Abstract
The range of potential applications of liquid biopsies for non-small cell lung cancer management is expanded by the use of circulating tumor deoxyribonucleic acid and circulating tumor cells. Principal studies have demonstrated the predictive accuracy of droplet digital polymerase chain reaction detection, next-generation sequencing, and circulating tumor cells detection in patients with non-small cell lung cancer. The translational potential of these liquid biopsy technologies promotes the improvement of sensitivity and specificity in genomic and molecular methods. Here, we highlight the realities and challenges associated with the use of liquid biopsies for the detection of non-small cell lung cancer in patients. However, liquid biopsy technologies including circulating tumor cells detection, droplet digital polymerase chain reaction detection, and next-generation sequencing detection for precision therapy in non-small cell lung cancer will show substantive clinical applications in the future.
Keywords: precision therapy, liquid biopsy, circulating tumor cells, circulating tumor DNA, non-small cell lung cancer
Introduction
Liquid biopsy (including droplet digital polymerase chain reaction [ddPCR] detection, next-generation sequencing [NGS] detection, and circulating tumor cells [CTCs] detection) has been developed as a novel diagnosis method to monitor tumor development in a noninvasive manner.1–3 If these technologies can provide enough sensitivity and specificity, liquid biopsy can be used as a platform for detecting tumor gene subtypes,4 monitoring drug resistance,5 and predicting cancer at an early stage.6 Therefore, liquid biopsy will essentially promote the development of cancer precision medicine. At present, cell-free DNA (cfDNA) detection and CTC detection are the 2 main research fields in liquid biopsy. Cell-free DNA is a chromatin DNA fragment, which is primarily derived from the apoptosis and necrosis of somatic cells or tumor cells. It mainly exists in plasma, circulating in the bloodstream, but its concentration is very low.7 Circulating tumor cells, the tumor cells existing in the bloodstream, originate from tumor tissues in situ. They play an important role in tumor metastasis since they mix with blood cells and have the ability to adhere and proliferate in any organ. In addition, cfDNA and CTCs also exist in other body fluids, such as pleural fluid,8,9 urine,10,11 saliva,12,13 cerebrospinal fluid,14 and so on.
Non-small cell lung cancer is the one of the most malignant cancers and is the leading cause of morbidity and mortality in the world.15 Due to the limitations of diagnosis technologies, it is difficult for patients with NSCLC to make a definite diagnosis of the gene subtype in a noninvasive manner.16 Presently, molecular–pathological diagnosis of the tumor sample in situ is known as the most effective way to diagnose NSCLC gene subtypes. Surgical resection, bronchoscopy, and percutaneous needle lung biopsy are the 3 direct methods of taking out tumor samples in situ.17 However, patients suffer pain when the tumor tissue is taken out of the lung via the abovementioned methods.
With the development of liquid biopsies, scientists are dedicated to use these technologies to determine the gene subtype of patients with lung cancer.18–21 Droplet digital PCR is one of the most promising diagnosis technologies to determine the gene subtype of patients with lung cancer.22–24 Circulating tumor cell capture technology also has promise in promoting clinical translation, due to the constant breakthrough in the detection of NSCLC.25–27 Next-generation sequencing for the detection of cfDNA and CTCs has also made great breakthroughs.16,21,28–30 All of these technologies will possibly be translated to clinical application in the next few years.
Although liquid biopsy technologies have made lots of advancements, the sensitivity and specificity still need to be further improved, and the operating steps will also need to be further simplified. This paper seeks to summarize the research findings on NSCLC detection by liquid biopsy, analyze the advantages and disadvantages of the present technologies, and propose improvements for the program, in hopes of lending a hand to the clinical translation of liquid biopsies in NSCLC precision therapy.
Tissue Biopsies Used for NSCLC Gene Subtype Diagnosis
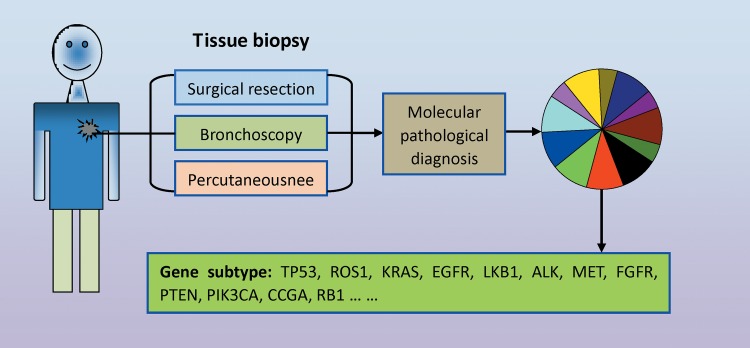
With the continuous development in molecular pathology, NSCLC is known as a driver gene-induced tissue malignant transformation31,32 (Figure 1). To date, NSCLC is divided into multiple driver gene subtypes, including TP53, ROS1, KRAS, EGFR, LKB1, ALK, MET, FGFR, PTEN, PIK3CA, CCGA, RB1. 33 Molecular pathological diagnosis plays an important role in distinguishing the abovementioned gene subtypes. To satisfy the reliability of molecular pathological diagnoses, tumor samples must be taken out from the primary location of the cancer. Various methods, including surgical resection, bronchoscopy, percutaneous needle, and so on, are used for tissue collection.17,33 Meanwhile, another problem comes to light: Due to the above mentioned methods being classified as invasive technologies, patients must suffer great harm when the tumor tissue is taken out in situ. Clinically, the majority of patients with NSCLC refuse the second or third tissue biopsy because of the unbearable pain. In advanced stages, the majority of patients with NSCLC choose imageological examination to monitor cancer development. As a consequence, this precludes the real-time monitoring of gene mutation subtypes during the diagnosis of either recurrence or drug resistance.
Figure 1.
Gene subtype detection in lung cancer tissue biopsy. Cancer tissue in situ is taken out via invasive technologies including surgical resection, bronchoscopy, and percutaneous needle. Molecular pathological diagnosis technologies are performed to test alterations in the lung cancer driver gene including gene mutation, copy number variations, and fusion genes.
Liquid Biopsy for Lung Cancer Gene Subtype Detection
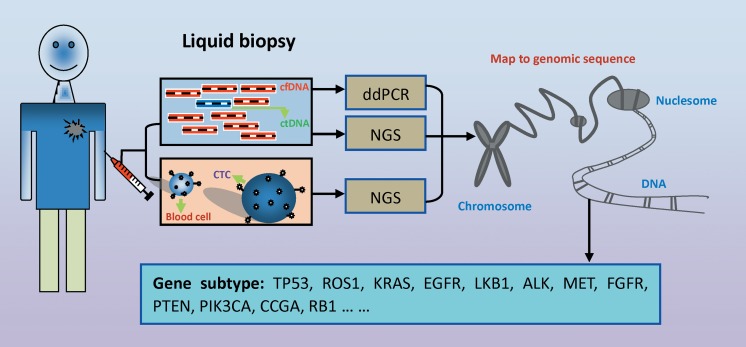
Studies have indicated that circulating tumor DNA (ctDNA) is presented in the bloodstream earlier than CTCs.34 The liquid biopsy technology, ddPCR, is a very useful method for detecting specific gene mutations22 (Figure 2 and 3). Furthermore, it can also be used for the detection of drug resistance-related gene mutations.35 Del Re et al performed ddPCR to detect cfDNA from epidermal growth factor receptor-tyrosine kinase receptor inhibitor-resistant patients and found that different patients with drug resistance have different gene mutations, such as T790M, KRAS, and so on. Droplet digital PCR characterizes a high sensitivity and specificity for detecting mutated genes.36 Usually, it is useful for the detection of gene subtypes and drug resistance-related mutation genes in patients with advanced NSCLC.22,35 However, one obvious disadvantage is that lots of infrequently mutated genes are missed and that it is hard to make a standard judgment in earlier stages of patients with NSCLC.
Figure 2.
Gene subtype detection in circulating tumor DNA (ctDNA). Cell-free DNA (cfDNA) is isolated from blood collected from patients with lung cancer. Circulating tumor DNA accounts for a small ratio of cfDNA fragments. Droplet digital PCR (ddPCR) and next-generation sequencing (NGS) are performed to test alterations in the lung cancer driver gene including gene mutation, copy number variations, and fusion genes.
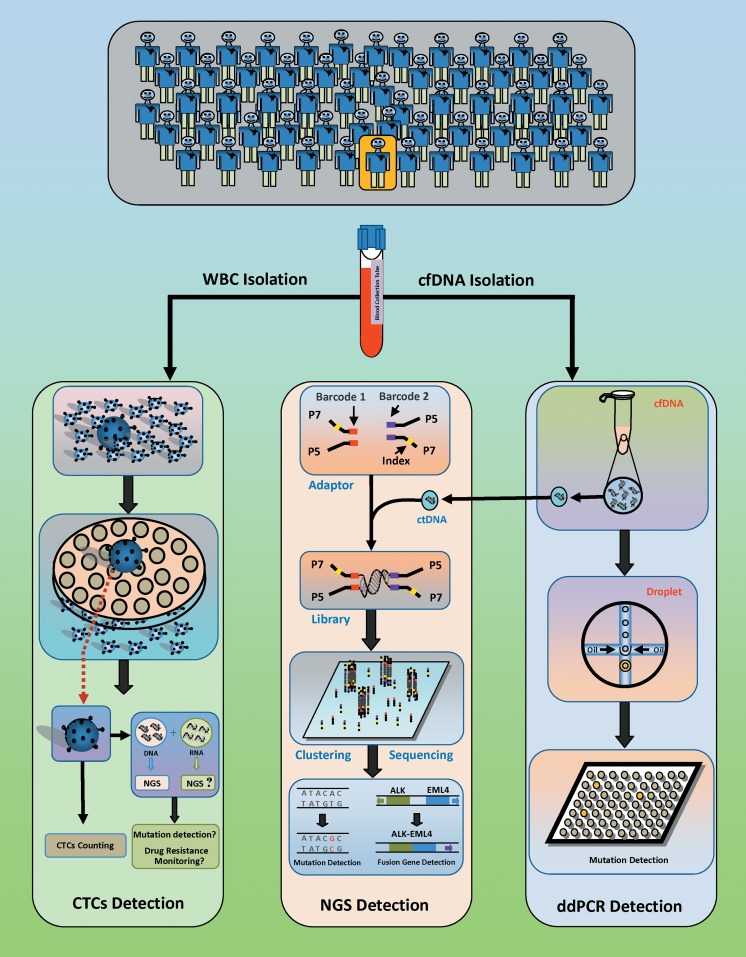
Figure 3.
Circulating tumor cell detection and ctDNA detection used for lung cancer diagnosis. Circulating tumor cell capture is performed to isolate cells with large sizes. The number of CTCs is used as a reference for predicting the prognosis and survival time of patients with lung cancer. Circulating tumor DNA (ctDNA) is isolated from the blood of patients with lung cancer. Digital PCR (ddPCR) detection, a fluorescent probe-based PCR assay, is partitioned into highly uniform 1-nL reverse micelles (water-in-oil). After PCR amplification, the fluorescence of each droplet is individually measured and defined as positive (presence of PCR product) or negative (absence of PCR product). For NGS detection, a pair of index-based adaptors are ligated at the ends of the cfDNA fragment. During high-throughput sequencing, adaptor-ligated DNA fragments undergo amplification, clustering, and sequencing. Data are used for analysis of ctDNA point mutations, copy number variations, and chromosomal rearrangements. NGS indicates next-generation sequencing; PCR, polymerase chain reaction.
As another liquid biopsy technology, NGS has advantages in multiple gene mutation detection, tumor heterogeneity detection, and drug resistance-related gene mutation detection16,21 (Figure 2). Whole genomic sequencing provides a comprehensive database for cfDNA and ctDNA. However, the present sensitivity and specificity of NGS do not meet clinical diagnosis criteria. Scientists have improved the accuracy rate to nearly 100% in stage II, stage III, and stage IV in patients with lung cancer. The accuracy rate dropped to nearly 50% in patients with stage I lung cancer,16,21 suggesting that the sensitivity and specificity of this method need to be further improved for early detection.
Circulating tumor cell capture technology characterizes the prediction of tumor metastasis and drug resistance28,37 (Figure 2). Scientists have separated CTCs from the blood of patients with NSCLC via various methods. Whole-exome sequencing has been performed on CTCs, and the analysis results contribute to distinguishing the attributes of NSCLC in situ.27 It is also helpful for recommending a treatment regimen for those patients. Due to the focus of CTC analyses on one or few cells, the objective state of the tumor in situ is not easily reflected. Therefore, because CTCs detect gene mutation subtype and tumor heterogeneity, many CTC captures taken from the same patient should be performed for NGS detection in the future.
Circulating Tumor Cell Capture, CTC Verification, and Clinical Application
Circulating tumor cell capture is a method for capturing rare CTCs from the blood of patients with cancer via a specific technology. Presently, scientists have developed several CTC capture technologies, such as the identification of protein markers on the cell surface, the filtration via the tumor cell volume (Figure 3), the detection of specific markers of fusion genes and metabolic pathways, and so on.25–27 These technologies may be developed as commercial instruments for taking part in clinical diagnosis.
There are many difficulties in the identification of CTCs, which are isolated from the blood using various capture technologies. To date, scientists have developed several technologies to get rid of fake CTCs. These technologies include the identification of specific tumor-related protein markers, the identification of specific mutated genes, NGS analysis, and so on.27,38–40 However, different capture technologies have different defects. It will be exciting if the accuracy of these capture technologies can be improved significantly in the future.
Circulating tumor cell capture has its advantages in predicting lung cancer progression. Studies have suggested that CTCs can be detected in blood derived from patients with lung cancer (diameter < 1.0 cm). For predicting the prognosis and survival time of patients with lung cancer, the number of CTCs has an important reference value41,42(Figure 3). For precision medicine development, CTC capture also has some advantages. If the CTCs can be cultured in vitro, different regimens of combined chemotherapy will be tested. In addition, then, the best chemotherapy regimen will be used for clinical treatment.43,44
In the areas of drug resistance detection and lung cancer recurrence monitoring, CTC capture also plays an important role.28 The disappearance of CTCs from the patient’s blood after surgical resection can last from a few months to a few years. It is suggested that lung cancer will recur in the next few months if CTCs are detected. At least, it is an indicator that the tumor has been growing in the body.41,42
Cell-Free DNA and Clinical Application
Cell-free DNA was discovered in the whole blood of healthy adults in 1948.45 More than 2 decades later, cfDNA isolated from patients with cancer was first reported.46 After that, scientists further found that the content of cfDNA from patients with cancer is higher than that from healthy participants.3 For cfDNA isolation (Figure 3), plasma was collected from the peripheral venous blood of patients with cancer. Cell-free DNA was isolated from the plasma using a specific circulating nucleic acid isolation kit. Studies have demonstrated that the ctDNA from tumor cells is shorter than the cfDNA from somatic cells.47 The difference in fragment length between cfDNA and ctDNA is approximately 20 bp. In addition, the longer ctDNA fragments (above 1000 bp) are contained in cfDNA.48 Based on the rare of ctDNA content, we should do our best to capture the overall ctDNA and analyze the information carried by them.
Cell-free DNA was first discovered in 1977 in the whole blood of patients with lung cancer.49 Scientists presumed that the content of cfDNA may be related to the progression of lung cancer.50 To date, studies have demonstrated that ctDNA (DNA fragments from the degradation of tumor cell chromatin) is part of cfDNA.6,51 The content of ctDNA is thought to be related to the stage of the tumor. Studies suggested that the total content of ctDNA in cfDNA is up to 5% to 10% in patients with advanced NSCLC, while the content of total ctDNA in cfDNA is less than 1% at early stages.6,21
The Challenges of Applying cfDNA Detection to NSCLC Diagnosis
Several groups have started to detect cfDNA using different technologies.16,19,35,52 Zhu et al have used ddPCR for cfDNA detection, and their results suggested that judging via the fluorescence intensity from amplified target mutated genes can enhance the sensitivity and specificity greatly.22 Next-generation sequencing has been used for cfDNA detection and has shown promise. Newman et al found that the success rate of mutation detection is up to 100% in patients with stage II and above lung cancer, while down to 50% in patients with stage I lung cancer.21 Improvement in the technology is needed to further carry forward. Two years later, they enhanced both the sensitivity and specificity to over 90% via the use of integrated digital error suppression.16 However, there is still not enough data to demonstrate similar results when cfDNA detection was used in patients with NSCLC at an early stage. Recently, Christopher Abbosh et al used phylogenetic ctDNA analysis to depict early stages of NSCLC evolution. Their results showed that ctDNA profiling tracks the subclonal nature of NSCLC relapse and metastasis.30
Next-Generation Sequencing Detection for Lung Cancer Diagnosis
Next-generation sequencing plays an important role in lung cancer diagnosis and treatment (Figure 3). Hagemann et al designed an experiment to verify the results from NGS analysis based on in situ tissue.53 Results suggested that NGS analysis provides reliable reference information for NSCLC diagnosis. In the field of lung cancer study, NGS has the advantage of detecting unknown mutated sites, while ddPCR focuses on individual known mutated sites. For example, a study focused on gene mutations of patients with lung cancer and patients with colonic adenocarcinoma revealed 3 new mutation sites on KRAS and EGFR. 54 Furthermore, targeting NGS has been used to characterize similar mutated sites in patients with lung cancer.55 Studies have shown that NGS detection of solid tumor samples can provide definite information on the mutated sites of squamous carcinoma or nonsquamous carcinoma, such as TP53, PIK3CA, CCND1, CDKN2A, SOX2, NOTCH1, and FBXW7.2 In a comparative study of whole-genome sequencing performed by The Cancer Genome Atlas, 178 NSCLC squamous carcinoma samples were compared with 230 NSCLC adenocarcinoma samples simultaneously.56 The study demonstrated that copies of the genes including SOX2, PDGFRA, KIT, EGFR, FGFR1, WHSC1L1, CCND1, and CDKN2A are increased significantly in NSCLC squamous carcinoma samples.57
Next-generation sequencing may be used as a conventional technology for the diagnosis of NSCLC gene mutation subtypes in the future. First, scientists developed a new targeting NGS system for mononucleotide mutation detection in lung cancer samples. In total, 168 genes (including the common mutations KRAS, TP53, EGFR, PIK3CA, BRAF, NRAS, JAK3, CTNNB1, and CKDN2A) were detected in the study.58 Second, whole-exome sequencing and transcriptome sequencing on small-cell lung cancer samples indicated that the highly mutated status of TP53, RB1, and PTEN simultaneously existed in tumor tissues in situ and metastatic tumor tissues. Similarly, some fusion genes were detected based on the NGS technology, such as ALK, ROS1, and RET. In brief, the data from NGS have been proved effectively in the noninvasive diagnosis of NSCLC.
Conclusions
Cell-free DNA detection has been developed as an important platform for NSCLC prediction and diagnosis. In recent years, scientists have developed a set of liquid biopsy technologies and experienced the evolution from low sensitivity to high sensitivity and from low specificity to high specificity. Although the abovementioned technologies have made an outstanding achievement in liquid biopsy, key limitations of the abovementioned technologies still need to be resolved. Next-generation sequencing analysis for CTCs needs to solve the problems of contamination and artificial mutation. How to resolve the single-cell genome or transcriptome amplification as well as the representative faithfulness of the original state of the CTCs still need to be further discussed. Although detection of ctDNA using ddPCR has made certain achievements, the detection of single or few mutations limits this application in detecting and monitoring tumors early and in tumor heterogeneity and drug resistance. Next-generation sequencing detection has the advantages of detecting multigene mutations, monitoring drug resistance-related gene mutations, and testing tumor heterogeneity, but the sensitivity and specificity has still not met the requirements of clinical application. In addition, simplifying and standardizing the procedures of the NGS analysis need to be further developed. Collectively, the achievements of the liquid biopsy provide us with a platform for precision therapy in NSCLC in the future.
Abbreviations
- CTCs
circulating tumor cells
- cfDNA
cell-free DNA
- ctDNA
circulating tumor DNA
- ddPCR
droplet digital PCR
- NGS
next-generation sequencing
- NSCLC
non-small cell lung cancer
Footnotes
Authors’ Note: All authors listed in the manuscript contributed to writing and proofreading. Jun Lu was responsible for most of the manuscript writing and figure generating. Baohui Han was involved in conception delineation, manuscript revision, and final approval of manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the program of Systems Biomedicine Innovation Centre at Shanghai Jiao Tong University (Project No. 15ZH4009); the key program of Translational Medicine Centre from Shanghai Jiao Tong University School of Medicine (Project No. 15ZH1008); and National Natural Science Foundation of China (Project No. 81673015).
ORCID iD: Baohui Han, MD, PhD  http://orcid.org/0000-0002-3950-3030
http://orcid.org/0000-0002-3950-3030
References
- 1. Wan JC, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–238. [DOI] [PubMed] [Google Scholar]
- 2. Bennett CW, Berchem G, Kim YJ, El-Khoury V. Cell-free DNA and next-generation sequencing in the service of personalized medicine for lung cancer. Oncotarget. 2016;7(43):71013–71035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diaz LA, Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lebofsky R, Decraene C, Bernard V, et al. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol Oncol. 2015;9(4):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120–124. [DOI] [PubMed] [Google Scholar]
- 6. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benlloch S, Marti-Ciriquian JL, Galbis-Caravajal JM, et al. Cell-free DNA concentration in pleural fluid and serum: quantitative approach and potential prognostic factor in patients with cancer and pleural effusions. Clin Lung Cancer. 2006;8(2):140–145. [DOI] [PubMed] [Google Scholar]
- 9. Chan MH, Chow KM, Chan AT, et al. Quantitative analysis of pleural fluid cell-free DNA as a tool for the classification of pleural effusions. Clin Chem. 2003;49(5):740–745. [DOI] [PubMed] [Google Scholar]
- 10. Reckamp KL, Melnikova VO, Karlovich C, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol. 2016;11(10):1690–1700. [DOI] [PubMed] [Google Scholar]
- 11. Fujii T, Barzi A, Sartore-Bianchi A, et al. Mutation-enrichment next-generation sequencing for quantitative detection of KRAS mutations in urine cell-free DNA from patients with advanced cancers. Clin Cancer Res. 2017;23(14):3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei F, Lin CC, Joon A, et al. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med. 2014;190(10):1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pu D, Liang H, Wei F, et al. Evaluation of a novel saliva-based epidermal growth factor receptor mutation detection for lung cancer: a pilot study. Thorac Cancer. 2016;7(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Springer S, Zhang M, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA. 2015;112(31):9704–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 16. Newman AM, Lovejoy AF, Klass DM, et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016;34(5):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerr KM, Bubendorf L, Edelman MJ, et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol. 2014;25(9):1681–1690. [DOI] [PubMed] [Google Scholar]
- 18. Qian X, Liu J, Sun Y, et al. Circulating cell-free DNA has a high degree of specificity to detect exon 19 deletions and the single-point substitution mutation L858R in non-small cell lung cancer. Oncotarget. 2016;7(20):29154–29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forshew T, Murtaza M, Parkinson C, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4(136):136ra168. [DOI] [PubMed] [Google Scholar]
- 20. Kimura H, Kasahara K, Kawaishi M, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res. 2006;12(13):3915–3921. [DOI] [PubMed] [Google Scholar]
- 21. Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20(5):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu G, Ye X, Dong Z, et al. Highly sensitive droplet digital PCR method for detection of EGFR-activating mutations in plasma cell-free DNA from patients with advanced non-small cell lung cancer. JMD. 2015;17(3):265–272. [DOI] [PubMed] [Google Scholar]
- 23. Pender A, Garcia-Murillas I, Rana S, et al. Efficient genotyping of KRAS mutant non-small cell lung cancer using a multiplexed droplet digital PCR approach. PLoS One. 2015;10(9):e0139074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Q, Yang X, He Y, et al. Droplet digital PCR for absolute quantification of EML4-ALK gene rearrangement in lung adenocarcinoma. JMD. 2015;17(5):515–520. [DOI] [PubMed] [Google Scholar]
- 25. Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7(2):306–315. [DOI] [PubMed] [Google Scholar]
- 26. Earhart CM, Hughes CE, Gaster RS, et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab on a chip. 2014;14(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang Y, Wang Z, Li Z, et al. High-throughput screening of rare metabolically active tumor cells in pleural effusion and peripheral blood of lung cancer patients. Proc Natl Acad Sci U S A. 2017;114(10):2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carter L, Rothwell DG, Mesquita B, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med. 2017;23(1):114–119. [DOI] [PubMed] [Google Scholar]
- 29. Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20(8):897–903. [DOI] [PubMed] [Google Scholar]
- 30. Christopher Abbosh NJB, Wilson GA, Mariam JH, et al. Phylogenetic ctDNA analysis depicts early stage lung cancer evolution. Nature. 2017;545(7655):446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warth A, Endris V, Penzel R, Weichert W. Molecular pathology of lung cancer. State of the art 2014 [in German]. Der Pathologe. 2014;35(6):565–573. [DOI] [PubMed] [Google Scholar]
- 32. Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiley CT, Le Quesne J, Santis G, et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet. 2016;388(10048):1002–1011. [DOI] [PubMed] [Google Scholar]
- 34. Deng X, Nakamura Y. Cancer precision medicine: from cancer screening to drug selection and personalized immunotherapy. Trends Pharmacol Sci. 2017;38(1):15–24. [DOI] [PubMed] [Google Scholar]
- 35. Ishii H, Azuma K, Sakai K, et al. Digital PCR analysis of plasma cell-free DNA for non-invasive detection of drug resistance mechanisms in EGFR mutant NSCLC: correlation with paired tumor samples. Oncotarget. 2015;6(31):30850–30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Del Re M, Tiseo M, Bordi P, et al. Contribution of KRAS mutations and c.2369C > T (p.T790M) EGFR to acquired resistance to EGFR-TKIs in EGFR mutant NSCLC: a study on circulating tumor DNA. Oncotarget. 2017;8(8):13611–13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009;15(2):6980–6986. [DOI] [PubMed] [Google Scholar]
- 38. Chen X, Zhou F, Li X, et al. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol. 2015;10(8):1163–1171. [DOI] [PubMed] [Google Scholar]
- 39. de Wit S, van Dalum G, Lenferink AT, et al. The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci Rep. 2015;5:12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Myung JH, Roengvoraphoj M, Tam KA, et al. Effective capture of circulating tumor cells from a transgenic mouse lung cancer model using dendrimer surfaces immobilized with anti-EGFR. Anal Chem. 2015;87(19):10096–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tognela A, Spring KJ, Becker T, et al. Predictive and prognostic value of circulating tumor cell detection in lung cancer: a clinician’s perspective. Crit Rev Oncol Hematol. 2015;93(2):90–102. [DOI] [PubMed] [Google Scholar]
- 42. Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol. 2012;7(3):512–519. [DOI] [PubMed] [Google Scholar]
- 43. Maheswaran S, Haber DA. Ex vivo culture of CTCs: an emerging resource to guide cancer therapy. Cancer Res. 2015;75(12):2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Z, Shiratsuchi H, Lin J, et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 2014;5(23):12383–12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mandel P, Metais P. Les acides nucleiques du plasma sanguin chez l’homme. C R Seances Soc Biol Fil. 1948;142(3-4):241–243. [PubMed] [Google Scholar]
- 46. Koffler D, Agnello V, Winchester R, Kunkel HG. The occurrence of single-stranded DNA in the serum of patients with systemic lupus erythematosus and other diseases. J Clin Invest. 1973;52(1):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Underhill HR, Kitzman JO, Hellwig S, et al. Fragment length of circulating tumor DNA. PLoS Genet. 2016;12(7):e1006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheng SH, Jiang P, Sun K, et al. Noninvasive prenatal testing by nanopore sequencing of maternal plasma DNA: feasibility assessment. Clin Chem. 2015;61(10):1305–1306. [DOI] [PubMed] [Google Scholar]
- 49. Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37(3):646–650. [PubMed] [Google Scholar]
- 50. Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–437. [DOI] [PubMed] [Google Scholar]
- 51. Pantel K. Blood-based analysis of circulating cell-free DNA and tumor cells for early cancer detection. PLoS Med. 2016;13(12):e1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Belic J, Koch M, Ulz P, et al. Rapid identification of plasma DNA samples with increased ctDNA levels by a modified fast-seqs approach. Clin Chem. 2015;61(6):838–849. [DOI] [PubMed] [Google Scholar]
- 53. Hagemann IS, O’Neill PK, Erill I, Pfeifer JD. Diagnostic yield of targeted next generation sequencing in various cancer types: an information-theoretic approach. Cancer Genet. 2015;208(9):441–447. [DOI] [PubMed] [Google Scholar]
- 54. Chevrier S, Arnould L, Ghiringhelli F, Coudert B, Fumoleau P, Boidot R. Next-generation sequencing analysis of lung and colon carcinomas reveals a variety of genetic alterations. Int J Oncol. 2014;45(3):1167–1174. [DOI] [PubMed] [Google Scholar]
- 55. Paweletz CP, Sacher AG, Raymond CK, et al. Bias-corrected targeted next-generation sequencing for rapid, multiplexed detection of actionable alterations in cell-free DNA from advanced lung cancer patients. Clin Cancer Res. 2016;22(4):915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Network CGAR. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Network CGAR. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao X, Wang A, Walter V, et al. Combined targeted DNA sequencing in Non-Small Cell Lung Cancer (NSCLC) using UNCseq and NGScopy, and RNA sequencing using UNCqeR for the detection of genetic aberrations in NSCLC. PloS One. 2015;10(6):e0129280. [DOI] [PMC free article] [PubMed] [Google Scholar]