Abstract
Objectives
We aimed to systematically assess the relationship between folic acid supplementation in pregnancy and risk of preeclampsia and gestational hypertension.
Methods
The relevant studies were included by retrieving the Embase, PubMed and Cochrane library databases. Data extraction was conducted by two investigators independently. The risk ratio (RR) and 95% confidence interval (CI) were used as effect indexes to evaluate the relationship between folic acid supplementation and risk of gestational hypertension or preeclampsia. A subgroup analysis was performed according to the supplementation patterns of folic acid. The homogeneity of the effect size was tested across the studies, and publication biases were examined.
Results
In total, 13 cohort studies and 1 randomized controlled trial study was included, containing 160,562 and 149,320 women with and without folic acid supplementation during pregnancy. Pooled results showed that risk of gestational hypertension was not associated with the supplementation of folic acid. However, folic acid supplementation during pregnancy could significantly reduce the risk of preeclampsia. Moreover, the results of subgroup analysis showed that the decreased preeclampsia risk was associated with supplementation of multivitamins containing folic acid rather than folic acid alone.
Conclusions
Our findings indicate that the supplementation of multivitamins containing folic acid during pregnancy could significantly lower preeclampsia risk.
Electronic supplementary material
The online version of this article (10.1007/s00404-018-4823-4) contains supplementary material, which is available to authorized users.
Keywords: Folic acid, Risk of preeclampsia, Risk of gestational hypertension, Meta-analysis
Introduction
Gestational hypertension and preeclampsia are common hypertensive disorders during pregnancy [1, 2]. Preeclampsia is a devastating complication of pregnancy responsible for maternal mortality and morbidity [3, 4]. Mothers with preeclampsia during pregnancy may result in neurocognitive dysregulation and suboptimal infant development in offspring [5]. During pregnancy, hypertension is a risk factor for diabetes and cardiovascular disease in later life [6, 7]. Preeclampsia is always caused by impaired placental perfusion, nevertheless, but other risk factors for preeclampsia remain unclear [8].
Accumulating evidences have suggested that elevated levels of blood homocysteine are a cause of gestational hypertension and preeclampsia [9, 10]. In addition, more and more studies confirm that folic acid supplementation can reduce blood homocysteine levels [11, 12]. Furthermore, the relationship between folic acid supplementation and preeclampsia risk has been investigated by several epidemiological studies, but their results are inconsistent. Some studies showed that the supplementation of folic acid is beneficial in decreasing the incidence of preeclampsia and gestational hypertension during pregnancy [13–15]. However, Li et al. demonstrated that there was no association between the occurrence of preeclampsia or gestational hypertension and the consumption of folic acid alone during early pregnancy [16].
In light of these inconsistent results, we conducted this meta-analysis to systematically analyze exploration of the relationship between folic acid supplementation in pregnancy and the risk of preeclampsia or gestational hypertension. This meta-analysis will provide significant clues for further clinical studies.
Materials and methods
Search strategy
All relevant published studies were retrieved by searching the databases of Embase (http://www.embase.com), PubMed (http://www.ncbi.nlm.nih.gov/pubmed), and Cochrane library (http://www.thecochranelibrary.com/view/0/index.html) updated to 21 Aug 2017 with the following keywords: pregnancy, folic acid, preeclampsia, and gestational hypertension. The search terms were: (folic acid) AND pregnancy AND {[hypertension OR (gestational hypertension)] OR [pre-eclampsia OR preeclampsia]}. Moreover, manual retrieval for hard copy literature review was also performed in this study for including more studies.
Inclusion and exclusion criteria
The inclusion criteria were: (1) the study population was pregnant women; (2) comparison group was folic acid supplementation versus folic acid absence; (3) outcome indicators included the risk of gestational hypertension or preeclampsia.
The exclusion criteria were: (1) review literature, conference abstracts, and comments; (2) the studies about the association between the levels of folic acid in serum and gestational hypertension or preeclampsia; (3) animal experiments; (4) gene polymorphism analysis; (5) pregnant women with other specific diseases.
Data extraction and quality assessment
Data extraction was conducted in all eligible studies by two investigators independently using a standardized protocol. The extracted characteristics included the name of the first author, study design, study period, publication year, demographic characteristics (such as gender and age) of study population, sample size, the supplementation patterns of folic acid, the period of folic acid use, and the incidence of preeclampsia and gestational hypertension After finishing the data extraction, the extraction table was exchanged for check, and the disagreements were settled by discussing. The quality assessments for randomized controlled trials (RCTs) and cohort study were then performed based on the Cochrane risk of bias tool [17] and Newcastle–Ottawa Scale (NOS) [18], respectively. NOS contained 9 points: ≥ 7, 5–6, and < 5 were considered methodologically to be excellent, fair, and poor.
Statistical analysis
The risk ratio (RR) and 95% confidence interval (CI) were applied as effect indexes to analyze the relationship between the supplementation of folic acid and risk of preeclampsia or gestational hypertension. The homogeneity of effect size across the studies was tested with Cochran Q statistics and the I2 statistics [19]. If P < 0.05 and I2 > 50%, the homogeneity was significant and the random effects model was then applied to pool results, otherwise, the fixed effects model was used [20]. Based on the supplementation patterns of folic acid (folic acid alone versus multivitamins containing folic acid), the subgroup and sensitivity analyses were carried out with Stata 13.0 (StataCorp, College Station, TX, USA). Publication biases were assessed by Egger’s regression test.
Results
Description of eligible studies
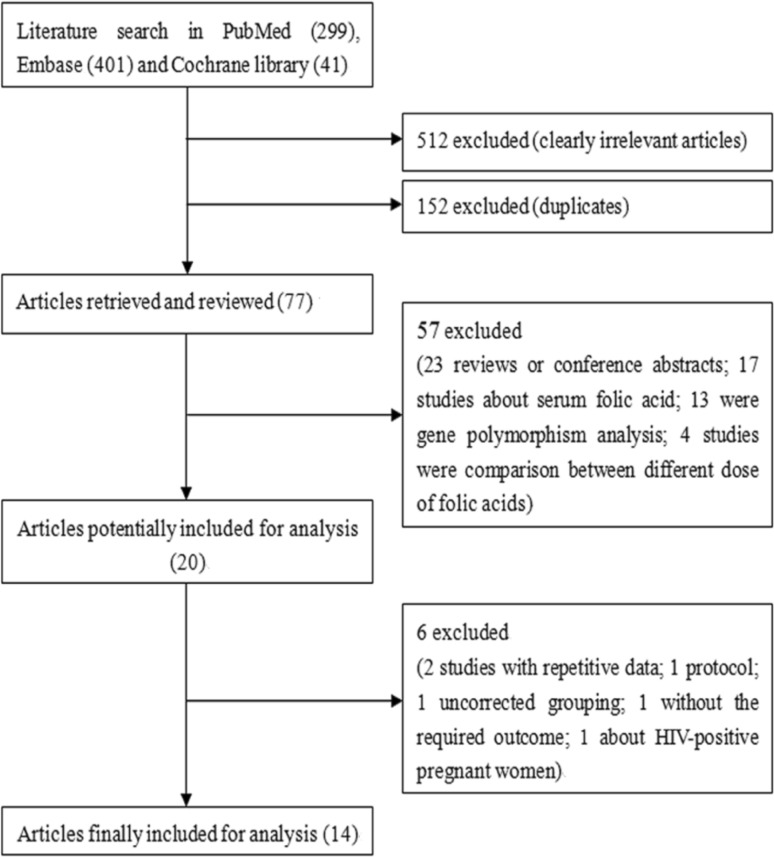
The flow chart for selection of eligible studies is shown in Fig. 1. In total, 741 articles, including 299 in PubMed, 401 in Embase and 41 in Cochrane library, were retrieved. After reviewing the title, 152 duplicates and 512 clearly irrelevant articles were excluded. The remaining 77 articles were then selected by reviewing the abstract, and 57 studies were excluded according to our exclusion criteria, including 23 reviews or conference abstracts, 17 about serum folic acid, 13 gene polymorphism analysis, and 4 about comparison between different doses of folic acids. After further reviewing of the full text, six studies were excluded, including two with repetitive data, one protocol, one uncorrected grouping, one without the required outcome, and one about HIV-positive pregnant women. At last, 14 eligible studies [13–16, 21–30] were included.
Fig. 1.
Flowchart of the literature search and study selection
The characteristics of included studies are shown in Table 1. There were 13 cohort studies (12–14, 20–22, 24–30) and 1 RCT [24], containing 160,562 and 149,320 women with or without folic acid supplementation during pregnancy. The period in which the women received folic acid was presented: some was in preconception, some were in early second trimester, and some were throughout pregnancy. The included studies were conducted in China, United States, UK, Australia, Denmark, Canada, and South Korea. The NOS assessment showed that the quality of the included 13 cohort studies was excellent (6–9 points). Moreover, RCT conducted by Charles et al. [24] showed that blinding of participants and personnel, allocation concealment, blinding of outcome assessment, and incomplete outcome data were all at the low risk (supplementary Fig. 1).
Table 1.
The characteristics of included studies
| Study | Study type | Country | Study period | The period of folic acid use | Folic acid supplement | No. | Quality assessment | |
|---|---|---|---|---|---|---|---|---|
| Suppl. | No suppl. | |||||||
| Bodnar et al. [21] | PCS | USA | 1997–2001 | In the past 6 months, daily use | Periconceptional multivitamin use | 860 | 975 | 7 |
| Bukowski et al. [22] | PCS | USA | 1999–2002 | In the past 1 year, daily use | Preconceptional folate supplementation | 12,444 | 15,259 | 7 |
| Catov et al. [23] | PCS | Denmark | 1997–2003 | 4 weeks before the LMP through 8 weeks after the LMP | Periconceptional multivitamin use | 21,785 | 11,503 | 6 |
| Folate-only users | 2609 | 11,503 | ||||||
| Charles et al. [24] | RCT | UK | 1966–1967 | < 37 weeks gestation | Periconceptional folic acid use | 1902 | 917 | |
| Hernandez-Diaz et al. [25] | RCS | USA, Canada | 1993–2000 | From 2 months before conception through the entire pregnancy | Periconceptional folic acid use | 1953 | 147 | 7 |
| Kim et al. [26] | RCS | South Korea | 2009–2010 | NR | Prenatal intake of folic acid | 134 | 81 | 8 |
| Li et al. [16, 31] | RCS | China | 1993–1996 | During early pregnancy | Folic acid supplementation | 92,731 | 100,823 | 9 |
| Liu et al. [27] | PCS | China | 2010–2012 | Pre- and post-conception | Folic acid intake | 7864 | 2315 | 7 |
| Martinussen et al. [28] | PCS | USA | 1996–2000 | First trimester overall | Folic acid supplementation in early pregnancy | 3301 | 346 | 8 |
| Timmermans et al. [29] | PCS | Netherlands | 2002–2006 | Before 8 weeks | Preconceptional folate supplementation | 2362 | 1770 | 9 |
| Vanderlelie et al. [13] | PCS | Australia | 2006–2011 | The first trimester of pregnancy | First trimester multivitamin/mineral use | 719 | 1066 | 7 |
| Folate-only users | 476 | 1066 | ||||||
| Wang et al. [15] | PCS | China | 2010–2012 | Before conception and/or during pregnancy | Dietary folate intake before conception and during pregnancy | 794 | 265 | 7 |
| Wen et al. [30] | PCS | Canada | 2002–2005 | In early second trimester | Prenatal multivitamin use | 2317 | 238 | 6 |
| Folate-only users | 421 | 238 | ||||||
| Wen et al. [14] | PCS | Canada | 2002–2008 | In early second trimester | Prenatal multivitamin use | 7265 | 404 | 6 |
| Folate-only users | 625 | 404 | ||||||
PCS prospective cohort study; RCS retrospective cohort study; RCT randomized controlled trail; Suppl supplementation; LMP last menstrual period
Relationship between supplementation of folic acid and risk of gestational hypertension
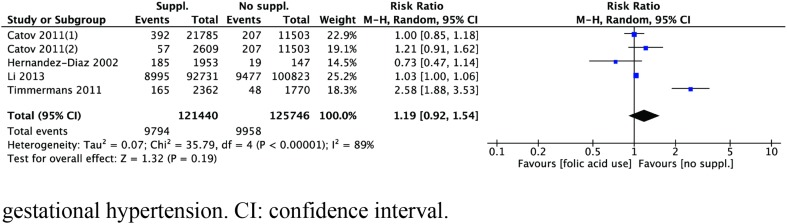
Four studies [23, 25, 29, 31] about the relationship between folic acid supplementation and gestational hypertension risk were included in this meta-analysis. The heterogeneity was high across the above four studies (I2 = 89%, P < 0.01). As shown in Fig. 2, there was no relationship between the supplementation of folic acid and gestational hypertension risk (RR = 1.19, 95% CI 0.92–1.54, P = 0.19).
Fig. 2.
Forest plot of the association between folic acid supplementation and risk of gestational hypertension. CI confidence interval
Relationship between supplementation of folic acid and risk of preeclampsia
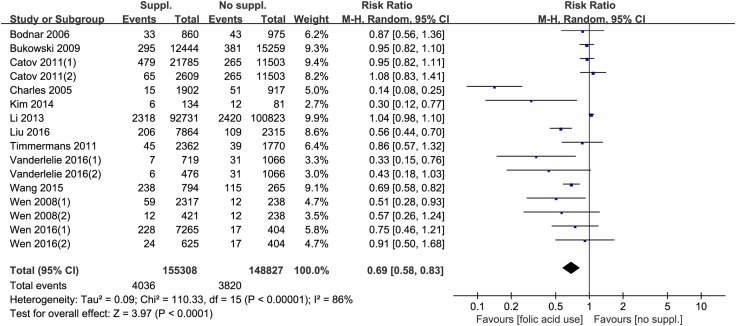
Twelve studies [13–15, 21–24, 26, 27, 29–31] were included to explore the relationship between folic acid supplementation and preeclampsia risk. The heterogeneity was also high across these studies (I2 = 86%, P < 0.01), and the pool results showed that the decreased risk of preeclampsia is related to folic acid supplementation during pregnancy (RR = 0.69, 95% CI 0.58–0.83, P < 0.01, Fig. 3).
Fig. 3.
Forest plot of the association between folic acid supplementation and risk of preeclampsia. CI confidence interval
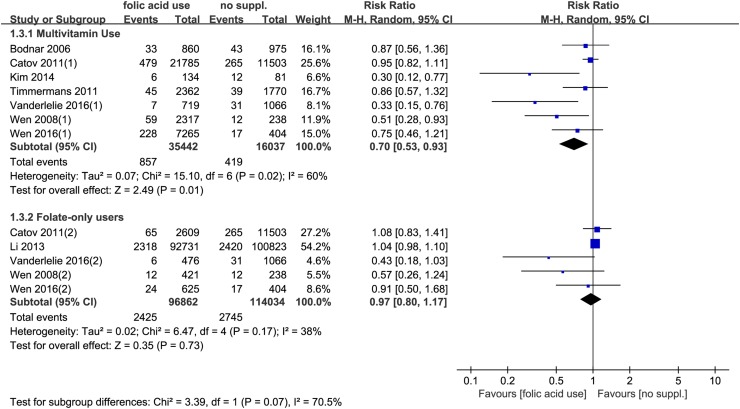
Furthermore, according to the patterns of supplementation of folic acid, a subgroup analysis was performed. The results showed that the supplementation of multivitamins containing folic acid significantly decreased the risk of preeclampsia (RR = 0.70, 95% CI 0.53–0.93, P = 0.01), while the supplementation of folic acid alone had no significant effects on preeclampsia risk (RR = 0.97, 95% CI 0.80–1.17, P = 0.73) (Fig. 4).
Fig. 4.
Forest plot of the association between different patterns of folic acid supplementation and risk of preeclampsia. CI confidence interval
Publication bias
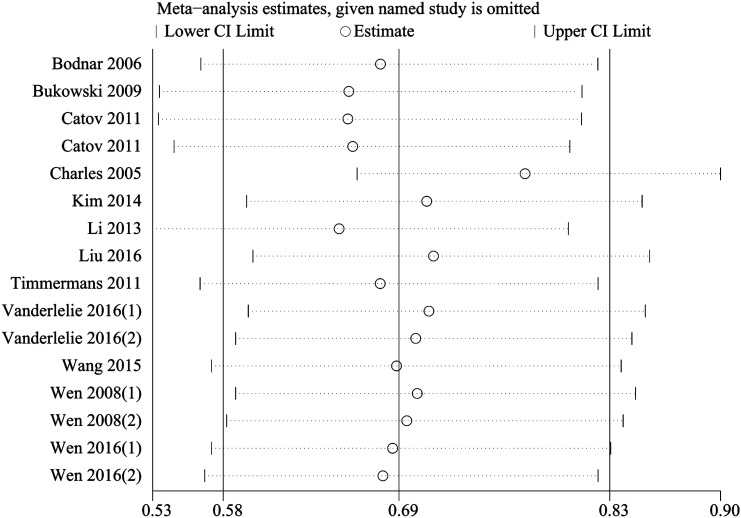
The results of Egger’s test showed that a significant publication bias existed among our included studies (P = 0.002). The publication bias was then verified using trim and fill method [32], and the obtained results were not changed. Moreover, a sensitivity analysis was performed by eliminating the included studies one by one, and eventually, the pooled results were also not significantly changed (Fig. 5).
Fig. 5.
A sensitivity analysis for publication bias of the included studies. CI confidence interval
Discussion
In this meta-analysis, 14 studies were included and pooled results showed that the supplementation of folic acid during pregnancy significantly reduced preeclampsia risk, but had no effects on gestational hypertension risk. Moreover, the decreased preeclampsia risk was associated with supplementation of multivitamins containing folic acid rather than folic acid alone. These results merit further discussion.
Supplementation of folic acid has been found to decrease preeclampsia risk. The possibility is that folic acid can affect the levels of hyperhomocysteinemia, which is suggested to damage the vascular endothelium of the developing placenta [33]. Moreover, a folate deficiency may induce the apoptosis of human cytotrophoblast cells, thus possibly affecting trophoblast invasion and placental development [34, 35]. Therefore, the supplementation of folic acid may improve placental implantation and subsequently affect the incidence of hypertensive pregnancy disorders. In this study, our results are consistent with the findings of a previous study reported by Catov et al. [36] that a decreased preeclampsia risk is related to multivitamin use, but there is no association between the risk of preeclampsia and folic acid supplement alone. Given the biologic rationale of folic acid in reducing the risk of developing preeclampsia, we speculate that folic acid may play a more important role in preeclampsia than other vitamins because the biologic mechanisms of other vitamins in reducing the risk of developing preeclampsia have not been fully explored. Moreover, previous RCTs have found that supplementation with vitamins C and E (without folic acid) during pregnancy has no protective effect on preeclampsia [37, 38]. Lv et al. also demonstrated that the risk for lower clinical pregnancy rate was not significantly correlated with deficient serum vitamin D level in infertile woman undergoing in vitro fertilization [39]. In addition to several studies that have confirmed a relationship between decreased preeclampsia risk and the supplementation of multivitamins containing folic acid [13, 14], we speculate that other vitamins may also play some roles in the prevention of preeclampsia, and the key roles of folic acid in preventing the risk of preeclampsia may be enhanced by other nutrients present in multivitamins. However, the ingredients of multivitamins in different included studies are largely unknown, hindering us to further explore the key other vitamins in preventing the risk of preeclampsia. More studies are still needed to confirm our observation.
Furthermore, strengths and limitations should be considered. Strengths of our study were that a total of 14 articles containing a larger number of patients were included in this study. The larger sample size increased the reliability of our results. In addition, this meta-analysis was conducted on a basis of good quality studies. Although publication bias existed among our included studies, the results of trim and fill method and a sensitivity analysis also confirmed that the pool results had no significant changes. Despite these, our results should be considered in light of limitations. First, a high heterogeneity existed in this study. One possibility for this was that the sample size differed among different studies, which may result in inconsistent 95% CI. Second, among all included studies, 13 were cohort studies and one was a RCT study. Third, we did not collect baseline nutritional data on the cohorts, especially the data on folic acid intake from food. In consideration of the findings of Ray and Mamdani [40] that folic acid from food intake was very low to make any impact on the risk of preeclampsia, we believe that supplementation of multivitamins containing folic acid during pregnancy may be beneficial. Fourth, the dosage of folic acid is largely unknown due to lack of related information in included studies and the period in which the women received folic acid was insistent, which may influence the effects of folic acid on the prevention of preeclampsia. Notably, previous findings showed that there was no significant association between dosage of folic acid supplementation and the strength of its effect [41, 42], but an inverse relationship exists between the duration of folic acid supplementation and the risk of stroke [42], suggesting that the supplementation duration of folic acid is more important than its dosage. A further analysis on exploring key period of folic acid supplementation may have important clinical significance. Considering the different study design among studies, our results are still needed to be further verified by more high-quality clinical studies.
In conclusion, the findings of this meta-analysis indicate that the supplementation of multivitamins containing folic acid during pregnancy may reduce preeclampsia risk. Multivitamin supplementation may be considered as a promising prevention strategy for preeclampsia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
CL: made the inclusion criteria and exclusion criteria, explored the literature, document selection, data extraction, data analysis, and composed the manuscript. CL: made the inclusion criteria and exclusion criteria, document selection, data analysis. QW: explored the literature, document selection, data extraction. ZZ: posed a question, made the inclusion criteria and exclusion criteria, and composed the manuscript
Funding
This study was funded by the International Cooperation Project with Russia (Grant Number 2015DFR31070) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Grant Number ZYLX201713).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Ayansina D, Black C, Hall S, Marks A, Millar C, Prescott G, Wilde K, Bhattacharya S. Long term effects of gestational hypertension and pre-eclampsia on kidney function: record linkage study. Pregnancy Hypertens. 2016;6(4):344–349. doi: 10.1016/j.preghy.2016.08.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masoudian P, Nasr A, de Nanassy J, Fung-Kee-Fung K, Bainbridge SA, El Demellawy D. Oocyte donation pregnancies and the risk of preeclampsia or gestational hypertension: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(3):328–339. doi: 10.1016/j.ajog.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 3.McGinnis R, Steinthorsdottir V, Williams NO, Thorleifsson G, Shooter S, Hjartardottir S, Bumpstead S, Stefansdottir L, Hildyard L, Sigurdsson JK. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat Genet. 2017;49(8):1255–1260. doi: 10.1038/ng.3895. [DOI] [PubMed] [Google Scholar]
- 4.Al-Jameil N, Khan FA, Khan MF, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6(1):1. doi: 10.5897/JCMR11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nomura Y, John RM, Janssen AB, Davey C, Finik J, Buthmann J, Glover V, Lambertini L. Neurodevelopmental consequences in offspring of mothers with preeclampsia during pregnancy: underlying biological mechanism via imprinting genes. Arch Gynecol Obstet. 2017;295(6):1319–1329. doi: 10.1007/s00404-017-4347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garovic VD, Bailey KR, Boerwinkle E, Hunt SC, Weder AB, Curb D, Mosley TH, Jr, Wiste HJ, Turner ST. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens. 2010;28(4):826. doi: 10.1097/HJH.0b013e328335c29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114(5):961–970. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41(3):437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez SE, Zhang C, Rene Malinow M, Ware-Jauregui S, Larrabure G, Williams MA. Plasma folate, vitamin B12, and homocyst(e)ine concentrations in preeclamptic and normotensive Peruvian women. Am J Epidemiol. 2001;153(5):474–480. doi: 10.1093/aje/153.5.474. [DOI] [PubMed] [Google Scholar]
- 10.Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen ALB, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71(4):962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- 11.Sudchada P, Saokaew S, Sridetch S, Incampa S, Jaiyen S, Khaithong W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;98(1):151–158. doi: 10.1016/j.diabres.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Rumbak I, Žižic V, Vrkic N, Baric IC. Folic acid supplementation impact on homocysteine and C-terminal cross-linking telopeptides of type I Collagen. Eur J Nutr Food Saf. 2015;5(5):1157–1158. doi: 10.9734/EJNFS/2015/21298. [DOI] [Google Scholar]
- 13.Vanderlelie J, Scott R, Shibl R, Lewkowicz J, Perkins A, Scuffham PA. First trimester multivitamin/mineral use is associated with reduced risk of pre-eclampsia among overweight and obese women. Matern Child Nutr. 2016;12(2):339–348. doi: 10.1111/mcn.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen SW, Guo Y, Rodger M, White RR, Yang Q, Smith GN, Perkins SL, Walker MC. Folic acid supplementation in pregnancy and the risk of pre-eclampsia—a cohort study. PLoS One. 2016;11(2):e0149818. doi: 10.1371/journal.pone.0149818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Zhao N, Qiu J, He X, Zhou M, Cui H, Lv L, Lin X, Zhang C, Zhang H, Xu R, Zhu D, Dang Y, Han X, Zhang H, Bai H, Chen Y, Tang Z, Lin R, Yao T, Su J, Xu X, Liu X, Wang W, Ma B, Liu S, Qiu W, Huang H, Liang J, Wang S, Ehrenkranz RA, Kim C, Liu Q, Zhang Y. Folic acid supplementation and dietary folate intake, and risk of preeclampsia. Eur J Clin Nutr. 2015;69(10):1145–1150. doi: 10.1038/ejcn.2014.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Ye R, Zhang L, Li H, Liu J, Ren A. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension. 2013;61(4):873. doi: 10.1161/HYPERTENSIONAHA.111.00230. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 2010;5(2):S38. [Google Scholar]
- 18.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng RN, Zhao C, Sun CH, Li Y. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. PLoS One. 2011;6(4):e18480. doi: 10.1371/journal.pone.0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodnar LM, Tang G, Ness RB, Harger G, Roberts JM. Periconceptional multivitamin use reduces the risk of preeclampsia. Am J Epidemiol. 2006;164(5):470–477. doi: 10.1093/aje/kwj218. [DOI] [PubMed] [Google Scholar]
- 22.Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GDV, Eddleman K, Gross SJ, Dugoff L, Craigo SD. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. 2009;6(5):e1000061. doi: 10.1371/journal.pmed.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catov JM, Bodnar LM, Olsen J, Olsen S, Nohr EA. Periconceptional multivitamin use and risk of preterm or small-for-gestational-age births in the Danish National Birth Cohort. Am J Clin Nutr. 2011;94(3):906–912. doi: 10.3945/ajcn.111.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles DHM, Ness AR, Campbell D, Smith GD, Whitley E, Hall MH. Folic acid supplements in pregnancy and birth outcome: re-analysis of a large randomised controlled trial and update of Cochrane review. Paediatr Perinat Epidemiol. 2005;19(2):112–124. doi: 10.1111/j.1365-3016.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Diaz S, Werler MM, Louik C, Mitchell AA. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am J Epidemiol. 2002;156(9):806–812. doi: 10.1093/aje/kwf129. [DOI] [PubMed] [Google Scholar]
- 26.Kim MW, Ahn KH, Ryu KJ, Hong SC, Lee JS, Nava-Ocampo AA, Oh MJ, Kim HJ. Preventive effects of folic acid supplementation on adverse maternal and fetal outcomes. PLoS One. 2014 doi: 10.1371/journal.pone.0097273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Lv L, Zhang H, Zhao N, Qiu J, He X, Zhou M, Xu X, Cui H, Liu S, Lerro C, Lin X, Zhang C, Zhang H, Xu R, Zhu D, Dang Y, Han X, Bai H, Chen Y, Tang Z, Lin R, Yao T, Su J, Wang W, Wang Y, Ma B, Huang H, Liang J, Qiu W, Liu Q, Zhang Y. Folic acid supplementation, dietary folate intake and risk of preterm birth in China. Eur J Nutr. 2016;55(4):1411–1422. doi: 10.1007/s00394-015-0959-1. [DOI] [PubMed] [Google Scholar]
- 28.Martinussen MP, Bracken MB, Triche EW, Jacobsen GW, Risnes KR. Folic acid supplementation in early pregnancy and the risk of preeclampsia, small for gestational age offspring and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2015;195:94–99. doi: 10.1016/j.ejogrb.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmermans S, Jaddoe VW, Silva LM, Hofman A, Raat H, Steegers-Theunissen RP, Steegers EA. Folic acid is positively associated with uteroplacental vascular resistance: the generation R study. Nutr Metab Cardiovasc Dis. 2011;21(1):54–61. doi: 10.1016/j.numecd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Wen SW, Chen XK, Rodger M, Rennicks White R, Yang Q, Smith GN, Sigal RJ, Perkins SL, Walker MC. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am J Obstet Gynecol. 2008;198(1):41–45. doi: 10.1016/j.ajog.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Ye R, Zhang L, Li H, Liu J, Ren A. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension. 2013;61(4):873–879. doi: 10.1161/HYPERTENSIONAHA.111.00230. [DOI] [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 33.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357(9249):53–56. doi: 10.1016/S0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 34.Steegers-Theunissen RPM, Smith SC, Steegers EAP, Guilbert LJ, Baker PN. Folate affects apoptosis in human trophoblastic cells. BJOG. 2000;107(12):1513–1515. doi: 10.1111/j.1471-0528.2000.tb11677.x. [DOI] [PubMed] [Google Scholar]
- 35.Di SN, Riccardi P, Maggiano N, Piacentani A, D’Asta M, Capelli A, Caruso A. Effect of folic acid on homocysteine-induced trophoblast apoptosis. Mol Hum Reprod. 2004;10(9):665–669. doi: 10.1093/molehr/gah091. [DOI] [PubMed] [Google Scholar]
- 36.Catov JM, Nohr EA, Bodnar LM, Knudson VK, Olsen SF, Olsen J. Association of periconceptional multivitamin use with reduced risk of preeclampsia among normal-weight women in the Danish National Birth Cohort. Am J Epidemiol. 2009;169(11):1304–1311. doi: 10.1093/aje/kwp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poston L, Briley A, Seed P, Kelly F, Shennan A, Consortium ViP-eT Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 38.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354(17):1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 39.Lv SS, Wang JY, Wang XQ, Wang Y, Xu Y. Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet. 2016;293(6):1339–1345. doi: 10.1007/s00404-016-4058-1. [DOI] [PubMed] [Google Scholar]
- 40.Ray JG, Mamdani MM. Association between folic acid food fortification and hypertension or preeclampsia in pregnancy. Arch Intern Med. 2002;162(15):1776–1777. doi: 10.1001/archinte.162.15.1776. [DOI] [PubMed] [Google Scholar]
- 41.Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;3:CD001056. doi: 10.1002/14651858.CD001056. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369(9576):1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.