Abstract
Background and aims
Corner’s rules describe a global spectrum from large-leaved plants with thick, sparingly branched twigs with low-density stem tissues and thick piths to plants with thin, highly branched stems with high-density stem tissues and thin piths. The hypothesis was tested that, if similar crown areas fix similar amounts of carbon regardless of leaf size, then large-leaved species, with their distantly spaced leaves, require higher stem growth rates, lower stem tissue densities and stiffnesses, and therefore thicker twigs.
Methods
Structural equation models were used to test the compatibility of this hypothesis with a dataset on leaf size, shoot tip spacing, stem growth rate and dimensions, and tissue density and mechanics, sampling 55 species drawn from across the angiosperm phylogeny from a morphologically diverse dry tropical community.
Key results
Very good fit of structural equation models showed that the causal model is highly congruent with the data.
Conclusions
Given similar amounts of carbon to allocate to stem growth, larger-leaved species require greater leaf spacing and therefore greater stem extension rates and longer stems, in turn requiring lower-density, more flexible, stem tissues than small-leaved species. A given stem can have high resistance to bending because it is thick (has high second moment of area I) or because its tissues are stiff (high Young’s modulus E), the so-called E–I trade-off. Because of the E–I trade-off, large-leaved species have fast stem growth rates, low stem tissue density and tissue stiffness, and thick twigs with wide piths and thick bark. The agreement between hypothesis and data in structural equation analyses strongly suggests that Corner’s rules emerge as the result of selection favouring the avoidance of self-shading in the context of broadly similar rates of carbon fixation per unit crown area across species.
Keywords: Adaptation, allometry, biomechanics, Corner’s rules, deciduous trees, evergreen trees, high wood density trees, leaf size–stem size spectrum, structural equation models, trade-offs, water-storing trees, wood density
INTRODUCTION
Understanding the causes of global patterns of cross-species trait covariation is one of the central aims of plant evolutionary ecology (Westoby et al., 2002). Of these patterns, one of the most often-documented and longest known is Corner’s rules, which consists of a series of leaf and stem features that covary (Westoby and Wright, 2003). Plants tend to be arrayed along a continuum of those with large leaves borne on thick, sparsely branched twigs at one extreme to those with small leaves borne on slender, intricately branched twigs at the other. Other traits are also involved, with large-leaved species having wide piths (the parenchymatous tissue at the centre of the stem, interior to the primary xylem), flexible, low-density wood (White, 1983a; Ackerly, 1996) and thick bark (Rosell et al., 2014).
Much progress has been made in documenting the extent of Corner’s rules as well as elucidating its causes. Corner’s original formulation (Corner, 1949), embedded in a soon discredited attempt to place plants along a linear primitive–advanced axis (Olson, 2012b), consisted of the qualitative observation that, across species, as twigs become larger, leaves become larger, wood becomes more flexible (of lower density) and branching more sparing. Since then, plant evolutionary ecologists have quantitatively detailed relationships of features such as total shoot leaf area and leaf length with stem diameter, wood density and stem resistance to bending. These efforts have shown that Corner’s rules are very widespread, being found across virtually all plants studied to date, across lineages, continents and vegetation types, both across and within species (White, 1983a, b; Ackerly, 1996; Ackerly and Donoghue, 1998; Brouat et al., 1998; Westoby and Wright, 2003; Sun et al., 2006; Wright et al., 2006; Olson et al., 2009; Leslie et al., 2014; Fajardo, 2016; Trueba et al., 2016). While various questions remain regarding its causes, the pattern seems likely to involve inevitable trade-offs in the allocation of finite amounts of fixed carbon and selection favouring avoidance of self-shading (Messier et al., 2017; Smith et al., 2017).
One aspect of Corner’s rules that remains puzzling is why variation in leaf size is related to variation in twig diameter and wood density (Westoby et al., 2002; Swenson and Enquist, 2008; Malhado et al., 2009). That selection should favour wide twigs in supporting large leaves is routinely cited as a cause for Corner’s rules (White, 1983b). However, why these twigs are inevitably made up of low-density wood has remained unclear. Large twigs are often cited as necessary for supplying large leaves with water (Tyree et al., 1991), but why these twigs should always have wide piths and thick bark, but never a central vascular cylinder or thin bark (Ackerly, 1996), is not explained by appeals to hydraulics (Fan et al., 2017). However, metabolic scaling theory (West et al., 1997, 1999; Enquist and Niklas, 2002) does provide the basis for an explanation.
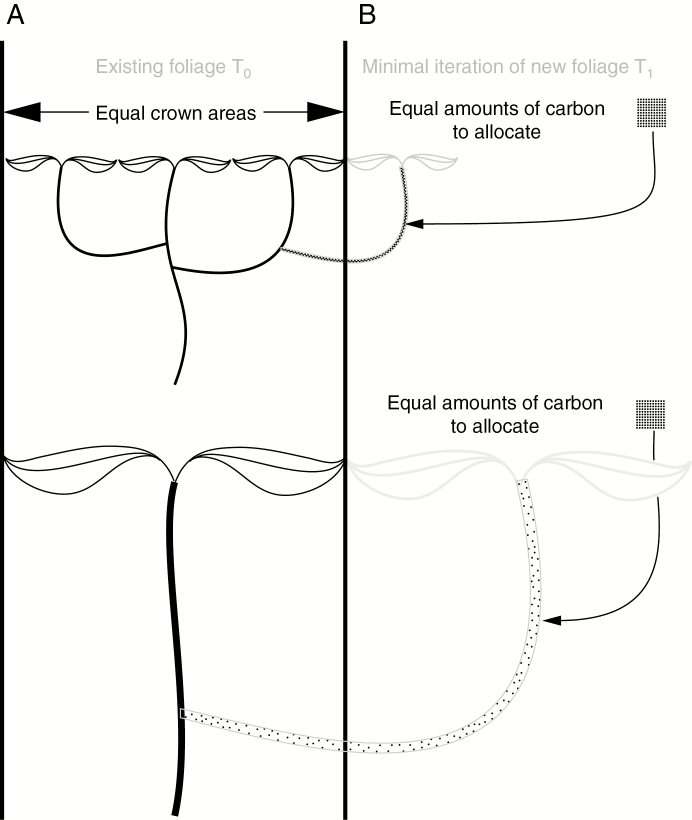
Metabolic scaling theory posits that plants in general should have similar amounts of crown area for a given basal stem diameter regardless of leaf size (Enquist and Niklas, 2002; Olson et al., 2009). Moreover, on average, plants should fix similar amounts of carbon per unit crown area irrespective of leaf size (West et al., 1997, 1999; Enquist et al., 1999; Enquist and Niklas, 2002; Valladares et al., 2002; Selaya and Anten, 2010; Michaletz et al., 2014; Stephenson et al., 2014), whether over a growing season carbon is fixed quickly in short-lived leaves or more slowly in longer-lived ones (Wright et al., 2004). As a result, two plants of identical crown area but differing leaf size will have on average similar amounts of carbon to invest in the next iteration of growth. In broad terms, larger leaves are deployed at wider spacings within canopies than small ones. This spacing is presumably the result of selection leading to avoidance of self-shading via factors such as leaf longevity and photosynthetic rate (Ackerly and Bazzaz, 1995a, b; Valladares et al., 2002; Kitajima et al., 2005; Duursma et al., 2012; Smith et al., 2017). Given greater leaf spacing, for the next iteration of growth, the large-leaved plant requires the production of a longer stem than the small-leaved plant does, but with the same amount of carbon as in the small-leaved plant (Messier et al., 2017). If a flush of leaves is produced in the same amount of time in both plants, then the large-leaved species will require a greater absolute rate of stem extension (Ackerly and Bazzaz, 1995a). The production of a greater amount of twig with the same amount of carbon necessarily results in stem tissues of lower density in the large-leaved plant. The scenario presented to this point is illustrated in Fig. 1.
Fig. 1.
Corner’s rules and carbon. Plants on average fix similar amounts of carbon per unit crown area regardless of leaf size, an essential condition for observing Corner’s rules. With black lines, this figure shows two plants with identical crown areas, one made up of small leaves on narrow terminal twigs (A) and the other of large leaves on thick twigs (B). Because identical crown areas fix similar amounts of carbon, both plants have the same amount of carbon (represented by black dots) to allocate to the next iteration of growth, outlined in grey. In plants with small leaves, the distance required to locate an apical meristem whose leaves will not be shaded by existing leaves is short, whereas it is much longer in a species with large leaves. Because the extension required is short, the tissue in the small-leaved species is dense, represented by units of carbon that are very close to one another (A). However, in the large-leaved plant (B), the same number of units of carbon as in (A) are necessarily farther apart from one another, i.e. make up tissues of low density. That large-leaved plants have low-density stem tissues is a well-known correlation and a vital link in the causal chain leading to Corner’s rules.
Why twigs should have hollow, rather than solid, vascular cylinders likely has to do with the mechanical consequences of variation in stem tissue density (Sterck et al., 2006). Lower-density tissues mean that stem materials have lower resistance to bending, as expressed by Young’s modulus E. How well stem tissues are deployed to resist bending can be quantified in terms of twig transectional size and geometry by the moment of inertia I (Niklas, 1992). Material at the centre of a beam contributes little to resisting bending, so even a moderately wide pipe is much stiffer than a solid cylinder of the same amount of material transectional area (Gere and Timoshenko, 1999). This is why so many structures, such as bicycle frames and metal lamp posts, are always hollow. Stems are no exception, and when E is low the I of the wood + bark complex is high due to the presence of a wide pith (Niklas, 1992, 1995; Hogan and Niklas, 2003; see also Ackerly, 1996). A given flexural rigidity EI can be achieved by a tissue of high E and low I or low E and high I. In biological terms, this means that twigs made up of high-density tissues should be slender, whereas twigs made up of low-density tissues should be thick. In this way, the so-called E–I trade-off is responsible for large-leaved plants having thick, stubby twigs. The considerations mentioned, based on carbon limitation and biomechanical considerations, can potentially account for the trait associations that make up Corner’s rules.
Although most of the trait associations that make up our scenario have been documented previously (Supplementary Data Fig. S1), stem growth rate has never had a central role in Corner’s rules studies. We suggest that growth rate (specifically, the rate of production of volume of terminal branches) is a key aspect of this constellation of associations, as shown in Fig. 2. We studied variation in growth rate in relation to other Corner’s variables with a comparative study across 55 species in 36 families from 22 angiosperm orders from a tropical dry forest with an exceptional range in leaf size. Because the species at our site begin their yearly pulse of growth in response to the same rainfall event, the growth accrued at the end of the rainy season can be used as a comparable measure of growth rate across species.
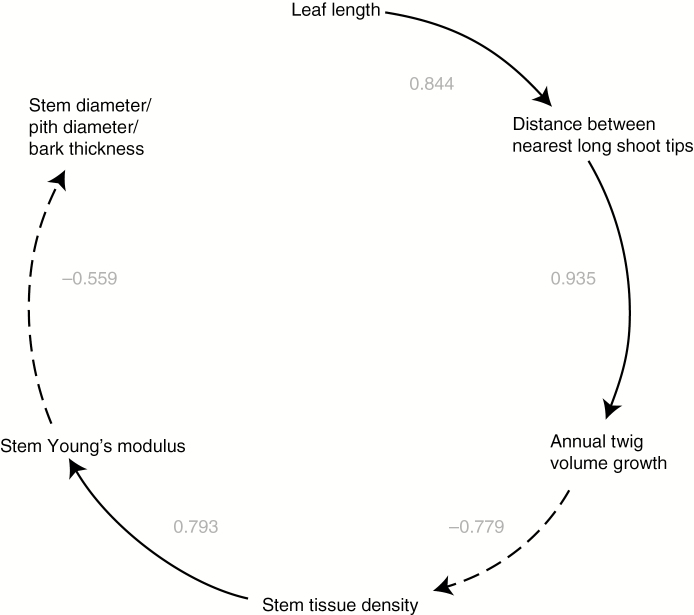
Fig. 2.
Important traits in the hypothesized causal chain for Corner’s rules. This diagram depicts the positive or negative correlations that lead to Corner’s rules. Positive correlations are shown with solid lines and negative correlations with dashed lines. Selection favours avoidance of self-shading via wider distances between stem tips in species with larger leaves (Fig. 1) (Smith et al., 2017), leading to the positive correlation between leaf length and distance between stem tips. Greater spacing between stem tips should predict faster growth rates, in terms of terminal stem length or volume. In turn, given carbon limitation, faster growth rates should be associated with lower stem tissue density (Fig. 1). Stem tissue density predicts tissue stiffness, as measured by Young’s modulus. For a given amount of carbon, twig stiffness can be achieved via slender stems made of stiff tissues, or thick twigs made of low-density tissue, leading to the expectation of a negative relationship between Young’s modulus, twig diameter, pith diameter and bark thickness, the so-called E–I trade-off. These relations together cause Corner’s rules. A version of the figure with references for previously documented correlations is given in Supplementary Data Fig. S1. Numbers are standardized coefficients from structural equation models (Table 3).
In addition to growth rate, we measured leaf length, which should determine the radius of the leaf-bearing cylinder surrounding stems, and thus the branch spacing that selection should favour (Smith et al., 2017). We measured distances between branch tips, with the expectation that larger-leaved species should have greater branch spacing and higher terminal twig growth rates. We then measured stem tissue density and resistance to bending, given that faster-growing species should have lower-density tissues that are mechanically more flexible (Ackerly, 1996). Because their tissues are more flexible, via the E–I trade-off, twigs of faster-growing species should be thicker, with thicker wood + bark cylinders and wider piths. In the Materials and methods section we discuss in more detail the rationale for measuring these variables. We used these data to test the hypothesis in Fig. 2 using structural equation models, an approach that makes it possible to test whether the data are compatible with a given causal hypothesis (Shipley, 2004). Our approach allowed us to show why tissue density and growth rates should vary across species of different leaf sizes, and why stem diameter, bark thickness and pith diameter should also covary with all the other traits.
MATERIALS AND METHODS
To test the proposed causal hypothesis, a comparative dataset with enough variation in the relevant traits was needed. Moreover, a strongly delimited growing season would be ideal to estimate growth rates across species. These conditions were met in the Chamela tropical dry forest on the Pacific coast of Jalisco, Mexico. The upland species of the forest apparently lack access to deep water sources (Méndez-Alonzo et al., 2013). As a result, most species begin growth simultaneously in response to the onset of heavy rains during the brief summer rainy season, which averages ~750 mm year–1 (Méndez-Alonzo et al., 2012). By sampling towards the end of the wet season, it was possible to compare how average terminal twig volume and biomass were accumulated on average across species (variables measured are described below). Most species were wild in the upland forest, but we also included cultivated but unwatered plants (Annona muricata and Moringa oleifera) and two plants from the margins of subdeciduous forests in swales (Licaria nayaritensis and Guaiacum coulteri) in the interest of ordinal-level diversity.
We selected species spanning the entire ordinal-level diversity of the woody self-supporting angiosperms, from shrubs to trees, of the area (Lott, 2002). We also made sure to select the maximal range of leaf sizes present. We measured the following variables, following the order in Fig. 2. We measured leaf length, including petioles, because it is the stem-to-leaf tip distance that defines the radius of the leaf-bearing cylinder surrounding stems (Smith et al., 2017). We measured leaf length in the same way in species with simple and compound leaves. We measured the lengths of all of the leaves derived from the apical meristems (i.e. excluding any from axillary meristems) from five randomly selected branches, mostly leading, and usually from five different individuals. In some cases there were very few leaves per shoot, so we measured leaves from additional shoots to ensure adequate sample sizes. The number of leaves measured per species ranged from 20 to 235, with a median of 50.
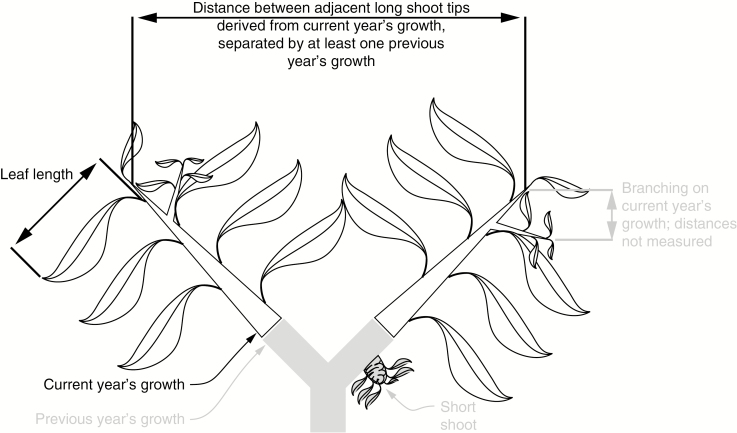
Presumably selection acts in such a way that the leaf-bearing cylinders of adjacent stems do not overlap excessively (Smith et al., 2017). Accordingly, we gathered data on the distances between adjacent shoot tips, which should be greater in larger-leaved species (Halloy and Mark, 1996; Read et al., 2006). We selected 25 long shoot tips randomly on five to ten individuals, and then measured the straight-line distance to the three closest shoot tips from the same individual, for a total of 75 measurements per species. We measured distances between different shoots, i.e. ones that were separated by at least 1 year’s worth of previous-season growth (Fig. 3). This sampling strategy meant that we did not measure distances between shoot tips within the same current season’s growth.
Fig. 3.
Diagram of selected variables measured. Stems in grey represent the previous year’s growth. White stems with black outline represent the current year’s growth and were the focus of the present study. Focal variables are labelled in black to distinguish them from ones not measured, which are in grey. We measured the distance between the tips of current year shoots that were separated by at least 1 year’s previous growth. Selection favouring the avoidance of self-shading predicts that this distance should correlate positively with leaf length as well as stem volume growth rate, both of which were also measured.
In accordance with Fig. 1, species with greater distances between shoot tips should have higher growth rates. We measured the length of the year’s growth, with the generally green, leaf-bearing portion of the shoots readily identifying the year’s growth increment. Using the diameter of the stem at the base of the current year’s growth, we calculated the terminal volume of the stems, using the formula for the volume of a cylinder. We used terminal volume rather than just length because volume expresses best the requirements facing a plant for deployment of carbon (Huang et al., 2016). We measured long shoots and avoided brachyblasts or short shoots (Fig. 3). Some species alternate between long- and short-shoot behaviour on the same branches, e.g. Amphypterygium adstringens, Comocladia engleriana and Plumeria rubra. In these species, new leaf whorls are deployed in the crown via long shoots. On these long shoots, the leaves are separated by visible internodes. Once the apical bud is deployed, its position remains relatively fixed, often for years, via the production of leaves that are not separated by internodes, equivalent to a short shoot (White, 1983a). Because the tissue density–growth rate relationship should be expressed most clearly during deployment growth, as in Fig. 1, we did not include short shoots or analogous structures in our stem extension rate measurements.
Species with greater stem extension rates are expected to have lower densities of stem tissues. We measured total stem density from 1-cm diameter portions of the five stems per species on which leaf length was measured, for a total of five total stem tissue density measurements per species. Stem tissue density measurements were conducted using the water displacement method. Given that water has a density of 1 g cm–3, the fresh volume of a stem portion was measured as the additional weight detected by an analytical balance when submerging the sample in water. After measuring volume, stem portions were dried for 4 d at 100 °C to measure their densities as the dry weight divided by fresh volume (Williamson and Wiemann, 2010). Although wood density is a commonly measured trait (Chave et al., 2009) with a long history in the forestry literature, total stem tissue density would seem to be the more biologically relevant measurement for our study, for two reasons. First, the pool of carbon available for investment in stem is deployed among bark and wood, and to a lesser extent pith (which is almost always of very low density), so the collective density of the whole stem represents the way that carbon is distributed in the whole structure. Second, it is in turn the mechanical behaviour of the whole structure that is important from the point of view of natural selection.
With regard to mechanical performance, species with stems of lower tissue density are expected to have tissues that are more flexible. We therefore performed mechanical tests on portions ~1 cm in diameter and 40 cm long of the five stems per species for which stem tissue density was measured. We measured the apical and basal total, xylem, bark and pith diameters of the mechanical segments. We averaged the total diameters and the pith diameters and used these values for calculation of the moment of inertia I using the formula for hollow beams (Niklas, 1992). The thickness of the wall of the beam was the sum of the thicknesses of xylem and bark. We tested each segment in three-point bending with an Instron 3345 testing machine fitted with a 5 kN load cell (Instron Corporation, MA, USA). The distance between supports ranged from 125 to 600 mm, varying with segment diameter in such a way as to maintain a minimum 1:20 diameter:length ratio to maximize bending as opposed to shear. Segments were bent until breakage, usually with a crosshead travel of 1–5 cm, at a crosshead speed of 2.5 mm min−1. With System IX software, we calculated the slope of the maximum deflection-versus-force curve to calculate the stem Young’s modulus E (Rosell and Olson, 2014).
Statistical analyses
After calculating means for each species, we checked the normality of each variable and log10-transformed all data. We calculated Pearson correlation coefficients to examine the associations between all pairs of traits. These pairs included the traits postulated in our Corner’s rules causal hypothesis, and also those involving indirect associations often documented in the literature, such as that between leaf length and stem tissue density, and Young’s modulus (Supplementary Data Fig. S1). We calculated phylogenetically independent contrasts (PICs) to recalculate correlations between pairs of traits, taking into account potential non-independence of our comparative data (Felsenstein, 1985). For this, we built a phylogeny based on the backbone of Stevens (2001 onwards) and resolved relationships within families and genera with reference to phylogenetic studies of specific groups (phylogeny shown in Supplementary Data Fig. S2). We assigned branch lengths based on the divergence times in Wikstrom et al. (2001) using Phylocom v. 4.2 (Webb et al., 2008). There was only one polytomy at the family level, in Malpighiales, that could not be resolved. We carried out phylogenetic analyses using the three possible totally resolved phylogenetic trees. We calculated PICs using the package picante (Kembel et al., 2010).
To test our hypothesis regarding the factors leading to Corner’s rules, we fitted structural equation models (Shipley, 2004) following the postulated causal relationships shown in Fig. 2. The causal chain starts with mean leaf length, variation in which causes changes in the distance between branch tips, growth rate, stem density, stem Young’s modulus and ultimately stem diameter. In this way, the strong association between leaf area and stem diameter, which lies at the core of Corner’s rules (White, 1983a, b; Ackerly and Donoghue, 1998), is mediated causally as depicted in Fig. 2. Presumably this chain of causality can go from leaf length to stem diameter and vice versa, for example when selection acts on stem diameter instead of on leaf length, or at any other point in the chain. The trait experiencing direct selection will cause changes in the other traits through the proposed causal chain. To reflect this range of possible directionalities, we used structural equation models to test the causal chain from leaf length to stem diameter (Model LL → SD) and in the reverse direction, from stem diameter to leaf length (Model SD → LL). Models were based on log10-transformed variables.
We evaluated the fit of the models using the χ2 test and goodness-of-fit indices such as the root mean square error of approximation (RMSEA) and its associated test, the standardized root mean square residual (SRMR), the comparative fit index (CFI) and the non-normed fit index (NNFI) (Kline, 2011). We concluded that a model fitted the data well when the χ2 test was not significant (P > 0.05) and when the listed indices indicated a good fit. We used a cut-off value of 0.05 for RMSEA and its associated test, a value of 0.08 for the SRMR and 0.95 for the CFI and the NNFI (Hu and Bentler, 1999). Given that both models were based on the same correlation structure, they both had the same value for the χ2 test and all goodness-of-fit indices. We also examined the stability of both models. Structural equation models were fitted using the R package lavaan (Rosseel, 2012) in R v.3.4.3 (www.r-project.org).
RESULTS
The 55 species collected belonged to 36 families in 22 orders of angiosperms. Species mean leaf length varied over two orders of magnitude, ranging from 8.6 to 816.9 mm (Table 1). Twig basal diameter (here measured as the diameter at the base of the stem annual increment), another core Corner’s variable, ranged from 8.9 to 22.4 mm. The other traits mediating the relationship between leaf length and stem diameter also had wide ranges of variation (Table 1).
Table 1.
Descriptive statistics of the variables involved in the causal chain of Corner’s rules (N = 55 species)
| Median | Range | |
|---|---|---|
| Leaf length (mm) | 119.7 | 8.6–816.9 |
| Distance between stem tips (mm) | 257.9 | 63.2–780.0 |
| Annual terminal twig volume growth (mm3) | 3506.6 | 26.8–205 918.7 |
| Stem tissue density (g cm−3) | 0.47 | 0.19–0.80 |
| Young’s modulus (N m−2) | 3915 | 1058–10253 |
| Stem diameter (mm) | 12.1 | 8.9–22.4 |
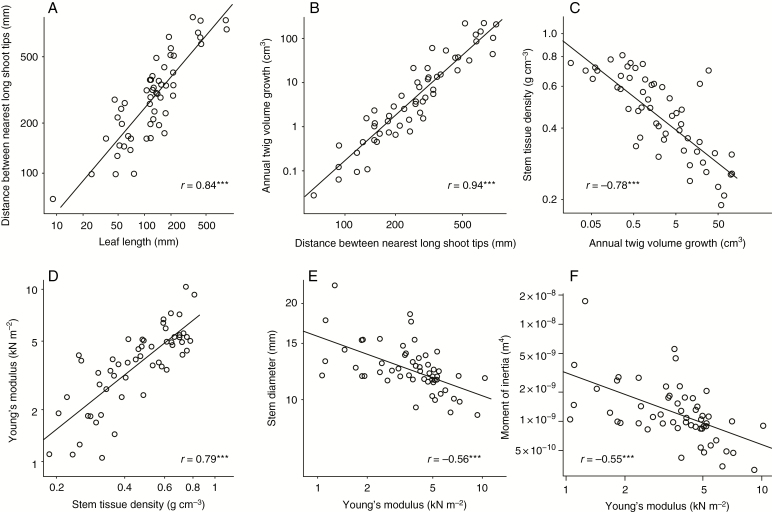
The Pearson correlation between log10-transformed leaf length and stem diameter was 0.43 (P < 0.001). All other variables were very closely associated with leaf length and stem diameter (Table 2). The variables hypothesized to be involved in the causal chain of Corner’s rules conformed with the predicted patterns of covariation. Leaf length was positively and strongly correlated with distance to nearest stem tips (r = 0.84, P < 0.001; Fig. 4A), which in turn was strongly and positively associated with annual terminal twig volume growth (r = 0.94, P < 0.001; Fig. 4B). Also, as predicted, species of faster growth had lower stem density (r = −0.78, P < 0.001; Fig. 4C), and species with lower stem density had more flexible stem tissues of lower Young’s modulus (r = 0.79, P < 0.001; Fig. 4D). Finally, stem tissue Young’s modulus was negatively correlated with stem diameter (r = −0.56, P < 0.001; Fig. 4E). All mediating variables in the causal chain were also strongly associated with one another (Table 2, Fig. 5). All correlations based on PICs showed the same patterns, having similar magnitudes and levels of significance as correlations based on raw data (Table 2). Correlations based on PICs had practically the same value for the three possible fully resolved phylogenetic trees.
Table 2.
Pearson correlations between traits (log10-transformed) involved in the causal chain of Corner’s rules
| Leaf length (mm) | Distance between stem tips (mm) | Annual terminal twig volume growth (mm3) | Stem tissue density (g cm−3) | Young’s modulus (N m−2) | Stem diameter (mm) | |
|---|---|---|---|---|---|---|
| Leaf length (mm) | 0.78*** | 0.69*** | −0.59*** | −0.48*** | 0.28* | |
| Distance between stem tips (mm) | 0.84*** | 0.91*** | −0.74*** | −0.56*** | 0.47*** | |
| Annual terminal twig volume growth (mm3) | 0.80*** | 0.94*** | −0.82*** | −0.67*** | 0.41** | |
| Stem tissue density (g cm−3) | −0.70*** | −0.74*** | −0.78*** | 0.81*** | −0.39** | |
| Young’s modulus (N m−2) |
−0.47*** | −0.47*** | −0.53*** | 0.79*** | −0.49*** | |
| Stem diameter (mm) |
0.43*** | 0.49*** | 0.47*** | −0.47*** | −0.56*** |
Correlations below the diagonal are based on raw data and those above the diagonal are based on phylogenetically independent contrasts.
*** P < 0.001, **P < 0.01, *P < 0.05.
Fig. 4.
Covariation of traits in the Corner’s rules causal chain. Congruent with the predictions in Fig. 2, we documented covariation between (A) distance between shoot tips and leaf length, (B) annual terminal twig volume growth and distance between shoot tips, (C) stem density and annual terminal twig volume growth, and (D) Young’s modulus and stem density. The negative relationships between stem diameter and Young’s modulus (E) and between the moment of inertia and Young’s modulus (F) illustrate the E–I trade-off, with thicker twigs being made up of more flexible tissues. Axes are log-scaled but labelled with the original values. ***P < 0.001, N = 55 species.
Fig. 5.
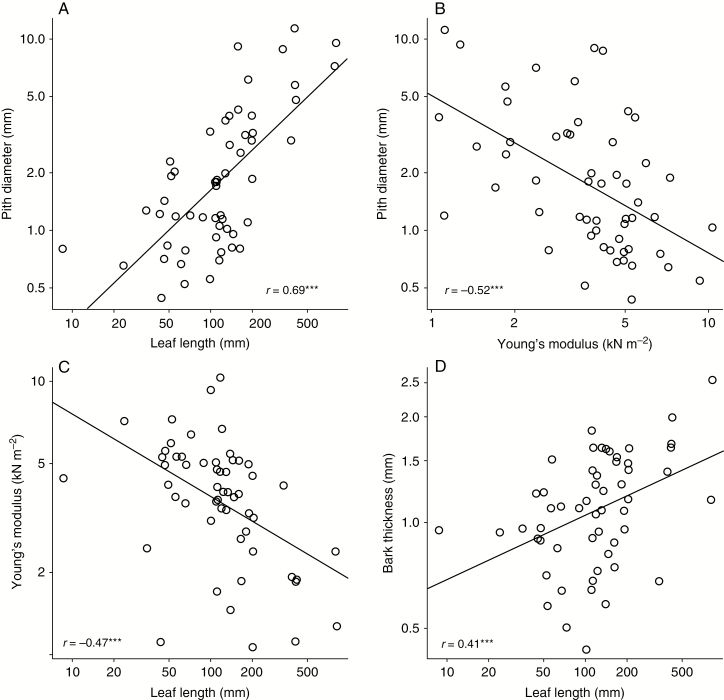
Corner’s rules and twig biomechanical characteristics. (A) Plants with large leaves have thick twigs that never have a solid vascular cylinder but instead always have wide piths, due to the E–I trade-off. (B) As part of this trade-off, the lower-density, more flexible tissues of fast-growing large-leaved plants must have higher moment of inertia I, achieved by deploying xylem and bark as a tube rather than as a solid cylinder, meaning that species with wider piths have predictably more flexible stem tissues. (C) Because of carbon limitation in the context of faster growth rates, large-leaved species have predictably more flexible stem tissues. (D) Also part of the E–I trade-off is that species with more flexible tissues must in compensation produce thicker structures to maintain mechanical rigidity. The bark is no exception, and species with large leaves, fast growth rates and flexible tissues have thicker bark. ***P < 0.001, N = 55 species.
The two structural equation models used to test the proposed causal chain for Corner’s rules fitted the data well. Both models shared the value of the χ2 test and of goodness-of-fit indices, given that they were based on the same correlation structure across variables. The structural equation models were not rejected by the data (χ2= 14.85, d.f. = 10, P = 0.138), suggesting that the proposed causal hypothesis is congruent with the observed covariation patterns between the traits in Fig. 2. The other criteria also suggested that both models fitted the data well. Although the RMSEA was 0.094, above the traditional cut-off of 0.05, the P-value of its associated test was 0.220, indicating that we cannot reject a value ≤0.05 for the RMSEA index, and thus a good fit of the model. Similarly, the SRMR was 0.059 (cut-off value = 0.080), the CFI was 0.984 and the NNFI was 0.976 (cut-off value for both indices = 0.950), all indicating good fit of the models. Structural equation modelling thus suggests that the hypothesis in Fig. 2 with causation flowing from leaf length to stem diameter, as well as that flowing from stem diameter to leaf length, is congruent with the data.
Parameter estimates for both structural equation models are shown in Table 3 and Fig. 2. All variables of the causal chain seem to have a similar importance within the models, as suggested by the similar magnitudes and statistical significances of the standardized coefficients (estimates within parentheses in Table 3). In both models, the standardized coefficient associated with distance from nearest long-shoot tips predicting annual terminal twig volume growth (and vice versa) was the highest (0.935; Table 3), but it was closely followed by most of the coefficients associated with the other variables. The standardized coefficient of Young’s modulus predicting stem diameter (and vice versa) was the smallest (−0.559), but was still high and highly significant (P < 0.001).
Table 3.
Structural equation models predicting stem diameter based on leaf length (Model LL → SD) and predicting leaf length based on stem diameter (Model SD → LL)1
| Model LL → SD | Slope ± s.e. | Intercept ± s.e. | Model SD → LL | Slope ± s.e. | Intercept ± s.e. |
|---|---|---|---|---|---|
| Distance between nearest long shoot tips – leaf length | 0.624 ± 0.139 (0.844 ± 0.187) |
1.111 ± 0.288 | Young’s modulus – stem diameter | −1.701 ± 0.518 (−0.559 ± 0.170) |
5.418 ± 0.562 |
| Annual terminal twig volume growth – distance between nearest long shoot tips | 3.454 ± 0.165 (0.935 ± 0.045) |
−4.689 ± 0.397 | Stem density – Young’s modulus | 0.593 ± 0.052 (0.793 ± 0.070) |
–2.457 ± 0.189 |
| Stem density – annual terminal twig volume growth | −0.137 ± 0.015 (−0.779 ± 0.083) |
0.145 ± 0.049 | Annual terminal twig volume growth – stem density | −4.420 ± 0.474 (−0.779 ± 0.083) |
2.057 ± 0.210 |
| Young’s modulus – stem density | 1.060 ± 0.122 (0.793 ± 0.091) |
3.926 ± 0.039 | Distance between nearest long shoot tips – Annual terminal twig volume growth | 0.253 ± 0.012 (0.935 ± 0.046) |
1.488 ± 0.045 |
| Stem diameter – Young’s modulus | −0.184 ± 0.047 (−0.559 ± 0.142) |
1.747 ± 0.169 | Leaf length – Distance between nearest long shoot tips |
1.141 ± 0.119 (0.844 ± 0.088) |
−0.671 ± 0.293 |
Standardized parameters are shown in parentheses.
1All estimates were significant (P < 0.005).
DISCUSSION
The causal hypothesis we present helps add important mechanistic detail to previous explanations for Corner’s rules. The hydraulic argument that large leaves require abundant water supply and therefore wide twigs is one example. Hydraulic arguments make no specification regarding the deployment of vascular tissue in the stem. A given leaf could be supplied from a solid vascular cylinder located centrally in the stem, but this is never the case. A pith is always present in seed plants (and in young stems of all plants, mechanically stiff tissues tend to be concentrated at the stem periphery), and the pith becomes wider, often becoming hollow, with increasing leaf size (we are aware of no counterexamples, and our data are no exception; r = 0.69, P < 0.001; Fig. 5A) (White, 1983a). We were unable to generate a plausible alternative causal hypothesis to that in Fig. 2 that linked hydraulic traits to pith dimensions. Only mechanical considerations appear able to provide an explanation for the presence of pith and its variation in diameter (Niklas, 1992, 1995; Ackerly, 1996; Hogan and Niklas, 2003). Pith diameter is predicted well by Young’s modulus E, with a negative relationship, reflecting a phenomenon known as the E–I trade-off.
The E–I trade-off refers to the relationship between the stiffness of the tissue making up a stem and the stem length–diameter relationship (Larjavaara and Muller-Landau, 2010). The product EI describes how well a given beam, here understood as a stem, resists bending under a given load. The E–I trade-off was readily observed in our dataset through the negative relationship between E and I (r = −0.55, P < 0.001; Fig. 4F). In Corner’s terms, fast-growing, large-leaved plants have tissues of lower density and therefore lower E. When E is low, in self-supporting plants selection appears always to favour maximal stem stiffness via higher I, given that the twigs of plants with tissues of low E have thick twigs with wide piths (Fig. 5A, B). This concentration of stiff material far from the centre of the stem is functionally very important because material very close to the centre of a beam contributes little to resistance to bending, whereas the capacity of material to resist bending increases with distance from the neutral axis to the fourth power (Gere and Timoshenko, 1999). As a result, the pith has very little capacity to contribute to resistance to bending (Niklas, 1995, 1999). This is certainly why pith in all but the narrowest stems is made up of very light parenchymatous tissue that has negligible E, or is even hollow (White, 1983a). Disregarding the pith, the bark + wood complex can be seen from the point of view of tube theory, the study of the mechanical behaviour of hollow cylinders. Tube theory shows that for a given E the resistance to bending of a hollow cylinder of constant material cross-sectional area rapidly increases as the inner diameter increases (Niklas, 1992; Hogan and Niklas, 2003). However, because the amount of material remains constant, as the inner diameter increases wall thickness begins to drop. For each E, a maximum is reached at which beam stiffness is maximal. Such a configuration necessarily entails a wider pith with decreasing E, and our data bear this out (r = −0.52, P < 0.001; Fig. 5A, B). This relationship explains the indirect relationship between wood density and stiffness and leaf size (Fig. 5C) (Swenson and Enquist, 2008). Our causal hypothesis, which explicitly includes the E–I trade-off, thus explains why twig diameter varies with leaf size, why stem tissues are more flexible in species with large leaves (r = −0.47, P < 0.001; Fig. 5C) and why pith diameter varies predictably across species (Fig. 5A, B).
Our causal hypothesis also helps explain why twig bark thickness varies with leaf size. Wood density is known to vary negatively with leaf size (Swenson and Enquist, 2008; Malhado et al., 2009). Bark density has been shown to correlate strongly with wood density (Rosell et al., 2014; Poorter et al., 2014). Because of the E–I trade-off, species with low-density wood have thick twigs with wide piths and relatively thick-walled xylem cylinders, as well as thick bark. In general, at any given site, the faster-growing species should have thicker bark of lower density and the slow-growing ones thin bark of higher density (Rosell et al., 2014). In this way, bark thickness is positively associated with leaf length (r = 0.41, P < 0.01; Fig. 5D).
Also in connection with leaf length, our hypothesis sheds light on apparent contradictions in the ecology of fast-growing pioneer species, which often have large leaves (Ackerly and Bazzaz, 1995a, b; Ackerly, 1996). One contradiction is that fast-growing species are usually pioneers of sunny gaps, but large leaves have thick boundary layers with high heat load. In such conditions, it would be thought that selection would favour small leaves (e.g. Horn, 1971; Parkhurst and Loucks, 1972). Fast-growing, early successional species rarely have small leaves (and those that do have moderately small ones often have plagiotropic lateral branches that act as large compound leaves, e.g. some Phyllanthus, Trema, etc.), a pattern accounted for by the hypothesis outlined here. Fast growth rates in the context of similar carbon supplies per unit crown area necessarily mean lower-density tissues. Lower-density tissues imply low E, requiring high I. Given a finite carbon supply, because twigs are necessarily thick they are also fewer, and therefore leaves must be relatively large in these fast-growing plants. Another potential paradox is that some authors expect large-leaved species to have stiffer tissues than those of small-leaved species as the result of selection favouring resistance of the greater associated mechanical loads (e.g. White, 1983a). However, the opposite is true, and species with large leaves always have tissues of low E (r = −0.47, P < 0.001; Fig. 5C) (Swenson and Enquist, 2008; Olson et al., 2009), a pattern that is explicable given the carbon scenario presented in Fig. 1.
Our hypothesis would seem to explain well why so many species lie along the Corner spectrum, and also why alternatives are rare, though not impossible. Our hypothesis posits that most of the Corner’s rules relationships are ones favoured by natural selection, not biophysical constraints that make alternatives developmentally impossible. This implies necessarily that heritable alternatives to the commonly observed configurations are possible (Olson, 2012a; Vasseur et al., 2012; Olson and Arroyo-Santos, 2015). In fact, likely deviations can be found in nature, but they are in more or less extreme situations (Niklas, 2004). For example, with their sclerophyllous and (usually) evergreen leaves, low-density stem tissues and slow growth rates, cycads and ponytail palms (Beaucarnea spp.) represent distinct exceptions to the typical Corner’s association of low stem density with fast growth rate that is so often associated with large, often deciduous leaves with low leaf mass per unit area (Méndez-Alonzo et al., 2012). The larger species of Didiereaceae of Malagasy dry forests seem candidates for examples of plants with low leaf area for their stem diameters. Some species, such as pendent-leaved epiphytic Anthurium spp. or Nephrolepis spp., seem to have much larger leaves than expected given their stem sizes. That alternatives to Corner’s ‘rules’ are possible underscores their likely adaptive nature. That is, alternatives are possible and even favoured in some situations, with the commonly observed relationships representing the generally favoured conditions.
Conclusions
Our data are congruent with hypotheses that posit that Corner’s rules involve selection favouring the avoidance of self-shading in the context of similar amounts of carbon being fixed per unit crown area regardless of leaf size (Olson et al., 2009; Smith et al., 2017). This means that plants of a given crown area will have similar amounts of carbon to allocate to the next iteration of growth. Selection favours wide distances between stem tips in the avoidance of leaf self-shading and mutual mechanical damage in the wind. At our seasonal tropical site, large-leaved species produced much greater terminal twig volume per unit time than small-leaved ones. Large-leaved plants, though, have less carbon available per unit stem volume, leading to the marked negative associations of both leaf size and growth rate with stem tissue density. The lower stiffness of less dense tissues, through the E–I trade-off, leads to species with large leaves having thick terminal twigs with thick bark and wide piths. Species with large leaves as a result have fast-elongating stems, tissues of low stiffness, and thick twigs with thick bark and wide piths, the central variables in the Corner’s rules and leaf size–twig size spectra. Our study thus emphasizes stem biomechanics and stem growth rate as important variables in explaining Corner’s rules, and that this pattern seems difficult to explain without the assumption of similar amounts of carbon being fixed per unit crown area regardless of leaf size.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: referenced version of Figure 2. Figure S2: phylogenetic relationships of the 55 sampled species.
ACKNOWLEDGEMENTS
This study was funded by Consejo Nacional de Ciencia y Tecnología projects 132404 and 237061, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, UNAM, project IA201415, and an MAB-Unesco award to J.A.R. We thank Gerardo Arévalo, Cipatli Jiménez, Calixto León, Enrique Ramírez, Katherine Renton and Jorge Vega for kind assistance. We thank Bill Shipley and two anonymous reviewers for their careful reading and their helpful suggestions.
LITERATURE CITED
- Ackerly DD. 1996. Canopy structure and dynamics: integration of growth processes in tropical pioneer trees. In: Mulkey SS, Chazdon RL, Smith AP, eds. Tropical forest plant ecophysiology. London: Chapman and Hall, 619–658. [Google Scholar]
- Ackerly DD, Bazzaz FA. 1995a. Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 101: 289–298. [DOI] [PubMed] [Google Scholar]
- Ackerly DD, Bazzaz FA. 1995b. Seedling crown orientation and interception of diffuse radiation in tropical forest gaps. Ecology 76: 1134–1146. [Google Scholar]
- Ackerly DD, Donoghue MJ. 1998. Leaf size, sapling allometry, and Corner’s rules: phylogeny and correlated evolution in maples (Acer). American Naturalist 152: 767–791. [DOI] [PubMed] [Google Scholar]
- Brouat C, Gibernau M, Amsellem L, McKey D. 1998. Corner’s rules revisited: ontogenetic and interspecific patterns in leaf-stem allometry. New Phytologist 139: 459–470. [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- Corner EJH. 1949. The Durian theory or the origin of the modern tree. Annals of Botany 13: 367–414. [Google Scholar]
- Duursma RA, Falster DS, Valladares F, et al. 2012. Light interception efficiency explained by two simple variables: a test using a diversity of small- to medium-sized woody plants. New Phytologist 193: 397–408. [DOI] [PubMed] [Google Scholar]
- Enquist BJ, Niklas KJ. 2002. Global allocation rules for patterns of biomass partitioning in seed plants. Science 295: 1517–1520. [DOI] [PubMed] [Google Scholar]
- Enquist BJ, West GB, Charnov EL, Brown JH. 1999. Allometric scaling of production and life-history variation in vascular plants. Nature 401: 907–911. [Google Scholar]
- Fajardo A. 2016. Are trait-scaling relationships invariant across contrasting elevations in the widely distributed treeline species Nothofagus pumilio?American Journal of Botany 103: 821–829. [DOI] [PubMed] [Google Scholar]
- Fan Z-X, Sterck F, Zhang S-B, Fu P-L, Hao G-Y. 2017. Tradeoff between stem hydraulic efficiency and mechanical strength affects leaf–stem allometry in 28 Ficus tree species. Frontiers in Plant Science 8. doi:10.3389/fpls.2017.01619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- Gere JM, Timoshenko SP. 1999. Mechanics of materials, 4th edn Cheltenham, UK: Nelson Thornes. [Google Scholar]
- Halloy SRP, Mark AF. 1996. Comparative leaf morphology spectra of plant communities in New Zealand, the Andes and the European Alps. Journal of the Royal Society of New Zealand 26: 41–78. [Google Scholar]
- Hogan CJ, Niklas KJ. 2003. On the economy and safety of hollow non-septate peduncles. American Journal of Botany 90: 356–363. [DOI] [PubMed] [Google Scholar]
- Horn HS. 1971. Adaptive geometry of trees. Princeton: Princeton University Press. [Google Scholar]
- Hu L, Bentler PM. 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling 6: 1–55. [Google Scholar]
- Huang Y, Lechowicz MJ, Price CA, Li L, Wang Y, Zhou D. 2016. The underlying basis for the trade-off between leaf size and leafing intensity. Functional Ecology 30: 199–205. [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR, et al. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Wright SJ. 2005. Variation in crown light utilization characteristics among tropical canopy trees. Annals of Botany 95: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. 2011. Principles and practice of structural equation modeling. New York: Guilford Press. [Google Scholar]
- Larjavaara M, Muller-Landau HC. 2010. Rethinking the value of high wood density. Functional Ecology 24: 701–705. [DOI] [PubMed] [Google Scholar]
- Leslie AB, Beaulieu JM, Crane PR, Donoghue MJ. 2014. Cone size is related to branching architecture in conifers. New Phytologist 203: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Lott E. 2002. Lista anotada de las plantas vasculares de Chamela-Cuixmala. In: Noguera FA, Vega-Rivera JA, García-Aldrete AN, Quesada-Avendaño M, eds. Historia natural de Chamela. Mexico City: Instituto de Biología, UNAM, 99–136. [Google Scholar]
- Malhado ACM, Malhi Y, Whittaker RJ, et al. 2009. Spatial trends in leaf size of Amazonian rainforest trees. Biogeosciences 6: 1563–1576. [Google Scholar]
- Méndez-Alonzo R, Paz H, Zuluaga RC, Rosell JA, Olson ME. 2012. Coordinated evolution of leaf and stem economics in tropical dry forest trees. Ecology 93: 2397–2406. [DOI] [PubMed] [Google Scholar]
- Méndez-Alonzo R, Pineda-García F, Paz H, Rosell JA, Olson ME. 2013. Leaf phenology is associated with soil water availability and xylem traits in a tropical dry forest. Trees 27: 745–754. [Google Scholar]
- Messier J, Lechowicz MJ, McGill BJ, Violle C, Enquist BJ. 2017. Interspecific integration of trait dimensions at local scales: the plant phenotype as an integrated network. Journal of Ecology 105: 1775–1790. [Google Scholar]
- Michaletz ST, Cheng D, Kerkhoff AJ, Enquist BJ. 2014. Convergence of terrestrial plant production across global climate gradients. Nature 512: 39–43. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago: University of Chicago Press. [Google Scholar]
- Niklas K. 1995. Plant height and the properties of some herbaceous stems. Annals of Botany 75: 133–142. [Google Scholar]
- Niklas KJ. 1999. The mechanical role of bark. American Journal of Botany 86: 465–469. [PubMed] [Google Scholar]
- Niklas KJ. 2004. Plant allometry: is there a grand unifying theory?Biological Reviews 79: 871–889. [DOI] [PubMed] [Google Scholar]
- Olson ME. 2012a. The developmental renaissance in adaptationism. Trends in Ecology & Evolution 27: 278–287. [DOI] [PubMed] [Google Scholar]
- Olson ME. 2012b. Linear trends in botanical systematics and the major trends of xylem evolution. Botanical Review 78: 154–183. [Google Scholar]
- Olson ME, Arroyo-Santos A. 2015. How to study adaptation (and why to do it that way). Quarterly Review of Biology 90: 167–191. [DOI] [PubMed] [Google Scholar]
- Olson ME, Aguirre-Hernández R, Rosell JA. 2009. Universal foliage-stem scaling across environments and species in dicot trees: plasticity, biomechanics and Corner’s rules. Ecology Letters 12: 210–219. [DOI] [PubMed] [Google Scholar]
- Parkhurst DF, Loucks OL. 1972. Optimal leaf size in relation to environment. Journal of Ecology 60: 505. [Google Scholar]
- Poorter L, McNeil A, Hurtado V-H, Prins HHT, Putz FE. 2014. Bark traits and life-history strategies of tropical dry- and moist forest trees. Functional Ecology 28: 232–242. [Google Scholar]
- Read C, Wright IJ, Westoby M. 2006. Scaling-up from leaf to canopy-aggregate properties in sclerophyll shrub species. Austral Ecology 31: 310–316. [Google Scholar]
- Rosell JA, Olson ME. 2014. The evolution of bark mechanics and storage across habitats in a clade of tropical trees. American Journal of Botany 101: 764–777. [DOI] [PubMed] [Google Scholar]
- Rosell JA, Gleason S, Méndez-Alonzo R, Chang Y, Westoby M. 2014. Bark functional ecology: evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytologist 201: 486–497. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. 2012. lavaan: an R package for structural equation modeling. Journal of Statistical Software 48. doi:10.18637/jss.v048.i02 [Google Scholar]
- Selaya NG, Anten NPR. 2010. Leaves of pioneer and later-successional trees have similar lifetime carbon gain in tropical secondary forest. Ecology 91: 1102–1113. [DOI] [PubMed] [Google Scholar]
- Shipley B. 2004. Cause and correlation in biology: a user’s guide to path analysis, structural equations and causal inference. Cambridge: Cambridge University Press. [Google Scholar]
- Smith DD, Sperry JS, Adler FR. 2017. Convergence in leaf size versus twig leaf area scaling: do plants optimize leaf area partitioning?Annals of Botany 119: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson NL, Das AJ, Condit R, et al. 2014. Rate of tree carbon accumulation increases continuously with tree size. Nature 507: 90–93. [DOI] [PubMed] [Google Scholar]
- Sterck FJ, Van Gelder HA, Poorter L. 2006. Mechanical branch constraints contribute to life-history variation across tree species in a Bolivian forest. Journal of Ecology 94: 1192–1200. [Google Scholar]
- Stevens PF. 2001. onwards. Angiosperm phylogeny website. Version 14, July 2017 [and more or less continuously updated since]. http://www.mobot.org/MOBOT/research/APweb/
- Sun S, Jin D, Shi P. 2006. The leaf size–twig size spectrum of temperate woody species along an altitudinal gradient: an invariant allometric scaling relationship. Annals of Botany 97: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson NG, Enquist BJ. 2008. The relationship between stem and branch wood specific gravity and the ability of each measure to predict leaf area. American Journal of Botany 95: 516–519. [DOI] [PubMed] [Google Scholar]
- Trueba S, Isnard S, Barthélémy D, Olson ME. 2016. Trait coordination, mechanical behaviour and growth form plasticity of Amborella trichopoda under variation in canopy openness. AoB Plants 8: plw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Snyderman DA, Wilmot TR, Machado JL. 1991. Water relations and hydraulic architecture of a tropical tree (Schefflera morototoni): data, models, and a comparison with two temperate species (Acer saccharum and Thuja occidentalis). Plant Physiology 96: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Skillman JB, Pearcy RW. 2002. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. American Journal of Botany 89: 1275–1284. [DOI] [PubMed] [Google Scholar]
- Vasseur F, Violle C, Enquist BJ, Granier C, Vile D. 2012. A common genetic basis to the origin of the leaf economics spectrum and metabolic scaling allometry. Ecology Letters 15: 1149–1157. [DOI] [PubMed] [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. 2008. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24: 2098–2100. [DOI] [PubMed] [Google Scholar]
- West GB, Brown JH, Enquist BJ. 1997. A general model for the origin of allometric scaling laws in biology. Science 276: 122–126. [DOI] [PubMed] [Google Scholar]
- West GB, Brown JH, Enquist BJ. 1999. A general model for the structure and allometry of plant vascular systems. Nature 400: 664–667. [Google Scholar]
- Westoby M, Wright IJ. 2003. The leaf size–twig size spectrum and its relationship to other important spectra of variation among species. Oecologia 135: 621–628. [DOI] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- White PS. 1983a. Corner’s rules in eastern deciduous trees: allometry and its implications for the adaptive architecture of trees. Bulletin of the Torrey Botanical Club 110: 203–212. [Google Scholar]
- White PS. 1983b. Evidence that temperate East North American evergreen woody plants follow Corner’s rules. New Phytologist 95: 139–145. [Google Scholar]
- Wikstrom N, Savolainen V, Chase MW. 2001. Evolution of the angiosperms: calibrating the family tree. Proceedings of the Royal Society B: Biological Sciences 268: 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson GB, Wiemann MC. 2010. Measuring wood specific gravity ... correctly. American Journal of Botany 97: 519–524. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Falster DS, Pickup M, Westoby M. 2006. Cross-species patterns in the coordination between leaf and stem traits, and their implications for plant hydraulics. Physiologia Plantarum 127: 445–456. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.