regulators of plant biotechnology.
MATERIALS AND METHODS
Plants
Seeds of wild brown mustard (Brassica juncea, 2n = 36, AABB) from a local field collection (Nanjing, China) were provided by Professor S. Qiang, Nanjing Agricultural University (NAU). Wild B. juncea is a known major weed in fields, infesting wheat (Triticum aestivum), barley (Hordeum vulgare), oilseed rape (Brassica napus) and some autumn crops, and it is a ruderal weed in empty lots and roadsides in China (Guo et al., 1998; FOC, 2001). Transgenic oilseed rape (OSR, B. napus ‘Westar,’ 2n = 38, AACC) was transformed with the pSAM12 plasmid harbouring genetically linked gfp (encoding a green fluorescent protein) and Bt Cry1Ac cassettes (GT) that are regulated by independent CaMV 35S promoters (Halfhill et al., 2001). The third generation of progeny of a single transgenic event (GT1) contained a single transgene insertion or multiple copies at a single locus (Halfhill et al., 2001). The Cry 1Ac protein of Bt B. napus plants of this generation was measured by ELISA and the protein content was 4.47 µg g−1 fresh weight on average in the flowering period (Liu et al., 2015b). Both brown mustard and OSR self-pollinate, but have mixed mating systems. Between 3 and 30 % of B. juncea seeds are derived from outcrossing in the field (Voskresenskaya and Lygina, 1973; Frello et al., 1995).
Experimental design of the field study
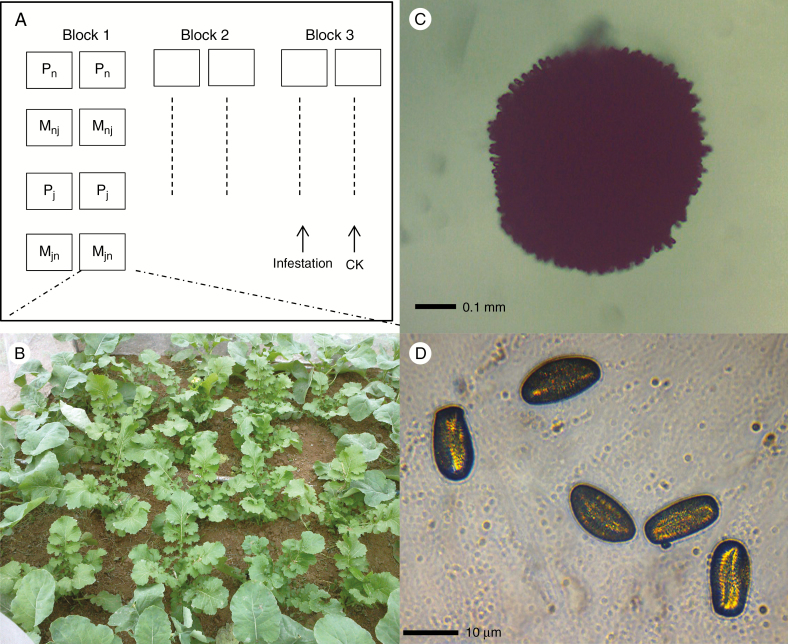
Since the flowering date of wild brown mustard is earlier than that of Bt OSR (Liu et al., 2015b), the seeds of Bt OSR were sown on 16 April 2013 and wild brown mustard seeds were sown on 23 April in Jiffy-7 pots in a greenhouse (22 °C under natural light), which facilitated an increased overlapped flowering time in the field. Seedlings were transplanted in enclosures (cages) 2 m wide × 2 m long × 2 m high and were wrapped in a 0.5-mm mesh nylon net 2 weeks after transplantation. Each cage included 36 plants arranged in six rows and six columns (Fig. 1). To study whether competition from sympatric presence affects the gene flow from Bt-transgenic plants to wild brown mustard, the two types of plants (Bt OSR and wild brown mustard) were cultivated in pure (Pn for Bt OSR and Pj for mustard) or mixed stands (Mnj and Mjn). In mixed stands, Mnj indicated Bt OSR plants in the cage centre (4 × 4 plants) and wild brown mustard (20 plants) in a circle around the Bt OSR, and Mjn indicated mustard in the cage centre and Bt OSR at the edge (Fig. 1). Cages of the four cultivation treatments were placed randomly in a column.
Fig. 1.
Map of field experiment and pollen and stigmas of Brassica napus. (A) Cage configuration. Pn, pure cages of Brassica napus; Pj, pure cages of Brassica juncea; Mnj, mixed cages with B. napus in the centre and B. juncea at the edge; Mjn, mixed cages with B. juncea in the centre and B. napus at the edge; infestation and CK, cages with and without Plutella xylostella. (B) Mjn, cage layout of B. juncea in the centre and B. napus at the edge. (C) One stigma under 4× objective. (D) Pollen under 40× objective. Black lines in (C) and (D) indicate scale length.
Twenty-four cages were deployed in four rows and six columns in the field, with 2 m distance between cages, which provided adequate light for plant growth. The experiment used three blocks, each of which contained two columns of cages. One column of cages in each block was infested with insects, whereas the other was not infested as control. Two second-instar larvae of Plutella xylostella were applied per plant on 30 May in the infestation treatment. No insecticide or fertilizer was applied during the experiment. Plants were watered and hand-weeded as needed. The weather was within normal regional ranges during the experiments. Wind pollination was permitted during the flowering period.
Measurements
We randomly selected three opened flowers per plant each day at 0800 h from 15 June to 12 July. Floral measurements from a total of 945 flowers from 315 randomly selected plants were taken immediately after sampling. The total pollen from each sampled flower was transferred to a 500-μL suspension containing a pollen stain of 0.5 % triphenyl tetrazolium chloride (TTC), and then 20 μL of the suspension was transferred to a counting plate. TTC stains viable pollen red, which was tallied under a microscope (Motic® AE31) using a 10× objective. Pollen size (projected area and perimeter) was measured using the Motic® Images Advanced 3.2 software and the 40× objective. Stigma size (projected area and perimeter) was measured under the 4× objective (Fig. 1). Style length and petal length and width were measured using a vernier calliper. Ovule number was counted by dissecting flowers, and the P/O and viable pollen frequency/total pollen (VPT) ratios were calculated.
Individual plants were harvested by hand upon maturity (i.e. siliques were golden brown in colour), which was defined as the onset of senescence. The above-ground biomass was separated into vegetative and reproductive tissues. The number of branches per plant was counted. Seeds were threshed by hand for each plant after air-drying for around 7 d, and seed mass was recorded. The remaining silique shells, stems and leaves were oven-dried at 80 °C for 48 h and weighed as biomass per plant. Estimated seed number per plant was obtained by dividing seed weight (g) per plant by the average 10 000-seed weight of the type of plant, which is 3.40 and 1.19 g for OSR and mustard, respectively (unpubl. data, Liu Y.). The survival percentage of OSR and mustard was calculated by dividing the number of mature plants by the number of transplanted seedlings in the centre or at the edge of the cage.
Germination experiments
The harvested seeds were sown in Jiffy-7 pots in a greenhouse (22 °C under natural light, starting in December 2013), and the emergence rate was calculated. In some cases few seeds were produced per plant and thus up to 45 seeds per plant were sown. A total of 14 130 seeds were sown from 340 plants, which resulted in 6710 seedlings.
To screen for the presence of transgenes, green fluorescent protein (GFP) fluorescence was measured using a GFP meter (Opti-Sciences), first on the uppermost fully expanded leaf on each plant and then three times at the four- to five-leaf stage, 4 weeks after seed germination, using an established method (Millwood et al., 2003). A total of 1617 Bt OSR and 4913 wild brown mustard seedlings were screened. Leaves from GFP-positive seedlings were subsequently sampled and confirmed by PCR with specific primers for GFP and Bt transgenes (Halfhill et al., 2001) and a Cry1Ab/Ac ELISA (Agidia EnviroLogix, USA) (Liu et al., 2015b) (Supplementary Data Fig. S1). Transgene flow rate was calculated by tallying the number of Bt seedlings versus total seedlings from the sown wild brown mustard seeds.
Statistical analyses
Mean values of variables of plants in the cage centre and at the edge were used for statistical analyses. Data distributions were tested for normality using the Shapiro–Wilk test, and the data were log-transformed to ensure a normal distribution of residuals whenever appropriate. A four-way mixed split-plot ANOVA model [Y ~ I× C × T + E + error (block/insect)] was employed to test the effects of insect infestation (I, infestation versus control), sympatric presence (C, mixed versus pure stands), plant type (T, Bt OSR versus wild brown mustard) and edge (E, cage edge versus centre) on plant fitness parameters and seed emergence rate. Since plant type had a significant effect on variables (see Results section), the data on Bt OSR and wild brown mustard were analysed separately using a three-way mixed split-plot ANOVA model [Y ~ I × C × E + error (block/insect)]. Variables were analysed separately for cage centre and edge when the edge effect was significant. To detect which characteristics are related to seed production, correlation indices were calculated between seed production and other fitness parameters using Kendall’s test. Plant size was tested for whether it had an effect on floral traits (pollen and ovule number, style length, petal width and P/O ratio), fitting data to a linear regression model in terms of floral traits, Y = a + b × X, where X is a floral trait. All statistical analyses were conducted using R software (R Development Core team, 2008).
RESULTS
Plant growth and reproduction in Bt OSR and wild brown mustard
The Bt OSR plants produced more branches and higher above-ground biomass than wild brown mustard (Tables 1 and 2). Bt OSR had greater petal length and width and stigma projected area than wild brown mustard. Each flower of Bt OSR produced more ovules and pollen than wild brown mustard flowers (Table 1). The P/O ratio was not significantly different between Bt OSR and wild brown mustard in this experiment, with an average value of 397.6 for the former and 393.7 for the latter. The style length of Bt OSR flowers was shorter than that of wild brown mustard. Pollen size (projected area) was not significantly different between Bt OSR (395 μm on average) and wild brown mustard (392 μm on average). Wild brown mustard produced 4.5-fold more seeds than Bt OSR: 6.95 × 103 versus 1.25 × 103 seeds per plant on average (Tables 1 and 2). In total, 744 plants survived, of which 351 were Bt OSR (a survival rate of 81 %, 351/432) and 393 were mustard plants (91 %).
Table 1.
Mean (± s.e.) values of fitness parameters of transgenic Brassica napus (n = 351) and wild Brassica juncea (n = 393) at the cage centre under Plutella xylostella infestation with pure and mixed cultivation patterns
| Brassica juncea | Brassica napus | |||||||
|---|---|---|---|---|---|---|---|---|
| Infestation | No | No | Yes | Yes | No | No | Yes | Yes |
| Cultivation | Pure | Mixed | Pure | Mixed | Pure | Mixed | Pure | Mixed |
| Petal length (cm) | 4.51 ± 0.08 | 4.34 ± 0.12 | 4.34 ± 0.05 | 4.94 ± 0.13 | 5.23 ± 0.08 | 5.16 ± 0.06 | 4.95 ± 0.15 | 5.77 ± 0.12 |
| Petal width (cm) | 3.63 ± 0.08 | 3.47 ± 0.05 | 3.45 ± 0.07 | 3.99 ± 0.12 | 4.35 ± 0.08 | 4.29 ± 0.05 | 3.9 ± 0.09 | 4.82 ± 0.12 |
| Style length (cm) | 1.92 ± 0.07 | 1.65 ± 0.10 | 1.98 ± 0.09 | 2.32 ± 0.10 | 0.98 ± 0.07 | 1.17 ± 0.06 | 1.23 ± 0.05 | 1.26 ± 0.05 |
| Stigma area (mm2) | 0.21 ± 0.03 | 0.20 ± 0.08 | 0.27 ± 0.10 | 0.22 ± 0.06 | 0.46 ± 0.11 | 0.48 ± 0.14 | 0.48 ± 0.08 | 0.49 ± 0.10 |
| Pollen area (μm2) | 421.29 ± 5.89 | 381.48 ± 9.66 | 393.95 ± 7.93 | 372.07 ± 8.97 | 367.00 ± 9.30 | 403.77 ± 5.58 | 396.92 ± 4.51 | 407.62 ± 3.38 |
| No. of ovules | 17.98 ± 0.53 | 19.67 ± 0.50 | 19.37 ± 0.47 | 19.35 ± 0.32 | 29.95 ± 0.57 | 26.47 ± 0.80 | 26.28 ± 1.09 | 29.4 ± 0.45 |
| No. of pollen grains (103) | 7.79 ± 0.44 | 6.46 ± 0.47 | 6.89 ± 0.48 | 5.44 ± 0.64 | 12.99 ± 1.18 | 11.12 ± 0.73 | 8.82 ± 0.50 | 10.7 ± 0.73 |
| P/O ratio | 446.61 ± 30.0 | 333.59 ± 26.6 | 358.53 ± 24.1 | 291.07 ± 35.9 | 432.04 ± 34.9 | 422.79 ± 24.4 | 350.45 ± 20.4 | 377.44 ± 29.1 |
| Viable pollen (%) | 52.53 ± 1.87 | 46.21 ± 2.63 | 48.73 ± 2.09 | 50.85 ± 4.41 | 41.43 ± 3.09 | 46.31 ± 1.75 | 41.81 ± 2.77 | 41.11 ± 3.18 |
| No. of branches | 6.7 ± 0.29 | 8.28 ± 0.38 | 7.54 ± 0.35 | 7.11 ± 0.36 | 11.73 ± 0.64 | 13.63 ± 0.32 | 10.58 ± 0.43 | 11.27 ± 0.52 |
| Biomass (g) | 28.01 ± 3.45 | 26.01 ± 2.35 | 29.79 ± 2.26 | 22.22 ± 1.82 | 39.47 ± 5.15 | 45.06 ± 3.02 | 35.42 ± 2.72 | 41.44 ± 4.53 |
| No. of seeds (103) | 7.01 ± 1.02 | 4.61 ± 0.57 | 8.49 ± 0. 76 | 3.96 ± 0.44 | 1.10 ± 0.20 | 1.25 ± 0.11 | 0.64 ± 0.06 | 1.06 ± 0.14 |
| Survival rate (%) | 95.83 ± 2.08 | 91.67 ± 8.33 | 77.08 ± 17.05 | 97.92 ± 2.08 | 68.75 ± 7.22 | 95.83 ± 2.08 | 68.75 ± 15.73 | 79.17 ± 9.08 |
Table 2.
F values of analysis of four-way mixed split-plot ANOVA for fitness parameters of transgenic Brassica napus (n = 351) and wild B. juncea (n = 393) under herbivory and cultivation treatments
| d.f. | Petal length | Petal width | Style length | No. of ovule | No. of pollen | P/O | Viable pollen percentage | No. of branch | Biomass | Seed weight | Seed number | Survival rate | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infestation (I) | 1 | 0.09 | 0.01 | 16.2* | 0.13 | 0.73 | 0.64 | 1.61 | 1.29 | 0.10 | 2.27 | 2.27 | 0.69 |
| Residuals | 2 | ||||||||||||
| Competition (C) | 1 | 0.88 | 2.68 | 0.14 | 0.25 | 3.11 | 4.61* | 0.03 | 3.33 | 0.17 | 1.01 | 1.01 | 12.4** |
| Plant type (P) | 1 | 88.5*** | 124*** | 212*** | 260*** | 38.3*** | 0.04 | 15.8*** | 213*** | 35.6*** | 48.2*** | 294*** | 4.38* |
| Edge (E) | 1 | 2.51 | 4.88* | 0.20 | 3.13 | 1.99 | 0.75 | 8.01** | 0.49 | 26.6*** | 12.6 | 12.6** | 0.26 |
| I × C | 1 | 12.6** | 10.9** | 1.82 | 0.02 | 2.20 | 2.76 | 11.0** | 4.34* | 1.98 | 0.01 | 0.00 | 0.43 |
| I × P | 1 | 0.68 | 0.02 | 0.74 | 2.17 | 0.16 | 0.09 | 0.95 | 0.01 | 0.44 | 0.27 | 0.27 | 0.11 |
| C × P | 1 | 0.02 | 0.04 | 0.34 | 0.10 | 0.58 | 0.06 | 1.84 | 0.14 | 12.1** | 12.3** | 12.3** | 1.80 |
| I × C× P | 1 | 0.00 | 0.02 | 0.03 | 2.16 | 0.42 | 0.03 | 0.44 | 2.74 | 1.00 | 1.99 | 0.17 | 1.18 |
| Residuals | 35 |
*P < 0.05; **P < 0.01; ***P < 0.001.
Seed production of Bt OSR was positively correlated with petal width and above-ground biomass, while seed production of wild brown mustard was positively correlated with style length, branch number and biomass (Supplementary Data Table S1).
Regression analysis showed no significant trends of plant size in terms of any floral traits measured for Bt OSR plants (P > 0.05). Wild mustard showed significant trends in plant size in terms of pollen number (Y = 23.8 + 0.002 × X, R2 = 0.07, P = 0.007), petal width (Y = −14.9 + 16.3 × X, R2 = 0.05, P = 0.04) and P/O ratio (Y = 24.8 + 0.04 × X, R2 = 0.07, P = 0.01), and no significant trends for ovule number (Y = 38.9 + 0.24 × X, R2 = 0.001, P = 0.831) or style length (Y = 37.9 + 2.78 × X, R2 = 0.002, P = 0.63).
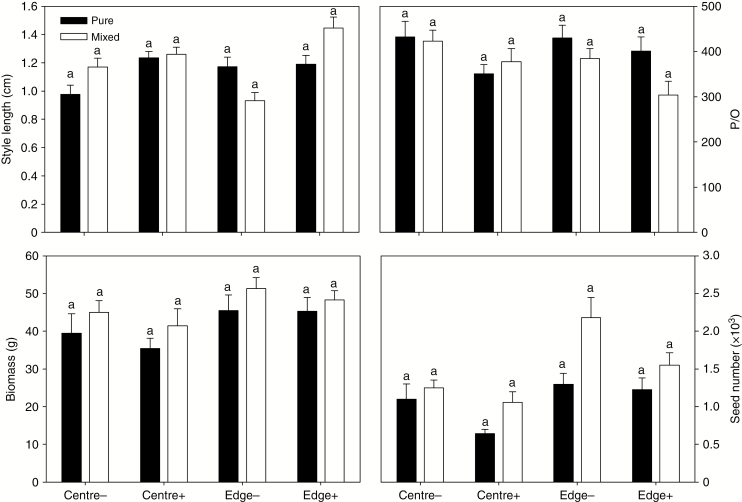
No effect of sympatric presence or low-level herbivore treatment on Bt OSR
There was no significant effect of low-level treatment with herbivores on fitness parameters of Bt OSR, except for increased style length (Table 1, Supplementary Data Table S2, Fig. 2). When fitness parameters were analysed separately for cage centre and edge, no effect of insect infestation was found for either location. Bt OSR had greater petal widths, with more seeds produced at the cage edge than at the cage centre; no edge effects were observed for other traits (Table 1, Supplementary Data Table S2, Fig. 2).
Fig. 2.
Fitness parameters of B. napus under herbivory and cultivation patterns. Cultivation patterns: Pure, all plants were B. napus; Mixed, B. napus mixed with B. juncea in cages. Centre-, wild B. juncea in cage centre under control without P. xylostella; Centre+, wild B. juncea in cage centre under P. xylostella infestation; Edge-, wild B. juncea in cage edge under control without P. xylostella; Edge+, wild B. juncea at cage edge under P. xylostella infestation. n = 351. Different letters above bars indicate significant differences at P < 0.05 for each group.
Sympatric presence had no significant effect on Bt OSR (P > 0.05). The frequency of survival of Bt OSR was significantly higher in the sympatric stands than in pure single-species stands (F1,12 = 7.83, P = 0.02; Supplementary Data Table S2). Only two characteristics, style length and the number of ovules, were affected by the interaction between infestation and sympatric presence and the edge effect (I × C × E).
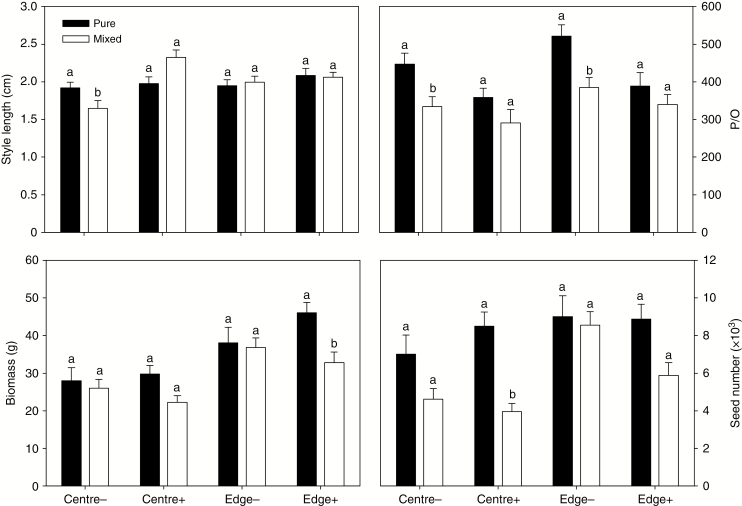
Sympatric presence affected wild brown mustard fitness but herbivory did not
Low levels of herbivores had no significant effect on fitness parameters of wild brown mustard (Table 1, Supplementary Data Table S3), which was also observed when the parameters were analysed separately in the cage centre and edge (Fig. 3). Wild brown mustard produced more ovules, pollen, branches and seeds and higher above-ground biomass at the cage edge than in the cage centre, but there were no observed edge effects for other traits (Fig. 3, Supplementary Data Table S3).
Fig. 3.
Fitness parameters of wild B. juncea under herbivory and cultivation patterns. Pure, all plants were B. juncea; mixed, B. juncea mixed with B. napus in cages. Axis labels and figure legends are the same as Fig. 2. N = 393. Different letters above bars indicate significant differences at P < 0.05 for each group.
Sympatric presence resulted in a significantly decreased P/O ratio, above-ground biomass and seed number in wild brown mustard (Table 1, Supplementary Data Table S3, Fig. 3). The number of pollen grains was lower in mixed stands than in pure stands of single species (Table 1, Supplementary Data Table S3). The number of mustard ovules was significantly higher in mixed cages than in pure cages in the absence of insects (Table 1). Sympatric presence resulted in decreased biomass and seed number of wild brown mustard in the presence of insect infestation but not in the absence of insects (Fig. 3). The survival rate of mustard was lower in pure-stand cages than in mixed cages in the presence of P. xylostella, but not in the absence of insects (Table 1). This means that competition from sympatric presence (mixed cages) and herbivory pressure had additive effects on wild brown mustard. There were interactions between infestation and sympatric presence (I × C) for branch number and above-ground biomass (Supplementary Data Table S3). There were no significant I × E, C × E and I × C × E interactions (Supplementary Data Table S3).
Gene flow rate from Bt OSR to wild brown mustard
Seeds were germinated from 116 Bt OSR and 224 wild brown mustard plants; 47 % emergence was observed for Bt OSR and 70 % for wild brown mustard in the greenhouse (Table 3). The emergence percentage of Bt OSR seeds was higher from mixed stands than from pure stands (two-way ANOVA; F1,4 = 16.8, P = 0.02), while there was no difference observed for mustard emergence percentage between mixed and pure stands.
Table 3.
Gene flow rate from Brassica napus to wild B. juncea and seed emergence rates
| Cultivation | Cage edge | Plutella xylostella infestation | Total no. of measured parents | No. of parents with Bt transgene | Percentage of parents with Bt transgene | Total no. of measured seedlings | No. of seedlings with Bt transgene | Percentage of progeny with Bt transgene | Emergence rate (±SE) |
|---|---|---|---|---|---|---|---|---|---|
| Pure | Centre | No | 28 | 0 | 0.00 | 665 | 0 | 0.00 | 0.69 ± 0.23 |
| Pure | Edge | No | 33 | 0 | 0.00 | 865 | 0 | 0.00 | 0.79 ± 0.15 |
| Mixed | Centre | No | 24 | 1 | 4.17 | 410 | 1 | 0.24 | 0.67 ± 0.19 |
| Mixed | Edge | No | 38 | 5 | 13.16 | 818 | 9 | 1.10 | 0.63 ± 0.14 |
| Pure | Centre | Yes | 15 | 0 | 0.00 | 288 | 0 | 0.00 | 0.69 ± 0.10 |
| Pure | Edge | Yes | 19 | 0 | 0.00 | 370 | 0 | 0.00 | 0.70 ± 0.14 |
| Mixed | Centre | Yes | 34 | 3 | 8.82 | 905 | 7 | 0.77 | 0.79 ± 0.13 |
| Mixed | Edge | Yes | 33 | 1 | 6.06 | 592 | 1 | 0.17 | 0.63 ± 0.16 |
| Total or mean | 224 | 10 | 4.46 | 4913 | 18 | 0.37 | 0.70 ± 0.16 | ||
In total, 4913 progeny seedlings of wild brown mustard were tested. Among the 224 mustard parent plants that were assayed for gene flow, ten plants produced 18 progeny seedlings that harboured the Cry1Ac gene, i.e. 4.46 % of mustard plants received pollen from Bt OSR and produced 0.37 % of Bt progeny (18/4913) (Table 3). No Bt seedlings were observed from seeds collected from mustard plants in pure stands. All Bt progeny from mustard plants were from mixed cages, with a gene flow frequency of 0.66 % (18/2725). The frequency of gene flow was similar in cages with insect infestation (0.53 %, 8/1497) and in the control (0.81 %, 10/1228). The frequency of gene flow from Bt OSR to wild brown mustard was lower when mustard was planted in the cage centre (0.24 %) than at the edge of the cage (1.10 %) in the absence of insects, while it was higher (0.77 versus 0.17 %) in the presence of insects (Table 3). This result indicates that pollination by adult insects (P. xylostella) facilitated pollen transfer to plants in the cage centre.
DISCUSSION
Transgene flow from transgenic crops to their wild relatives results in the presence of transgenes in the natural environment (sympatric presence), which is a potential interspecies coexistence issue in the biosafety assessment of GM plants (Ceddia et al., 2007; Devos et al., 2008; Ellstrand et al., 2013). The interspecies competition and selective pressures in unmanaged environments might determine not only the fate of transgenes but also the population dynamics of wild species. Our sympatric presence experiments were designed to test the hypothesis that competition from the Bt crop and low herbivore presence can increase the frequency of transgene flow to wild relatives.
The frequency of transgene flow from transgenic donor B. napus to wild brown mustard B. juncea was 0.66 % in the present study. It is consistent with other studies. In insect-proof cages continuously provided with houseflies for random pollination, the gene flow frequency from a mutant B. napus resistant to chlorsulfuron to B. juncea was 0.13 % (11 out of 8238 seedlings) (Liu et al., 2010). Bing et al. (1996) found five hybrids between B. napus and B. juncea out of 469 (1.07 %) tested plants in a field experiment. Higher gene flow rate (3 % spontaneous hybridization) of B. napus × B. juncea was found in the field (Jørgensen et al., 1996).
Low gene flow can still conceivably result in adventitious transgene presence in volunteer populations in unmanaged ecosystems, although there is a high frequency of aneuploidy and unviable progeny resulting from the B. napus (AACC) and B. juncea (AABB) cross (Liu et al., 2010). Several studies have already shown introgression for B. napus × B. juncea (Frello et al., 1995; Jørgensen et al., 1998; Liu et al., 2010), as largely demonstrated for B. napus × B. rapa (AA, 2n = 20) (Hansen et al., 2001, 2003) and for B. napus × Raphanus raphanistrum (RrRr, 2n = 18) (Adamczyk-Chauvat et al., 2017). In addition, the Brassica group is prone to producing unreduced gametes, so that many chromosome formulae are possible with viable introgressed progeny (Chèvre et al., 1997). Advanced backcrosses of B. napus × B. juncea were generated and research showed that plant production increased from BC1 to BC6 populations (Cao et al., 2014). Gene flow from glyphosate-resistant B. napus to its weedy relative B. rapa induced herbicide-resistant hybrids and backcross volunteers in fields (Warwick et al., 2008). The volunteers could act as a source of further transgene flow, but the application of control measures could limit the adventitious presence of GM material (Begg et al., 2006).
Herbivores could be expected to change gene flow frequency by consuming the flowers and decreasing flower number, affecting flowering time of wild species (Liu et al., 2015b). However, our results demonstrated that low herbivory did not act as sufficient selection pressure to affect gene flow rates. This result is in agreement with a previous study showing that high herbivory (>50 larvae of P. xylostella per plant) did not alter gene flow frequency from insect-resistant and glyphosate-resistant OSR to B. rapa (Londo et al., 2011b), although high herbivory seriously damaged the plants (Londo et al., 2011a).
Besides consuming reproductive tissues, herbivores could be expected to affect gene flow rate by reducing the release of transgenic pollen from herbivore-susceptible genotypes. In this study, the pollen production of Bt OSR and wild brown mustard was not affected by low-level herbivory. In another study, it was found that defoliation could increase both pollen and ovule number per flower of Hosta ventricosa, but did not affect the P/O ratio per flower (Cao et al., 2011). We found that the presence of insects stimulated Bt OSR to increase the length of flower styles and facilitated the movement of pollen among plants, and thus increased the gene flow rate in the cage centre.
Unlike herbivory pressure, the presence of Bt OSR near wild brown mustard significantly affected mustard plants. That is, there was a relative competitive advantage of Bt OSR compared with wild brown mustard, which was congruent with results from a previous study (Liu et al., 2015b). The superiority of the transgenic plants was obvious under insect herbivory, as expected from the role of the Bt protein under moderate or higher insect pressure (Moon et al., 2007; Letourneau and Hagen, 2009; Liu et al., 2015a).
Our results demonstrated that the presence of Bt OSR near wild brown mustard significantly decreased the P/O ratio of wild brown mustard. The P/O ratio is used as an indirect measure in plant breeding systems (Cruden, 1977; Galloni et al., 2007); a lower P/O ratio indicates a shift in sex allocation towards greater female investment (Burd, 2011). Since the P/O is negatively correlated with pollen transfer among plants (Gong and Huang, 2014), the presence of Bt OSR could increase pollen transfer efficiency between Bt OSR and wild brown mustard. Thus, the presence of Bt-transgenic plants near wild brown mustard is expected to affect gene flow by altering sex allocation.
Sex allocation may differ for genetic and/or environmental reasons. Models of sex allocation in plants predict that large plants should have higher female investment than small plants. For outcrossing plants, most studies showed that more resources were allocated to female function in larger plants compared with smaller plants (Cao and Kudo, 2007; Delesalle and Mazer, 2009), but wind-pollinated plants are expected to increase male investment with plant height (Nakahara et al., 2018). For selfing plants, P/O does not vary with plant size, with pollen and ovule production increasing commensurately with plant size (Delesalle and Mazer, 2009). Environmental conditions, including resource availability, soil nutrient and root competition, can affect sex allocation in plants (Lankinen et al., 2013). Therefore, the competitive advantage of Bt OSR changed sex allocation in brown mustard towards a relatively higher investment in female function.
Thanks to the decreased male investment (less pollen) of wild brown mustard, the presence of Bt OSR could result in increased gene flow if wild brown mustard receives pollen from transgenic plants within the community. Moreover, the pollen number of transgenic plants was not decreased in the mixed stands. In addition, although sympatric presence increased female investment in ovule production, we observed a reduction in reproductive fitness in wild brown mustard as a result of competitive inferiority relative to Bt OSR. Thus, the presence of transgenic plants near wild plants has the potential to significantly reduce the total number of wild mustard seeds that enter the seed bank in the following year, as well as to reduce the total number of transgenic seeds produced by wild mustard plants. However, competition pressure on wild brown mustard in mixed Bt OSR communities could also contribute to an increase in the ratio of transgenic seeds produced by first-generation hybrids by changing the reproductive strategy.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: results of PCR and ELISA experiments to detect Cry1Ac DNA and protein in transgenic and non-transgenic seedlings. Table S1: Z values of Kendall’s correlation between seed number and fitness parameters for Brassica napus and wild Brassica juncea. Table S2: F values for analysis of three-way mixed split-plot ANOVA for fitness parameters of transgenic Brassica napus under Plutella xylostella infestation and different cultivation patterns. Table S3: F values from analysis of three-way mixed split-plot ANOVA for fitness parameters of wild Brassica juncea under Plutella xylostella infestation and under cultivation patterns.
ACKNOWLEDGEMENTS
We thank Ms X. Zhang, F. Liu and Mr H. Huang for their assistance in experimental manipulations. This work was financially supported by the Natural Science Foundation of China (grant 31200288) and the Beijing Nova Program (xx2016B070; Z161100004916112).
LITERATURE CITED
- Adamczyk-Chauvat K, Delaunay S, et al. . 2017. Gene introgression in weeds depends on initial gene location in the crop: Brassica napus– Raphanus raphanistrum model. Genetics 206: 1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg GS, Hockaday S, McNicol JW, Askew M, Squire GR. 2006. Modelling the persistence of volunteer oilseed rape (Brassica napus). Ecological Modelling 198: 195–207. [Google Scholar]
- Bing DJ, Downey RK, Rakow FW. 1996. Hybridization among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinapis arvensis under open pollination conditions in the field. Plant Breeding 115: 470–473. [Google Scholar]
- Burd M. 2011. Are relationships between pollen-ovule ratio and pollen and seed size explained by sex allocation?Evolution 65: 3002–3005. [DOI] [PubMed] [Google Scholar]
- Cao D, Stewart CN Jr, Zheng M, et al. . 2014. Stable Bacillus thuringiensis transgene introgression from Brassica napus to wild mustard B. juncea. Plant Science 227: 45–50. [DOI] [PubMed] [Google Scholar]
- Cao G-X, Kudo G. 2007. Size-dependent sex allocation in a monocarpic perennial herb, Cardiocrinum cordatum (Liliaceae). Plant Ecology 194: 99–107. [Google Scholar]
- Cao G, Xue L, Li Y, Pan K. 2011. The relative importance of architecture and resource competition in allocation to pollen and ovule number within inflorescences of Hosta ventricosa varies with the resource pools. Annals of Botany 107: 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceddia MG, Bartlett M, Perrings C. 2007. Landscape gene flow, coexistence and threshold effect: the case of genetically modified herbicide tolerant oilseed rape (Brassica napus). Ecological Modelling 205: 169–180. [Google Scholar]
- Chèvre AM, Eber F, Baranger A, Renard M. 1997. Gene flow from transgenic crops. Nature 389: 924. [Google Scholar]
- Cruden RW. 1977. Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants Evolution 31: 32–46. [DOI] [PubMed]
- D’Hertefeldt T, Jorgensen RB, Pettersson LB. 2008. Long-term persistence of GM oilseed rape in the seedbank. Biology Letters 4: 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmency H. 1994. The impact of hybrids between genetically modified crop plants and their related species: introgression and weediness. Molecular Ecology 3: 37–40. [Google Scholar]
- Delesalle VA, Mazer SJ. 2009. Size-dependent pollen:ovule ratios and the allometry of floral sex allocation in Clarkia (Onagraceae) taxa with contrasting mating systems. American Journal of Botany 96: 968–978. [DOI] [PubMed] [Google Scholar]
- Devos Y, Demont M, Sanvido O. 2008. Coexistence in the EU – return of the moratorium on GM crops? Nature Biotechnology 26: 1223–1225. [DOI] [PubMed] [Google Scholar]
- Ellstrand N, Meirmans P, Rong J, et al. . 2013. Introgression of crop alleles into wild or weedy populations. Annual Review of Ecology, Evolution and Systematics 44: 325–345. [Google Scholar]
- FitzJohn RG, Armstrong TT, Newstrom-Lloyd LE, Wilton AD, Cochrane M. 2007. Hybridisation within Brassica and allied genera: evaluation of potential for transgene escape. Euphytica 158: 209–230. [Google Scholar]
- FOC 2001. Flora of China. Committee FOC, ed. Beijing: Science Press; St Louis: Missouri Botanical Garden Press. [Google Scholar]
- Ford CS, Allainguillaume J, Grilli-Chantler P, Cuccato G, Allender CJ, Wilkinson MJ. 2006. Spontaneous gene flow from rapeseed (Brassica napus) to wild Brassica oleracea. Proceedings of the Royal Society B: Biological Sciences 273: 3111–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frello S, Hansen KR, Jensen J, Jørgensen RB. 1995. Inheritance of rapeseed (Brassica napus)-specific RAPD markers and a transgene in the cross B. juncea × (B. juncea × B. napus). Theoretical and Applied Genetics 91: 236–241. [DOI] [PubMed] [Google Scholar]
- Galloni M, Podda L, Vivarelli D, Cristofolini G. 2007. Pollen presentation, pollen-ovule ratios, and other reproductive traits in Mediterranean legumes (fam. Fabaceae - Subfam. Faboideae). Plant Systematics and Evolution 266: 147–164. [Google Scholar]
- Gong YB, Huang SQ. 2014. Interspecific variation in pollen-ovule ratio is negatively correlated with pollen transfer efficiency in a natural community. Plant Biology 16: 843–847. [DOI] [PubMed] [Google Scholar]
- Guo QY, Tu HL, Qiu XL, Xin CY. 1998. Study of the occurrence dynamics and control technique for wild mustard (Brassica juncea L.) in fields. [In Chinese.] Science and Technology of Qinhai Agriculture and Forestry 18: 38–41. [Google Scholar]
- Halfhill MD, Richards HA, Mabon SA, Stewart CN Jr. 2001. Expression of GFP and Bt transgenes in Brassica napus and hybridization with Brassica rapa. Theoretical and Applied Genetics 103: 659–667. [Google Scholar]
- Hansen LB, Siegismund HR, Jørgensen RB. 2001. Introgression between oilseed rape (Brassica napus L.) and its weedy relative B. rapa L. in a natural population. Genetic Resources and Crop Evolution 48: 621–627. [Google Scholar]
- Hansen LB, Siegismund HR, Jørgensen RB. 2003. Progressive introgression between Brassica napus (oilseed rape) and B. rapa. Heredity 91: 276–283. [DOI] [PubMed] [Google Scholar]
- van Heerwaarden J, Ortega Del Vecchyo D, Alvarez-Buylla ER, Bellon MR. 2012. New genes in traditional seed systems: diffusion, detectability and persistence of transgenes in a maize metapopulation. PLoS ONE 7: e46123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen RB, Andersen B, Hauser TP, Landbo L, Mikkelsen TR, Ostergard H. 1998. Introgression of crop genes from oilseed rape (Brassica napus) to related wild species – an avenue for the escape of engineered genes. Acta Horticulture 459: 211–217. [Google Scholar]
- Jørgensen RB, Andersen B, Landbo L, Mikkelsen TR. 1996. Spontaneous hybridization between oilseed rape (Brassica napus) and weedy relatives. Acta Horticulturae 407: 193–200. [Google Scholar]
- Lankinen Å, Larsson MC, Fransson A-M. 2013. Allocation to pollen competitive ability versus seed production in Viola tricolor as an effect of plant size, soil nutrients and presence of a root competitor. Oikos 122: 779–789. [Google Scholar]
- Letourneau DK, Hagen JA. 2009. Plant fitness assessment for wild relatives of insect resistant crops. Environmental Biosafety Research 8: 45–55. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei W, Ma K, Darmency H. 2010. Backcrosses to Brassica napus of hybrids between B. juncea and B. napus as a source of herbicide-resistant volunteer-like feral populations. Plant Science 179: 459–465. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei W, Ma K, Darmency H. 2013a Spread of introgressed insect-resistance genes in wild populations of Brassica juncea: a simulated in-vivo approach. Transgenic Research 22: 747–756. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei W, Ma K, Li JS, Liang Y, Darmency H. 2013b Consequences of gene flow between oilseed rape (Brassica napus) and its relatives. Plant Science 211: 42–51. [DOI] [PubMed] [Google Scholar]
- Liu Y, Darmency H, Stewart CN Jr, Wei W, Tang ZX, Ma KP. 2015a The effect of Bt-transgene introgression on plant growth and reproduction in wild Brassica juncea. Transgenic Research 24: 537–547. [DOI] [PubMed] [Google Scholar]
- Liu Y, Stewart CN Jr, Li J, Huang H, Zhang X. 2015b The presence of Bt-transgenic oilseed rape in wild mustard populations affects plant growth. Transgenic Research 24: 1043–1053. [DOI] [PubMed] [Google Scholar]
- Londo JP, Bollman MA, Sagers CL, Lee EH, Watrud LS. 2011a Changes in fitness-associated traits due to the stacking of transgenic glyphosate resistance and insect resistance in Brassica napus L. Heredity 107: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londo JP, Bollman MA, Sagers CL, Lee EH, Watrud LS. 2011b Glyphosate-drift but not herbivory alters the rate of transgene flow from single and stacked trait transgenic canola (Brassica napus) to nontransgenic B. napus and B. rapa. New Phytologist 191: 840–849. [DOI] [PubMed] [Google Scholar]
- Marquardt P, Krupke C, Johnson WG. 2012. Competition of transgenic volunteer corn with soybean and the effect on western corn rootworm emergence. Weed Science 60: 193–198. [Google Scholar]
- Millwood RJ, Halfhill MD, Harkins D, Russotti R, Stewart CN Jr. 2003. Instrumentation and methodology for quantifying GFP fluorescence in intact plant organs. BioTechniques 34: 638–643. [DOI] [PubMed] [Google Scholar]
- Moon HS, Halfhill MD, Good LL, Raymer PL, Stewart CN Jr. 2007. Characterization of directly transformed weedy Brassica rapa and introgressed B. rapa with Bt cry1Ac and gfp genes. Plant Cell Reports 26: 1001–1010. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Fukano Y, Hirota SK, Yahara T. 2018. Size advantage for male function and size-dependent sex allocation in Ambrosia artemisiifolia, a wind-pollinated plant. Ecology and Evolution 8: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team 2008. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.r-project.org/. [Google Scholar]
- Ramachandran S, Buntin GD, All JN, Raymer PL, Stewart CN Jr. 2000. Intraspecific competition of an insect-resistant transgenic canola in seed mixtures. Agronomy Journal 92: 368–374. [Google Scholar]
- Reuter H, Menzel G, Pehlke H, Breckling B. 2008. Hazard mitigation or mitigation hazard? Would genetically modified dwarfed oilseed rape (Brassica napus) increase feral survival? Environmental Science and Pollution Research 15: 529–535. [DOI] [PubMed]
- Santa-Maria MC, Lajo-Morgan G, Guardia L. 2014. Adventitious presence of transgenic events in the maize supply chain in Peru: a case study. Food Control 41: 96–101. [Google Scholar]
- Schafer MG, Ross AA, Londo JP, et al. . 2011. The establishment of genetically engineered canola populations in the U.S. PLoS ONE 6: e25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler JA, Dale PJ. 1994. Opportunities for gene transfer from transgenic oilseed rape (Brassica napus) to related species. Transgenic Research 3: 263–278. [Google Scholar]
- Song X, Wang Z, Zuo J, Huangfu C, Qiang S. 2010. Potential gene flow of two herbicide-tolerant transgenes from oilseed rape to wild B. juncea var. gracilis. Theoretical and Applied Genetics 120: 1501–1510. [DOI] [PubMed] [Google Scholar]
- Stewart CN Jr, Halfhill MD, Warwick SI. 2003. Transgene introgression from genetically modified crops to their wild relatives. Nature Reviews Genetics 4: 806–817. [DOI] [PubMed] [Google Scholar]
- Vacher C, Weis AE, Hermann D, Kossler T, Young C, Hochberg ME. 2004. Impact of ecological factors on the initial invasion of Bt transgenes into wild populations of birdseed rape (Brassica rapa). Theoretical and Applied Genetics 109: 806–814. [DOI] [PubMed] [Google Scholar]
- Voskresenskaya GS, Lygina LM. 1973. Cross pollination of leaf mustard. Doklady Vsesoyuznoi Akademii Sel’skokhozyaistvennykh Nauk Im V. I. Lenina 6: 16–17. [Google Scholar]
- Warwick SI, Légère A, Simard MJ, James T. 2008. Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Molecular Ecology 17: 1387–1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.