Abstract
Malaria is one of the most severe health problems facing the world today. Until the mid-twentieth century, Europe was an endemic area of malaria, with the Balkan countries being heavily infested. Sibling species belonging to the Anopheles maculipennis complex are well-known as effective vectors of Plasmodium in Europe. A vast number of human malaria cases in the past in the former Yugoslavia territory have stressed the significance of An. maculipennis complex species as primary and secondary vectors. Therefore, the present study evaluates the species composition, geographic distribution and abundance of these malaria vector species. Mosquitoes were collected in the northern Serbian province of Vojvodina and analysed by PCR-RFLP, multiplex PCR and sequencing of the ITS2 intron of genomic rDNA. Four sibling species of the An. maculipennis complex were identified. Both larvae and adults of the recently described species An. daciae were identified for the first time in Serbia. In 250 larval samples, 109 (44%) An. messeae, 90 (36%) An. maculipennis s.s., 33 (13%) An. daciae and 18 (7%) An. atroparvus were identified. In adult collections, 81 (47%) An. messeae, 55 (32%) An. daciae, 33 (19%) An. maculipennis s.s., and 3 (2%) An. atroparvus were recorded. The most abundant species in Vojvodina was An. messeae, whereas An. atroparvus was confirmed a rare species in all parts. Since this species is a potentially, highly competent malarial vector, low population density could be crucial to prevent a new establishment of endemic malaria transmission in Serbia.
Keywords: Anopheles maculipennis complex, Malaria, Anopheles daciae, ITS2
Introduction
The discovery that mosquitoes can transmit microfilariae and malarial protozoa at the end of the nineteenth century initiated the collection, naming and classification of Anopheles species after the genus was introduced by Johann Wilhelm Meigen in 1818. Anopheles maculipennis was first recognised by van Thiel (1927) to be a complex of sibling species or races, since larvae, pupae and adults are mostly indistinguishable from each other by morphological characters. Eleven species of the An. maculipennis complex (AMC) are formally considered in the Palaearctic region: Anopheles artemievi Gordeyev, Zvantsov, Goryacheva, Shaikevich and Yezhov, 2005; Anopheles atroparvus van Thiel, 1927; Anopheles beklemishevi Stegnii & Kabanova (1976); Anopheles daciae Linton, Nicolescu and Harbach, 2004; Anopheles labranchiae Falleroni, 1926; Anopheles maculipennis s.s. Meigen, 1818; Anopheles martinius Shinagarev, 1926; Anopheles melanoon Hackett, 1934; Anopheles messeae Falleroni, 1926, Anopheles persiensis Linton, Sedaghat and Harbach, 2003 and Anopheles sacharovi Favre, 1903 (Harbach 2004; Harbach 2015; Linton et al. 2007; White 1978).
At the territory of Serbia following AMC, species have been recorded so far: An. atroparvus, An. labranchiae, An. maculipennis s.s., An. melanoon, An. messeae and An. sacharovi, of which An. atroparvus, An. maculipennis s.s., An. melanoon and An. messeae are present in Vojvodina Province (north Serbia), formerly an area of widespread endemic malaria (Zgomba et al. 2002; Kostić 1946).
Malaria was a widespread disease in Europe until the second half of the twentieth century. Historic and also current endemic infections in Europe, particularly transmitted by An. maculipennis complex species, have been caused regularly by Plasmodium vivax, whose sporozoites readily develop also in temperate climates (Marí and Peydró 2012). However, there has been a substantial number of imported tropical malaria (P. falciparum) which accounts for about 77% of tropical disease cases in Europe (65,596 infections reported between 2000 and 2009, TropNetEurop 2010).
The high density of Anopheles species in many southern European regions (Romi et al. 1997; Ponçon et al. 2007; Marí and Peydró 2010) and the increasing importation of malaria infections in the last two decades have led to the reappearance of autochthonous malaria cases in Italy (Baldari et al. 1998), Greece (Kampen et al. 2002), France (Doudier et al. 2007) and Spain (Santa-Olalla Peralta et al. 2010). As a consequence of mass immigration and travel from malaria-endemic countries to Greece, 85 human malaria cases were recorded in 2015 (six locally acquired), 88 in 2016 (five locally acquired) and 75 in 2017 (five locally acquired) (HCDCP 2015, 2016, 2017). Recently, The Netherlands and France have reported malaria in the patients without any previous travel history (ECDC 2014, 2015).
After the discovery of An. daciae (Nicolescu et al. 2004), the new member of AMC, identification of complex members is based on the nucleotide sequence differentiation of the internal transcribed spacer 2 (ITS2) region of genomic rDNA genes. Sequence analyses reduced probable misidentification of AMC species, due to overlapping egg characters, in different geographic regions. Since then, the species of AMC were identified by ITS2 analyses in many countries: England (Linton et al. 2002; Danabalan et al. 2013), Greece (Linton et al. 2001; Patsoula et al. 2007), Germany (Proft et al. 1999; Weitzel et al. 2012; Kronefeld et al. 2012; Kronefeld et al. 2014), Iran (Sedaghat et al. 2003), Italy (Marinucci et al. 1999; Di Luca et al. 2004), Poland (Rydzanicz et al. 2017), Romania (Nicolescu et al. 2004) and Turkey (Simsek et al. 2011), but not in Serbia. Our study applies ITS2 sequence analyses for molecular An. maculipennis complex identification.
Serbia’s Anopheles fauna has been studied based on morphological characteristics of adults and eggs (Adamovic 1975a, b, c, 1982, 1983; Dakic et al. 2008). The main vector of malaria in Serbia (in the Belgrade region) was An. maculipennis s.s. Secondary vectors were An. messeae and An. atroparvus (Simic 1948; Vukasovic 1950; Sitar 1977). Anopheles maculipennis s.s. was the predominant anopheline mosquito in the hilly areas of the Vojvodina province (Adamovic 1982), while An. messeae was by far the most abundant in the villages near marshes in the alluvial plain of the Danube, Sava and Tisa rivers. A similar species distribution in comparable landscapes was found in Hungary, Romania and Germany (Weyer 1938).
Species records of An. messeae prior to 2003 most likely comprised An. daciae, the most similar sibling species. Considering the species identification according to egg shape and colouration, differences between An. messae and An. daciae are minor and statistically insignificant (i.e. to be outside the range of natural phenotypic variation within a species) (Nicolescu et al. 2004; Kronefeld et al. 2012; Jetten and Takken 1994; Hackett et al. 1932). Therefore, morphological identification methods cannot be considered as reliable.
Consequently, historical data about the distribution, ecology and malaria vector potential of An. messeae could be imprecise. According to recent studies, the ecological demands and the spatial distribution of both species seem to be widely overlapping (Weitzel et al. 2012; Danabalan et al. 2013; Kronefeld et al. 2014; Lühken et al. 2016). An. atroparvus was found as a predominant species in the areas of alkaline soils in East Vojvodina, particularly in the lowlands of the Tisa and Tamis rivers (Adamovic 1980).
The discovery of An. daciae in neighbouring Romania and Greece indicates the potential occurrence of the species in Serbia. Accordingly, studies on ecological characters such as primarily host and breeding site preferences could refer to both species to an unknown extent so far.
This study aims to assess the species occurrence, geographical distribution and abundance of An. maculipennis complex species and the degree of overlap of breeding site preferences of larval populations in light of the potential occurrence of An. daciae in northern Serbia. This is the first study in Serbia in which molecular identification was used to separate the species of An. maculipennis complex.
Material and method
Study area and mosquito collection
Mosquitoes were collected in the northern Serbian province Vojvodina, located in the lowest part of the Pannonian Plain. It has a total surface area of 21,500 km2, which accounts for 24% of Serbia’s territory. The mountains surrounding this lowland, mainly Fruska Gora (539 m) and Vrsac Hill (641 m), have a significant impact on its climate characteristics.
Vojvodina is rich in fertile loamy loess soil, brown forest soil with patches of alkaline soil and black hydromorphic mineral soil, which is periodically flooded by the Danube river and its tributaries. Alkaline soils were formed in shallow depressions of this area.
The climate of Vojvodina is moderate continental, with cold winters and hot and humid summers with a huge range of extreme temperatures and featuring inconsistent amounts of rainfall over the course of months, which led to different values of aridity types. The mean annual temperature is 11 °C, and the mean annual precipitation is 602 mm (Mihailovic et al. 2004).
The most capacious anopheline breeding sites are extensive oxbow marshes, swamps and old riverbeds in the alluvial plains of the rivers Sava, Tisa and Danube. They are characterised by permanently stagnant, fresh water and dense hydrophilic vegetation.
Mosquitoes were collected from the 30th of May until the 13th of August 2015. Adults were collected biweekly and larvae monthly. An. maculipennis complex species were separated through the morphological identification of all specimens (Becker et al. 2010; Schaffner et al. 2001).
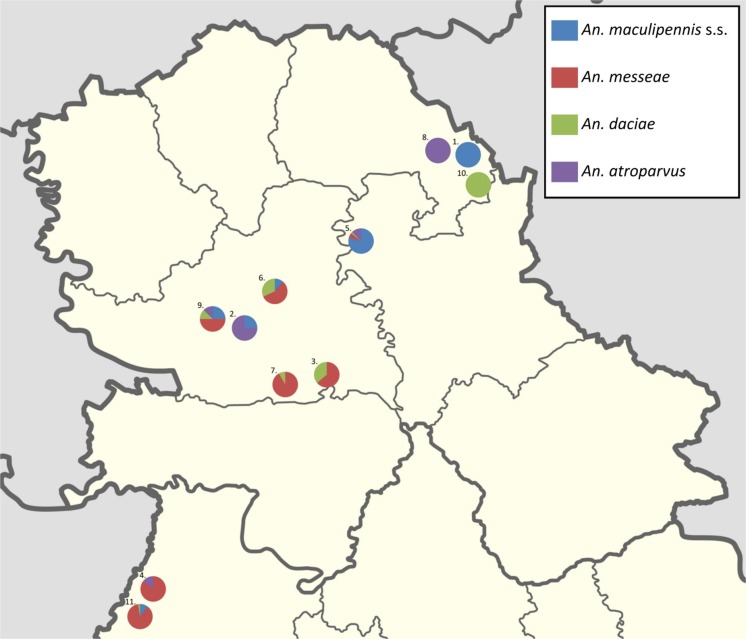
Larvae were sampled at 11 localities in rural and semi urban areas. In majority localities from one breeding site, but, at three of 11 localities, samples were taken from two breeding sites (Fig. 1, Table 1). Different types of larval breeding sites were chosen, as ditches, ponds, forest path filled with rainwater, swamps and marshes. The main criterion for the selection of sampling sites was the ecological diversity, aiming to determine the most attractive or typical for different An. maculipennis complex species. Larvae were collected by WHO dippers (350 ml volume); each sample consisted of ten dipps/breeding site. Larvae of L2, L3 and L4 instars were kept in 70% ethanol until molecular identification. Types and geographical coordinates of breeding sites, and species association indices are presented in Tables 1 and 2.
Fig. 1.
Records of An. maculipennis complex larvae in Vojvodina, Serbia
Table 1.
Sampling locations and breeding sites in Vojvodina and respective species composition of 250 larval samples
| No. | Locality | Geocoordinates | Breeding site | mac 1 | mess 2 | dac 3 | atr 4 | ∑ |
|---|---|---|---|---|---|---|---|---|
| 1 | Banatsko Veliko S. | 45° 48′ 56″ N 20° 36′ 32″ E | Artificial lake | 1 | 0 | 0 | 0 | 1 |
| 2 | Kisač | 45° 21′ 31″ N 19° 43′ 43″ E | Ditch | 1 | 0 | 0 | 3 | 4 |
| 3 | Kovilj | 45° 13′ 28″ N 20° 1′ 9″ E | Ditch | 0 | 23 | 13 | 0 | 36 |
| 4 | Lešnica | 44° 38′ 46″ N 19° 17′ 39″ E | Ditch | 0 | 6 | 0 | 1 | 7 |
| 5 | Novi Bečej | 45° 35′ 40″ N 20° 8′ 33″ E | Ditch | 42 | 6 | 0 | 4 | 52 |
| 5 | Novi Bečej | 45° 35′ 50″ N 20° 8′ 28″ E | Ditch | 35 | 0 | 2 | 8 | 45 |
| 6 | Sirig | 45° 26′ 40″ N 19° 49′ 8″ E | Ditch | 5 | 25 | 14 | 0 | 44 |
| 7 | Petrovaradin | 45° 15′ 16″ N 19° 53′ 35″ E | Forest path | 0 | 11 | 1 | 0 | 12 |
| 8 | Kikinda | 45° 48′ 11″ N 20° 26′ 56″ E | Marsh | 0 | 0 | 0 | 1 | 1 |
| 9 | Bački Petrovac | 45° 22′ 27″ N 19° 35′ 13″ E | Pond | 0 | 1 | 0 | 0 | 1 |
| 10 | Kozarci | 45° 46′ 51″ N 20° 36′ 38″ E | Pond | 0 | 0 | 1 | 0 | 1 |
| 11 | Lipnički Šor | 44° 35′ 41″ N 19° 14′ 1″ E | Pond | 4 | 32 | 1 | 0 | 37 |
| 11 | Lipnički Šor | 44° 36′ 18″ N 19° 13′ 43″ E | Pond | 0 | 2 | 0 | 0 | 2 |
| 9 | Bački Petrovac | 45° 21′ 6″ N 19° 35′ 41″ E | Swamp | 2 | 3 | 1 | 1 | 7 |
| Total | 90 | 109 | 33 | 18 | 250 | |||
mac1 An. maculipennis s.s., mess2 An. messeae, dac3 An. daciae, atr4 An. atroparvus
Table 2.
Species association indices (Dice 1945) based on the species occurrence in the eight most productive breeding sites
| An. maculipennis s.s. | An. messeae | An. daciae | An. atroparvus | |
|---|---|---|---|---|
| An. maculipennis s.s. | 0.57 | 0.67 | 0.75 | |
| An. messeae | 0.80 | 0.83 | 0.75 | |
| An. daciae | 0.80 | 0.71 | 0.50 | |
| An. atroparvus | 0.60 | 0.43 | 0.33 | |
| Breeding sites positive | 5 | 7 | 6 | 4 |
| Breeding site index | 0.63 | 0.88 | 0.75 | 0.50 |
| Avg. no. of Anopheles species per site | 2.20 | 1.71 | 1.83 | 2.00 |
Two reciprocal values above and below the diagonal are obtained. Below, the number of positive breeding sites for the respective species, the breeding site index and the average number of Anopheles species in the respective breeding sites are quantified
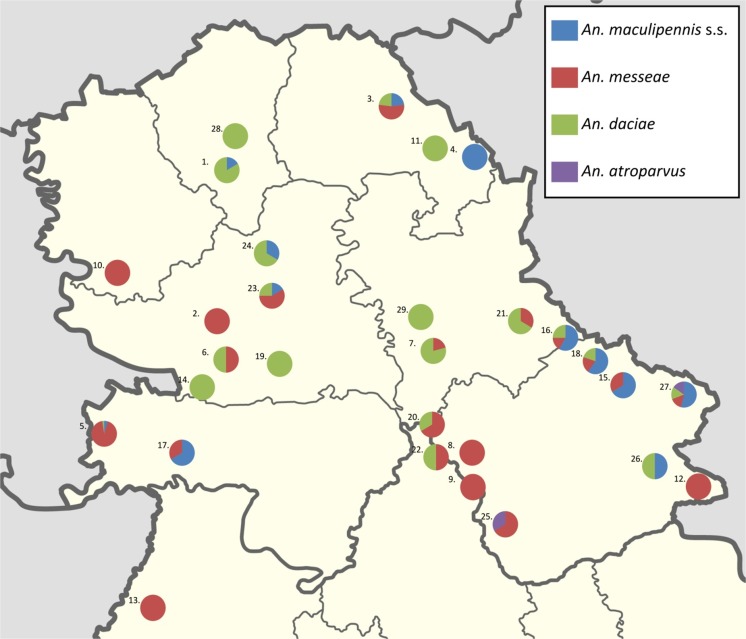
Adults were collected at 29 different urban and rural localities (Fig. 2, Table 3). The majority of localities (24) had only one collecting place. However, four localities had one additional collecting site and one location had three collecting sites. Adults were collected by CDC CO2 traps filled with dry ice (John W. Hock Company, Florida). Most of the traps were positioned near domestic animal stables, operating overnight. Traps were set up in the late afternoon and were collected in the morning. After morphological identification to the species complex level (Becker et al. 2010), all adults were conserved in vials containing 70% ethanol for molecular analysis.
Fig. 2.
Distribution of the An. maculipennis complex adults in Vojvodina, Serbia
Table 3.
Sampling locations of adult trap collections in Vojvodina and respective species composition of 172 adult samples
| Site no. | Locality | Collection place | Geocoordinates | mac 1 | mess 2 | dac 3 | atr 4 | ∑ Total |
|---|---|---|---|---|---|---|---|---|
| 1 | Bačka Topola | Home garden | 45° 49′ 33.77″ N 19° 37′ 57.41″ E | 1 | 0 | 5 | 0 | 6 |
| 2 | Bački Petrovac | Next to the pond | 45° 21′ 5.48″ N 19° 35′ 41.47″ E | 0 | 1 | 0 | 0 | 1 |
| 3 | Banatski Monoštor | Henhouse | 45° 57′ 25.78″ N 20° 16′ 41.16″ E | 3 | 7 | 3 | 0 | 13 |
| 4 | Banatsko Velikoselo | Pigsties, dog | 45° 48′ 55.72″ N 20° 36′ 33.33″ E | 2 | 0 | 0 | 0 | 2 |
| 5 | Batrovci | Forest | 45° 2′ 46.57″ N 19° 6′ 35.27″ E | 2 | 39 | 1 | 0 | 42 |
| 6 | Begeč | Home garden | 45° 14′ 4.30″ N 19° 37′ 25.23″ E | 0 | 0 | 2 | 0 | 2 |
| Begeč | Home garden | 45° 14′ 0.55″ N 19° 37′ 39.48″ E | 0 | 2 | 0 | 0 | 2 | |
| 7 | Ečka–Lukino Selo | Home garden | 45° 18′ 39.55″ N 20° 25′ 23.01″ E | 0 | 5 | 19 | 0 | 24 |
| 8 | Glogonj | Home garden | 44° 58′ 53.98″ N 20° 32′ 0.59″ E | 0 | 1 | 0 | 0 | 1 |
| 9 | Jabuka | Horse barn | 44° 57′ 19.27″ N 20° 37′ 56.57″ E | 0 | 1 | 0 | 0 | 1 |
| Jabuka | Home garden | 44° 56′ 51.67″ N 20° 35′ 6.24″ E | 0 | 1 | 0 | 0 | 1 | |
| 10 | Karavukovo | Home garden | 45° 30′ 21.42″ N 19° 11′ 20.40″ E | 0 | 1 | 0 | 0 | 1 |
| 11 | Kikinda | Next to the pond | 45° 48′ 8.99″ N 20° 26′ 50.36″ E | 0 | 0 | 1 | 0 | 1 |
| 12 | Kruščica | Henhouse | 44° 55′ 22.84″ N 21° 27′ 58.91″ E | 0 | 1 | 0 | 0 | 1 |
| 13 | Lipnički Šor | Next to the pond | 44° 35′ 41.80″ N 19° 14′ 0.11″ E | 0 | 1 | 0 | 0 | 1 |
| 14 | Lug | Henhouse | 45° 11′ 15.26″ N 19° 32′ 35.07″ E | 0 | 0 | 1 | 0 | 1 |
| 15 | Margita | Henhouse | 45° 12′ 52.25″ N 21° 10′ 42.36″ E | 2 | 1 | 0 | 0 | 3 |
| 16 | Markovićevo | Henhouse, dog | 45° 19′ 29.62″ N 21° 1′ 59.76″ E | 7 | 2 | 3 | 0 | 12 |
| 17 | Martinci | Hen/goat/pigs house | 45° 0′ 46.31″ N 19° 26′ 44.60″ E | 2 | 1 | 0 | 0 | 3 |
| 18 | Miletićevo | Goat pens, hens | 45° 51′ 28.03″ N 19° 12′ 39.85″ E | 0 | 1 | 0 | 0 | 1 |
| Miletićevo | Henhouse | 45° 18′ 13.71″ N 21° 3′ 34.87″ E | 3 | 0 | 1 | 0 | 4 | |
| 19 | Novi Sad | Home garden | 45° 15′ 16.08″ N 19° 48′ 55.95″ E | 0 | 0 | 1 | 0 | 1 |
| 20 | Opovo | Home garden | 45° 3′ 12.18″ N 20° 25′ 4.26″ E | 0 | 1 | 0 | 0 | 1 |
| Opovo | Home garden | 45° 0′ 59.41″ N 20° 28′ 21.58″ E | 0 | 1 | 0 | 0 | 1 | |
| Opovo | Home garden | 45° 3′ 11.98″ N 20° 25′ 4.60″ E | 0 | 0 | 1 | 0 | 1 | |
| 21 | Sečanj | Henhouse | 45° 21′ 39.19″ N 20° 46′ 20.50″ E | 0 | 1 | 0 | 0 | 1 |
| Sečanj | Henhouse | 45° 21′ 59.92″ N 20° 46′ 21.04″ E | 0 | 1 | 4 | 0 | 5 | |
| 22 | Sefkerin | Home garden | 44° 59′ 4.64″ N 20° 31′ 18.69″ E | 0 | 1 | 1 | 0 | 2 |
| 23 | Sirig | Home garden | 45° 26′ 41.82″ N 19° 48′ 58.92″ E | 2 | 7 | 3 | 0 | 12 |
| 24 | Srbobran | Home garden | 45° 32′ 52.69″ N 19° 47′ 31.64″ E | 1 | 0 | 2 | 0 | 3 |
| 25 | Starčevo | Home garden | 44° 49′ 14.67″ N 20° 43′ 40.75″ E | 0 | 2 | 0 | 1 | 3 |
| 26 | Straža | Henhouse, dog | 44° 58′ 42.79″ N 21° 18′ 10.81″ E | 1 | 0 | 1 | 0 | 2 |
| 27 | Veliko Središte | Henhouse, rabbits | 45° 11′ 48.49″ N 21° 23′ 44.01″ E | 7 | 2 | 2 | 2 | 13 |
| 28 | Zobnatica | Forest | 45° 52′ 0.38″ N 19° 38′ 1.50″ E | 0 | 0 | 3 | 0 | 3 |
| 29 | Zrenjanin | Home garden | 45° 21′ 33.00″ N 20° 23′ 29.77″ E | 0 | 0 | 1 | 0 | 1 |
| Total | 33 | 81 | 55 | 3 | 172 | |||
mac1 An. maculipennis s.s., mess2 An. messeae, dac3 An. daciae, atr4: An. atroparvus
DNA extraction and analyses
DNA was extracted individually from 422 mosquito samples using QuickExtract DNA Extraction Solution 1.0 (Biozym, Germany). According to the producer’s protocol, whole larvae or adults were homogenised using a pestle in a reaction tube before QuickExtract™ DNA Extraction Solution 1.0 was added. The volume of extract solution was 50 μL per adult and the same volume was used for the L3 and L4 larval stages. Lower amounts of solutions (25 μL) were used for 2nd instar larvae, respectively.
Smashed tissue was vortexed for 15 s, centrifuged for 1 min, incubated for 6 min at temp 65 °C and then vortexed again for 15 s, centrifuged for 1 min, incubated for 2 min at temp 98 °C and centrifuged for 1 min. Samples were stored in a freezer at − 20 °C until processed.
Standard PCR was carried out as described (Linton et al. 2001), utilising 5.8SF (5′-ATC ACT CGG CTC GTG GAT CG-3′) and 28SR primers (5′-ATG CTT AAA TTT AGG GGG TAG TC-3′) (VBC Biotech, Vienna, Austria) (Collins and Paskewitz 1996; Danabalan et al. 2013). PCR products were purified (PCR Clean Up extraction kit, GeneON, Germany) and then separated by 3% agarose gel electrophoresis (high resolution agarose, Roth, Germany), stained with Gelstar (Lonza, USA) and sized with Quantitas DNA low ladder (Biozym, Germany).
PCR products were further analysed by RFLP (BstUI, New England BioLabs, Germany) (Danabalan et al. 2013). Different numbers of BstUI recognition sites based on the diagnostic nucleotide sequence differences in the respective ITS2 regions permit the identification of An. daciae, An. messeae/maculipennis s.s. and An. atroparvus by diagnostic size and the number of fragments by 3% agarose gel electrophoresis (Danabalan et al. 2013).
Additionally, multiplex PCR (Kronefeld et al. 2014) was used to confirm the reliability of the PCR-RFLP assay for the differentiation of An. messeae from An. maculipennis s.s. The DNA of several samples was sequenced by Eurofins Medigenomix GmbH (Germany). DNA alignments were performed with CLC sequence viewer (CLC bio, Denmark; http://www.clcbio.com/products/clc-sequence-viewer) and BLAST (https://www.ebi.ac.uk/Tools/sss/ncbiblast/nucleotide.html).
Following the obtained results, species association indices were calculated by Dice (1945), based on the species occurrence in the eight most productive breeding sites (Table 2). Species association indices quantify the proportion of places where two species were found in combination compared to the total number of places where one of the respective species was found. From species association indices, the extent of overlapping demands on the biotic and abiotic structure of breeding sites may be estimated. Generally, more abundant and ubiquitous species may be found more frequently associated to others (Dice 1945).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
In 24 larval samples, 250 of them were successfully identified by molecular methods. Trap collections provided 47 samples with 172 adult specimens that were all morphologically identified to the species complex level and were analysed by PCR. Altogether, 422 specimens were successfully determined to the species level.
Larvae and adults of An. daciae were recorded for the first time in Serbia at 25 different locations in the Vojvodina province (Table 1, Table 3). In addition, three other An. maculipennis complex species were identified in different areas of the province: An. maculipennis s.s., An. messeae and An. atroparvus (Table 1, Table 3). Single-species records (diagonal) and species-combined records (above the diagonal) at 29 localities in 47 adult trap collections are presented in Table 4.
Table 4.
Single-species records (diagonal) and species-combined records (above the diagonal) at 29 geographical locations in 47 adult trap collections
| An. maculipennis s.s. | An. messeae | An. daciae | An. atroparvus | |
|---|---|---|---|---|
| An. maculipennis s.s. | 0 | 8 | 9 | 1 |
| An. messeae | 7 | 11 | 2 | |
| An. daciae | 4 | 1 | ||
| An. atroparvus | 0 |
Larval populations
In the collections of 250 larval specimens 109 (44%) An. messeae, 90 (36%) An. maculipennis s.s., 33 (13%) An. daciae and 18 (7%) An. atroparvus were identified (Table 1). An. messeae and An. maculipennis s.s. were the predominant in all larval samples from 14 breeding sites and 11 geographic locations in Vojvodina (Table 1). Also, An. daciae was found widespread in various breeding sites but less numerous. An. atroparvus was least abundant and mostly found associated with other sibling species (Table 2).
Each of the identified species was found in a variety of breeding sites at different geographical places across the region of Vojvodina.
Larvae of all species were found in each month during the sampling interval from the beginning of June to the middle of August 2015.
The distribution of all species within the complex was not only geographically overlapping, but the larvae of each species were found associated with other complex species in those breeding sites where numerous larvae could be collected (Table 2). An. messeae and An. daciae were very much connected to each other, and An. maculipennis s.s. was most universal, but was quantitatively dominant in ditches.
Adult populations
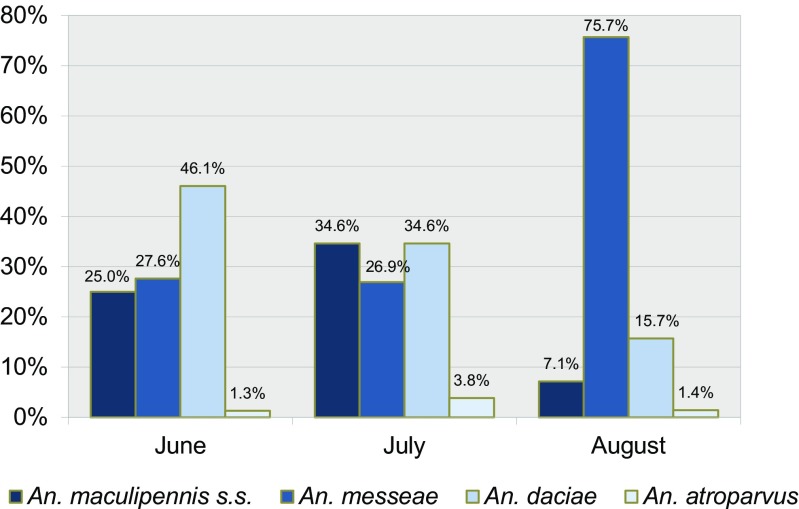
In 47 trap collections of females at 29 locations, 81 An. messeae, 55 An. daciae, 33 An. maculipennis s.s. and 3 An. atroparvus were determined (Table 3). The average number of specimens per trap/night was 3.2 in June, 3.3 in July and 4.7 in August (Fig. 3).
Fig. 3.
Species proportions of 47 adult samplings by CO2 traps during summer in the region Vojvodina, Serbia
Females of An. messeae were most widely distributed, found at 22 of 35 (66.7%) collecting places. The largest collection in one trap, 39 females/trap/night was also made for An. messeae in the forest region of Batrovci, indicating a considerable abundance. An. daciae was caught at 19 (57.6%) collecting sites and was the most abundant in Ecka–Lukino Selo, Banat village, close to Romania, where 13 females per trap/night were caught. An. maculipennis s.s. females were present in 14 (42.4%) collecting places by low numbers in various collections, whereas females of An. atroparvus were only present at two localities, Veliko Srediste and Starcevo village, with each one specimen per trap/night.
Females of An. maculipennis s.s. and An. messeae were included already in the first collections in May, whereas An. daciae and An. atroparvus were recorded in the traps in June (Fig. 3).
Discussion
The study of various larval breeding sites and CO2-trap collections in the geographic region of Vojvodina, Serbia, gave a comprehensive insight into the species abundance in different areas, which were heavily infested by malaria until the mid-twentieth century. The recognition of An. daciae at various places and its occurrence in combination with other complex species complements and completes previous studies on the species composition and distribution of the An. maculipennis complex in Serbia. The finding of a wide overlap of breeding site specificity, particularly of An. messeae and An. daciae, supports the assumption that both species had previously been admixed and recorded together as An. messeae. Although An. atroparvus was described to occur mainly at coastal regions and in brackish waters (Adamovic 1983; Dakic et al. 2008; Vujic et al. 2010), this species could also be found associated with other complex species in the same breeding sites in Vojvodina, but not numerously. In particular, An. messeae could be found frequently in ponds filled up with clean water. Due to the size and availability of such waters in Vojvodina, it is not surprising that An. messeae is the most abundant species of the complex at many places. In contrast, An. maculipennis s.s. larvae were recorded predominantly in manmade or humanly affected breeding sites. Unselective choice of breeding sites and particularly the use of manmade, contaminated and also small waters may be crucial aspects in terms of association to livestock husbandry. Blood meals could be provided in traditional stables, serving as well-tempered refugium for oogenesis and simultaneously for the sporogonic cycle of Plasmodium, which made An. maculipennis s.s. the most capacious vector in wide parts of Serbia. During the last decades, some of these favourable conditions may have been reduced as a result of landscape management, structural changes in agriculture and human lifestyle. Overall, analysis of the species association in breeding sites revealed widely overlapping breeding site requirements of An. maculipennis s.s., An. messeae, An. daciae and An. atroparvus that results in a wide geographic and spatial distribution of the four species, but with different abundance.
The CO2 trap collections conducted within this study confirmed the occurrence of the same four sibling species in Vojvodina. Only An. atroparvus was recorded very rare to receive sufficient geographic information. Close placement of the traps to livestock and stables revealed a variable connection of those species to such facilities. Compared to larval collections, adult An. daciae were more abundant than An. maculipennis s.s., which could be a consequence caused by sampling variance, but also by a strong and unselective association of An. daciae to various animal stocks. Concerning seasonality, conclusions are very limited, except the finding that An. messeae records increased by season progression that could be in relation to the size of breeding sites combined with increased reproduction.
Dakic et al. (2008) collected adult mosquitoes inside animal shelters at eight different localities within the area of the Danube and Sava river basin near Belgrade (Serbia) between June and October in 2003. Females were determined by egg morphology, hence not discriminated between An. messeae and An. daciae. Three species of the An. maculipennis complex, An. messeae, An. atroparvus and An. maculipennis s.s., were identified. The most abundant species was An. messeae (64%, maybe including unrecognised An. daciae specimens), which is in accordance with the results of this study. The second most abundant species was An. atroparvus, with a proportion of 21%, and the least abundant species was An. maculipennis s. s. with 8%. An. messeae was equally prevalent in animal shelters with different animal hosts such as cows, pigs, sheep, goats and turkeys. It remains unclear to what extent the mode of sampling, in or close to animal shelters, is representative of the species abundance, since the species records might be influenced by host selectivity and specific habits of invasion into stables. An. atroparvus was very rare in animal shelters with different kinds of domestic animals, especially turkeys and goats, while An. maculipennis s.s. prefers animal shelters with cows and pigs (Dakic et al. 2008). The same authors described characteristics of breeding sites, where larvae of the An. maculipennis complex were sampled. An. messeae was mostly recorded in clean, alkaline waters (pH = 9), followed by fresh waters with a lower quantity of chloride (40–50 mg/L) and a minimum quantity of bicarbonate of 1 mg/L. The preference of An. messeae for clean waters was also confirmed by Weitzel et al. (2012). Furthermore, An. atroparvus preferred clean, alkaline water, but tolerated a greater quantity of chloride (60–90 mg/L) and bicarbonate (500 mg/L). An. maculipennis s.s. was mainly found in waters with higher quantity of ammonia (47.44 mg/L) and mud, which is in accordance with the findings of this species predominantly in manmade and contaminated ditches (Dakic et al. 2008).
Adamovic (1982) studied Anopheles species in Srem, Vojvodina, which was according to Simic (1957) region of endemic malaria. He found An. maculipennis s.s. at all examined sites in a range from 11.8 to 95.9% and An. messeae with a slightly lower relative proportion of 4.1 to 86.7%. An. maculipennis s.s. was also common in our study, but An. messeae was more abundant, especially in adult trap collections on the territory of Vojvodina. The present results of An. atroparvus larval collections are in accordance with adult abundance obtained in the traps of this study. Species was rare sampled in both stages (larvae and adults). However, An. atroparvus was earlier found in low numbers, but widespread at 7 of 11 examined sites (Adamovic 1982).
In previous papers by Guelmino et al. (1951) and Adamovic (1975a), An. atroparvus was recorded as the predominant species near Smederevo and in the north part of the lowland of the Tisa river in the Pannonian Plain. They concluded that water management and drainage of wetlands during the second half of the twentieth century had impact on the abundance of An. atroparvus.
An. daciae is present in Germany (Kronefeld et al. 2012; Weitzel et al. 2012) and widely distributed (Kronefeld et al. 2014). In some regions of Wales and England, it was found more abundant than An. messeae (Danabalan et al. 2013), which was not the case in our study area.
Novikov and Vaulin (2014) found An. messeae/An. daciae to be the most widely distributed in European Russia and also most associated with other species, as An. maculipennis s.s. and An. beklemishevi. They concluded that combinations of coinhabiting species indicate the widest ecological niche of An. messeae/An. daciae compared to the other two.
Conclusion
Although malaria’s receptivity is still high in different parts of Europe, Marí and Peydró (2012) concluded that the malariogenic potential for endemic transmission is low. More attention should be paid to the increasing trend of malaria importation due to the increase of tourist and refugees mobility. Corresponding prophylactic measures during their travels to/from endemic areas should be taken in consideration.
Laboratory tests carried out on European populations of the An. maculipennis complex demonstrated that An. atroparvus can transmit Asian strains of P. vivax and African strains of P. ovale but is refractory to African strains of P. falciparum (James et al. 1932; Garnham et al. 1954; Ramsdale and Coluzzi 1975; Ribeiro et al. 1989; Teodorescu 1983).
In the present study, four sibling species of the An. maculipennis complex were identified on the territory of Vojvodina: An. messeae, An. maculipennis s.s., An. daciae and An. atroparvus. The newly described species An. daciae could be found both as larvae and as adults for the first time in Serbia.
An. daciae was differentiated from An. messeae for the first time in the region of Vojvodina in this study. Both species have a sympatric distribution in Europe so far. Due to limited access to the molecular tools for species differentiation, An. daciae was inevitably overseen. Consequently, potential An. daciae specimens have been most likely recorded as An. messeae in all studies prior to 2004. Thus, ecological, biogeographical and epidemiological data established for An. messeae in the past have to be attributed to either one or both species. This study contributes to a new assessment of An. maculipennis complex species distribution and abundance in Vojvodina. Besides abundance and geographic distribution of An. daciae records, changes in the abundance of all complex species in comparison to historical data are also very important and require further, complex assessment. At present, the most abundant species in Vojvodina is An. messeae. In order to estimate vector competence, An. daciae should be tested to its susceptibility to Plasmodium. Marí and Peydró (2012) believe that An. daciae, as a recently described species of An. maculipennis complex, could had played a role in malaria transmission earlier, but attributed as vector was An. messeae. As An. daciae is widespread in eastern Europe, our study demonstrates that the Balkan countries should be included in the area of its distribution.
Acknowledgements
The authors gratefully acknowledge the German Mosquito Control Association (KABS) and Institute for Dipterology-Gesellschaft zur Förderung der Stechmückenbekämpfung e.V.-GFS Speyer, Germany, for supporting and funding the study. We would like to thank Aleksandra Ignjatovic Cupina, Dubravka Pudar and Slavica Vaselek for assisting in the morphological identification of adult mosquitoes and Vid Srdic and Dragan Dondur for all the technical support during the field work. Authors acknowledge Christina Czajka for assistance in DNA sequencing.
Author contributions
MZ, DP and NB conceived the idea for this research. MZ and NB developed the experimental design and protocols. MK and DP collected material. MK and CM conducted the experiments. TW and MK conducted the data analysis. MK and TW wrote the paper. All authors contributed to the final draft and read and approved the manuscript.
Funding
This research was funded by the German Mosquito Control Association (KABS).
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Mihaela Kavran, Email: mihaela.kavran@polj.edu.rs.
Marija Zgomba, Email: mzgomba@polj.uns.ac.rs.
Thomas Weitzel, Email: thomas.weitzel@kabs-gfs.de.
Dusan Petric, Email: dusanp@polj.uns.ac.rs.
Christina Manz, Email: christina_Manz@gmx.de.
Norbert Becker, Phone: + 49 6232/990 950, Email: NorbertFBecker@web.de.
References
- Adamovic Z. A preliminary survey of the mosquitoes (Diptera, Culicidae) of Obedska bara, Serbia. Glas Prir Muz Beogr, Ser B Biol Nauke. 1975;30(1):75–80. [Google Scholar]
- Adamovic Z (1975b) Anopheline mosquitoes (Diptera, Culicidae) recorded in Potisje of the Pannonian plain. Second Eur Multicolloq Parasitol Trogir 399–403
- Adamovic Z. Distribution of Anopheline mosquitoes (Diptera, Culicidae) in Negotinska Krajina, Serbia. Acta Veterinaria. 1975;25(6):307–313. [Google Scholar]
- Adamovic Z. Über die Verbreitung und Bevökerungsdichte von Anopheles atroparvus Van Thiel (Diptera, Culicidae) in Serbien und Mazedonien, Jugoslawien. Anzeiger Schädlingskde. Pflanzenschutz. Umweltschutz. Berlin und Hamburg. 1980;53:83–86. [Google Scholar]
- Adamovic Z. Anopheline populations (Diptera, Culicidae) in Srem, Serbia. Acta Veterinaria. 1982;32(2–3):131–138. [Google Scholar]
- Adamovic Z. Anopheline species (Diptera, Culicidae) in Jasenica and Lepenica, Serbia. Glasnik Prirodnjackog Muzeja Beograd Serija B Knjiga. 1983;38:81–88. [Google Scholar]
- Baldari M, Tamburro A, Sabatinelli G, Romi R, Severini C, Cuccagna P, Fiorilli G, Allegri MP, Buriani C, Toti M. Malaria in Maremma, Italy. Lancet. 1998;351(9111):1246–1247. doi: 10.1016/S0140-6736(97)10312-9. [DOI] [PubMed] [Google Scholar]
- Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A. Mosquitoes and their control. 2. Heidelberg: Springer; 2010. [Google Scholar]
- Collins FH, Paskewitz SM. A review of the use of ribosomal DNA (rDNA) to differentiate among cryptic Anopheles species. Insect Mol Biol. 1996;5:1–9. doi: 10.1111/j.1365-2583.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Dakic Z, Kulisic Z, Stajkovic N, Pelemis M, Cobeljic M, Stanimirovic Z, Inđić N, Poluga J, Pavlović M. Ecology of Anopheles mosquitoes in Belgrade area: estimating vector potential for malaria retransmission. Acta Vet. 2008;58(5–6):603–614. [Google Scholar]
- Danabalan R, Monaghan MT, Ponsonby DJ, Linton YM. Occurrence and host preferences of Anopheles maculipennis group mosquitoes in England and Wales. Med Vet Entomol. 2013;28(2):169–178. doi: 10.1111/mve.12023. [DOI] [PubMed] [Google Scholar]
- Di Luca M, Boccolini D, Marinucci M, Romi R. Intra-population polymorphism in Anopheles messeae (Anopheles maculipennis complex) ınferred by molecular analysis. J Med Entomol. 2004;41:582–586. doi: 10.1603/0022-2585-41.4.582. [DOI] [PubMed] [Google Scholar]
- Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
- Doudier B, Bogreau H, De Vries A, Ponçon N, Stauffer WM, Fontenille D. Possible autochthonous malaria from Marseille to Minneapolis. Emerg Infect Dis. 2007;13(8):1236–1238. doi: 10.3201/eid1308.070143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European centre for disease prevention and control, ECDC (2014) Annual epidemiological report 2014 - emerging and vector-borne diseases. Stockholm
- European centre for disease prevention and control, ECDC (2015) Risk of importation and spread of malaria and other vector-borne diseases associated with the arrival of migrants to the EU – 21 October 2015, Stockholm
- European Network on Imported Infectious Disease Surveillance, TropNetEurop (2010) Friend and observers sentinel surveillance report. http://www.tropnet.net/reports_friends/reports_friends_index.html. Accessed 14th March 2011
- Garnham PCC, Bray RS, Cooper W, Lainson R, Awad FI, Williamson J. Pre-erythrocytic stages of human malaria: Plasmodium ovale. Trans R Soc Trop Med Hyg. 1954;49(1):158–167. doi: 10.1016/0035-9203(55)90042-0. [DOI] [PubMed] [Google Scholar]
- Guelmino DI, Kostic D, Jevtic M. Contribution to the study of Anopheles of Serbia. Glas Srpske akademije nauka CC/V, Odeljenje Medicinskih Nauka. 1951;4:153–166. [PubMed] [Google Scholar]
- Hackett LW, Martini E, Missiroli A. The races of Anopheles maculipennis. Am J Hyg. 1932;16:137–162. [Google Scholar]
- Harbach R. The classification of genus Anopheles (Diptera: Culicidae): a working hypothesis of phylogenetic relationships. Bull Entomol Res. 2004;94:537–553. doi: 10.1079/BER2004321. [DOI] [PubMed] [Google Scholar]
- Harbach R (2015) Mosquito taxonomic inventory. http://mosquito-taxonomic-inventory.info/sites/mosquito-taxonomic-inventory.info/files/Subgenus%20Anopheles%20classification_12.pdf. Accessed 20 Oct 2015
- Hellenic Center For Disease Control and Prevention, HCDCP (2015) Epidemiological surveillance report malaria in Greece, 2015, up to 16/10/2015
- Hellenic Center For Disease Control and Prevention, HCDCP (2016) Epidemiological surveillance report Malaria in Greece, 2016, up to 12/09/2016
- Hellenic Center For Disease Control and Prevention, HCDCP (2017) Epidemiological surveillance report malaria in Greece, 2017, up to 17/08/2017
- James SP, Nicol WD, Shute PG. P. ovale passage through mosquitoes and successful transmission by their bites. Ann Trop Med Parasitol. 1932;26:139–145. doi: 10.1080/00034983.1932.11684711. [DOI] [Google Scholar]
- Jetten TH, Takken W (1994) Anophelism without malaria in Europe. A review of the ecology and distribution of the genus Anopheles in Europe. Wageningen Agricultural University Papers, pp 94–5. Agricultural University Wageningen, The Netherlands
- Kampen H, Maltezos E, Pagonaki M, Hunfeld KP, Maier WA, Seitz HM. Individual cases of autochthonous malaria in Evros Province, northern Greece: serological aspects. Parasitol Res. 2002;88(3):261–266. doi: 10.1007/s00436-001-0530-2. [DOI] [PubMed] [Google Scholar]
- Kostić D. Survey, examination and control of malaria in the epidemiological territory of Central Institute for Hygiene in 1939 and 1940. Narodno Zdravlje Beograd. 1946;2(1):93–148. [Google Scholar]
- Kronefeld M, Dittmann M, Zielke D, Werner D, Kampen H. Molecular confirmation of the occurrence in Germany of Anopheles daciae (Diptera, Culicidae) Parasit Vectors. 2012;5:250. doi: 10.1186/1756-3305-5-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronefeld M, Werner D, Kampen H. PCR identification and distribution of Anopheles daciae (Diptera, Culicidae) in Germany. Parasitol Res. 2014;113:2079–2086. doi: 10.1007/s00436-014-3857-1. [DOI] [PubMed] [Google Scholar]
- Linton YM, Samanidou-Voyadjoglou A, Smith L, Harbach RE. New occurrence records for Anopheles maculipennis and An. messeae in northern Greece based on DNA sequence data. Eur Mosq Bull. 2001;11:31–36. doi: 10.1046/j.1365-2583.2002.00338.x. [DOI] [PubMed] [Google Scholar]
- Linton YM, Smith L, Harbach RE. Molecular confirmation of sympatric populations of Anopheles messeae and Anopheles atroparvus overwintering in Kent, southeast England. Eur Mosq Bull. 2002;13:1–7. [Google Scholar]
- Linton Y, Smith L, Koliopoulos G, Athanassios KZ, Samanidou Voyadjoglou A, Patsoula E, Harbach RE. The Anopheles (Anopheles) maculipennis complex (Diptera: Culicidae) in Greece. J Nat Hist. 2007;41:2683–2699. doi: 10.1080/00222930701403255. [DOI] [Google Scholar]
- Lühken R, Czajka C, Steinke S, Jöst H, Schmidt-Chanasit J, Pfitzner WP, Becker N, Kiel E, Krüger A, Tannich E. Distribution of individual members of the mosquito Anopheles maculipennis complex in Germany identified by newly developed real-time PCR assays. Med Vet Entomol. 2016;30:144–154. doi: 10.1111/mve.12161. [DOI] [PubMed] [Google Scholar]
- Marí RB, Peydró RJ. New anopheline records from the Valencian Autonomous Region of Eastern Spain (Diptera: Culicidae: Anophelinae) Eur Mosq Bull. 2010;28:148–156. [Google Scholar]
- Marí RB, Peydró RJ. Re-emergence of malaria and dengue in Europe. In: Rodriguez-Morales A, editor. Current topics in tropical medicine. Croatia: InTech. Rijeka; 2012. pp. 483–512. [Google Scholar]
- Marinucci M, Romi R, Mancini P, Di Luca M, Severini C. Phylogenetic relationships of seven Palaearctic members of the maculipennis complex inferred from ITS2 sequence data. Insect Mol Biol. 1999;8:469–480. doi: 10.1046/j.1365-2583.1999.00140.x. [DOI] [PubMed] [Google Scholar]
- Meigen JW. Systematische Beschreibung der bekannten europäischen zweiflügeligen Insekten. Vol. 1. Aachen: Bei Friedrich Wilhelm Forstmann: Gedrukt bei Beaufort Sohn; 1818. p. 333. [Google Scholar]
- Mihailovic DT, Lalic B, Arsenic I, Malinovic S, et al. Climate conditions for seed production. In: Milosevic M, Malesevic M, et al., editors. Seed breeding. 1. Novi Sad: Institute for Field and Vegetable Crops; 2004. pp. 243–266. [Google Scholar]
- Nicolescu G, Linton YM, Vladimirescu AF, Howard TM, Harbach RE. Mosquitoes of the Anopheles maculipennis group (Diptera: Culicidae) in Romania, with the discovery and formal recognition of a new species based on molecular and morphological evidence. Bull Entomol Res. 2004;94:525–535. doi: 10.1079/BER2004330. [DOI] [PubMed] [Google Scholar]
- Novikov YM, Vaulin OV. Expansion of Anopheles maculipennis s.s. (Diptera: Culicidae) to northeastern Europe and northwestern Asia: causes and consequences. Parasit Vectors. 2014;7:389. doi: 10.1186/1756-3305-7-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoula E, Samanidou-Voyadjoglou A, Spanakos G, Kremastinou J, Nasioulas G, Vakalis NC. Molecular characterization of Anopheles maculipennis complex during surveillance for the 2004 Olympic Games in Athens. Med Vet Entomol. 2007;21:36–43. doi: 10.1111/j.1365-2915.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- Ponçon N, Toty C, L’Ambert G, Le Goff G, Brengues C, Schaffner F, Fontenille D. Biology and dynamics of potential malaria vectors in Southern France. Malar J. 2007;6(1):18. doi: 10.1186/1475-2875-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft J, Maier WA, Kampen H. Identification of six sibling species of the Anopheles maculipennis complex (Diptera: Culicidae) by a polymerase chain reaction assay. Parasitol Res. 1999;85:837–843. doi: 10.1007/s004360050642. [DOI] [PubMed] [Google Scholar]
- Ramsdale CD, Coluzzi M. Studies on the infectivity of tropical African strains of Plasmodium falciparum to some southern European vectors of malaria. Parasitologia. 1975;17(1–3):39–48. [PubMed] [Google Scholar]
- Ribeiro H, Batista JL, Ramos HC, Pires CA, Champalimaud JL, Costa JM, Araújo C, Mansinho K, Pina MC. An attempt to infect Anopheles atroparvus from Portugal with African Plasmodium falciparum. Revista Portuguesa de Doenças Infecciosas. 1989;12:81–82. [Google Scholar]
- Romi R, Pierdominici G, Severini C, Tamburro A, Cocchi M, Menichetti D, Pili E, Marchi A. Status of malaria vectors in Italy. J Med Entomol. 1997;34(3):263–271. doi: 10.1093/jmedent/34.3.263. [DOI] [PubMed] [Google Scholar]
- Rydzanicz K, Czułowska A, Manz C, Jawień P. First record of Anopheles daciae (Linton, Nicolescu & Harbach, 2004) in Poland. J Vector Ecol. 2017;42(1):196–199. doi: 10.1111/jvec.12257. [DOI] [PubMed] [Google Scholar]
- Santa-Olalla Peralta P, Vazquez-Torres MC, Latorre-Fandós E, Mairal-Claver P, Cortina-Solano P, Puy-Azón A, Adiego Sancho B, Leitmeyer K, Lucientes-Curdi J, Sierra-Moros MJ. First autochthonous malaria case due to Plasmodium vivax since eradication, Spain, October 2010. Euro Surveillance: Bulletin Eur Commun Dis Bull. 2010;15(41):19684. doi: 10.2807/ese.15.41.19684-en. [DOI] [PubMed] [Google Scholar]
- Schaffner F, Angel G, Geoffroy B, Hervy JO, Rhaeim A. The mosquitoes of Europe/Les moustiques d’ Europe [CD-ROM] France: IRD Éditions and EID Méditerranée, Montpellier; 2001. [Google Scholar]
- Sedaghat MM, Linton YM, Oshaghi MA, Vatandoost H, Harbach RE. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterisation and recognition of a new species. Bull Entomol Res. 2003;93:527–535. doi: 10.1079/BER2003272. [DOI] [PubMed] [Google Scholar]
- Simic C. Malaria. Belgrade-Zagreb: Medicinska knjiga; 1948. [Google Scholar]
- Simic Z (1957) L’etat du paludismeenYougoslavieavantetapresI’application de DDT Communications de Congres National des Sciences Medicales, Bucharest. 755–765e
- Simsek FM, Ulger C, Akiner MM, Tuncay SS, Kiremit F, Bardakci F. Molecular identification and distribution of Anopheles maculipennis complex in the Mediterranean region of Turkey. Biochem Syst Ecol. 2011;39:258–265. doi: 10.1016/j.bse.2011.08.010. [DOI] [Google Scholar]
- Sitar A. Examination of anophelism in the areas previously contaminated with malaria. Acta Parasitol Jugoslavica. 1977;8(2):97–104. [Google Scholar]
- Teodorescu C. Experimental infection of an indigenous strain of Anopheles atroparvus with imported species of Plasmodium. Arch Roum Pathol Exp Microbiol. 1983;42(4):365–370. [PubMed] [Google Scholar]
- Van Thiel PH. Sur l’origine des variations de taille de l’ A. maculipennnis dans les Pays-Bas. Bull Soc Pathol Exot. 1927;20:366–390. [Google Scholar]
- Vujic A, Stefanovic A, Dragicevic I, Matijevic T, Pejcic L, Knezevic M, Krasic D, Veselic S. Species composition and seasonal dynamics of mosquitoes (Diptera: Culicidae) in flooded areas of Vojvodina, Serbia. Arch Biol Sci. 2010;62(4):1193–1206. doi: 10.2298/ABS1004193V. [DOI] [Google Scholar]
- Vukasovic P. Supplement to the examination of anophelism on the outskirts of Belgrade. Hygiene. 1950;5-6:507–526. [Google Scholar]
- Weitzel T, Gauch C, Becker N. Identification of Anopheles daciae in Germany through ITS2 sequencing. Parasitol Res. 2012;111:2431–2438. doi: 10.1007/s00436-012-3102-8. [DOI] [PubMed] [Google Scholar]
- Weyer F. Die geographische Verbreitung der Rassen von Anopheles maculipennis in Deutschland. Z F Parasitenk. 1938;10:437–463. doi: 10.1007/BF02123845. [DOI] [Google Scholar]
- White GB. Systematic reappraisal of the Anopheles maculipennis complex. Mosq Syst. 1978;10:13–44. [Google Scholar]
- Zgomba M, Petric D, Cupina A, Dmitrovic R (2002) Yugoslavia. In: World Health Organisation. Epidemiological surveillance of malaria in countries of central and eastern Europe and selected newly independent states: report on a WHO inter-country meeting, Sofia, Bulgaria
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.