Abstract
Objective. The use of cerebrospinal shunts is the standard of care for hydrocephalus. However, shunts are extremely vulnerable to failure and lack noninvasive methods to monitor their viability. We review current shunt technologies and attempts to improve their function. Methods. A PubMed search was performed to find literature on shunts and shunt function. Company brochures and websites were also used. Results. Fixed and variable pressure valves from four major companies are discussed. Also reviewed are siphon resistive devices, intracranial pressure sensors, and recent attempts on the development of cerebrospinal fluid sensors, including a micromechanical flow sensor we have recently developed. Conclusions. While variable pressure valves and siphon resistive devices have both had considerable success in dealing with variable intracranial pressure, a more sophisticated, continuous monitoring system is needed to ensure shunt viability and patient safety. An integrated flow sensor may provide the ability to track fluid flow and determine shunt functionality.
Keywords: Hydrocephalus, shunt valves, intracranial pressure sensors, flow sensors
Introduction
Hydrocephalus is caused by an accumulation of cerebrospinal fluid (CSF) within the ventricles of the brain. It has also been defined as active distension of the ventricles of the brain due to inadequate passage through the ventricular system [1]. Hydrocephalus is divided into two broad categories: communicating and obstructive. Communicating hydrocephalus occurs when CSF is unable to be reabsorbed back into the body. Congenital hydrocephalus, which falls under this category, may be the result of genetic or birth abnormalities and is often characterized by prominent enlargement of the head [2]. On the other hand, obstructive hydrocephalus is a result of a physical blockage within the ventricles [3]. Hydrocephalus is a prevalent condition, affecting 4 to 6 people per 1000 [4]. The prevalence underscores the need for better solutions to address this poorly treated affliction. Because hydrocephalus can vary depending on causes and locations of obstructions, the use of shunts began to emerge as the most common treatment.
To reduce accumulation, the shunt moves CSF to another part of the body (Figure 1). The general design of a shunt incorporates two catheters and a one-way valve [5]. A small catheter is first implanted into the ventricle of the brain, which is then connected to the main valve. The valve is responsible for the release of CSF from the brain into the second catheter that drains the CSF to the absorption site. Typically, CSF fluid is absorbed within the peritoneum or abdomen; however, lung and heart cavities may also be used [6].
Figure 1.
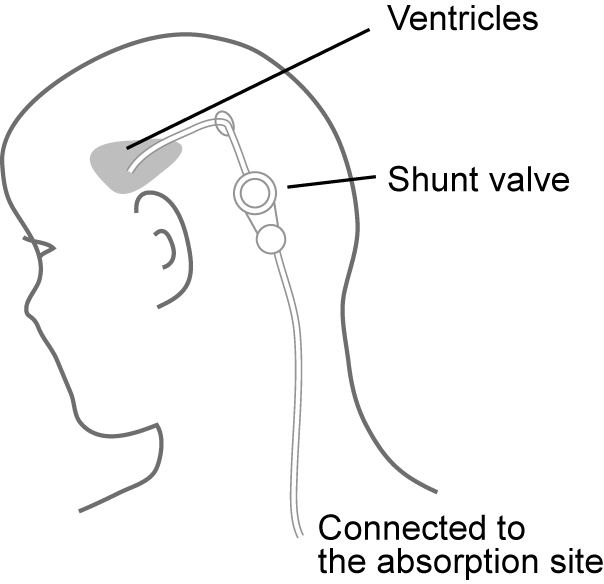
Implantation of a shunt, which is designed to move excess CSF from ventricles to another part of the body.
Despite the characterization and treatment of hydrocephalus for over 50 years, shunt designs which date from the 1950s have undergone few changes. The implantation of the shunt requires surgery, which has inherent risks, including infection. Shunts are prone to failure with issues ranging from shunt obstruction, disconnection, fracture, overdrainage, as well as underdrainage. Approximately 40 percent of shunts fail within two years of implantation and 98 percent fail after a 10-year span [6], which represents a major limitation of the current technology. With this in mind, novel developments of “smart shunts” are desirable to provide improved reliability, control, precision, and monitoring.
Methods
We performed PubMed literature searches with the following search terms: hydrocephalus, fixed pressure valve, variable pressure valve, transcutaneous pressure-adjustable valves, siphon resistive devices, Medtronic Strata, Codman-Medos Programmable Hakim valve, Aesculap MIETHKE, Tesla, MRI, and intracranial pressure. The search was limited to English language publications which were documented in PubMed before 11/30/2017. In addition to academic publications, we used company brochures and websites for information on previously and currently available shunt valves. These include Medtronic, DePuy Synthes, Aesculap, and Sophysa (Sophy) devices. We also reviewed recent efforts of integration of flow and pressure sensing in shunts. Finally, we introduce results of a CSF flow sensor we have developed.
Results
Shunt Usage in the USA
A study published in 1995 found that approximately 69,000 discharges occur each year in the USA relating to a hydrocephalus diagnosis for patients, while 36,000 hospital visits specifically involve shunt-related procedures. Of these 36,000 visits, 33,000 involved shunt placement [7]. Data retrieved from the year 2000 indicates that nearly 27,870 patients had shunt-related procedures [8]. Of these, 55.1 percent of visits involved emergent and urgent admissions while 43 percent constituted elective admissions. The two most common procedures were ventriculoperitoneal shunt placement (43.4 percent) and ventricular shunt replacement (42.8 percent). A newer report between 2001 and 2005 found that of the 7071 shunt placements, after a two-year period there were 4434 shunt-related procedures due to shunt malfunction [9]. CSF shunting procedures cost nearly $100 million in the USA, with almost half of this cost reflecting shunt revision. Regarding pediatric hydrocephalus, in a cross-sectional study conducted in 1997, 2000, and 2003, between 38,200 to 39,900 admissions were recorded each year totaling 391,000 to 433,000 hospital days. These admissions resulted in total hospital costs in the range of $1.4 to 2.0 billion, with hydrocephalus accounting for 0.6 percent of all 2003 pediatric hospital admissions [10]. Interestingly, over the course of this study, there was an increase in the age distribution of children with admittance for CSF shunt related malfunctions, with 21.6 percent of patients aged 11 to 18 years admitted in 1997, increasing to 23.2 percent and 25.9 percent in 2000 and 2003, respectively. These numbers suggest that older children were being admitted for chronic hydrocephalus conditions over time, increasing by approximately 1.3 percent over the course of the cross-sectional study.
Fixed Pressure Valves
Most shunts fall into one of two categories: a single setting fixed pressure valve or an adjustable variable pressure valve. The type of shunt is selected based on a combination of style, comfort, past experience, training, brand loyalty, advertising, and scientific evidence [11]. Fixed differential pressure valves, first used clinically in the 1950s, are non-adjustable valves that drain fluid at one setting. This type of valve is also commonly referred to as a mono pressure valve. It contains a ball and spring mechanism which has a static tension, as opposed to adjustable valves. Typically, a synthetic ruby ball connected to a flat metal spring is implemented in a cone mechanism. When the CSF reaches a high enough pressure, the ball compresses the spring, and CSF flows into the distal catheter. The valve has a one-way flow setting, draining excess CSF into the distal catheter where it is reabsorbed by the body at the end of the shunt, usually within the peritoneal cavity [12]. This type of valve is composed largely of a reservoir dome which allows physicians to sample cerebrospinal fluid via needle insertion and test shunt function. Called an “antechamber,” this volume can be filled manually by physicians to help keep the shunt open and check for the possibility of an obstruction in the proximal catheter (i.e., the reservoir is not filling) or an obstruction in the distal catheter (the reservoir remains stiff and does not respond to downward depressions). The benefits are a lack of components that may impact magnetic resonance imaging (MRI). Also, fixed valves can be small and are sometimes preferred for younger pediatric patients. Commonly used fixed differential pressure valves are the PS Medical Delta® and the Codman Hakim Precision Valve®, as well as the Aesculap MIETHKE. The Codman Hakim Precision Valve by DePuy Synthes, comes in eight different pre-set configurations, each of which can be separately purchased for implantation to ensure a correct pressure setting depending on the needs of the patient. The Precision Valve comes equipped with suture holes on the back for securing the valve to the pericranium, and the valve is equipped with an optional SIPHONGUARD® device which is described below. Because it is difficult to determine the valve pressure setting and it often requires adjustment, this type of valve is less commonly used as compared to variable pressure valves.
Variable Pressure Valves
The greatest difficulty with shunt valves is selecting the correct valve opening pressure for each patient at the time of implantation, and correction is often needed postoperatively. Variable pressure valves have the ability to change pressure to address over drainage and under drainage issues. Since variable pressure valves were introduced in the 1980s, numerous programmable valves have been developed including the Medtronic Strata, the Codman Hakim Programmable Valve, and the Aesculap MIETHKE.
Variable pressure valves use a magnetic torque to allow adjustment and measurement of the valve position. A magnet with the magnetization M experiences a torque (rotational force) T = Mmagnet × Hfield under the magnetic field Hfield. This torque always works in the direction that aligns the magnetization with an applied magnetic field. This mechanism is illustrated in Figure 2. The valve contains a magnetized material. To adjust the valve, a magnetic field is applied by an external magnet to rotate the valve. For measurement, a small and easily rotatable magnet placed above the valve works like a compass, and aligns to the magnetic field of the valve.
Figure 2.
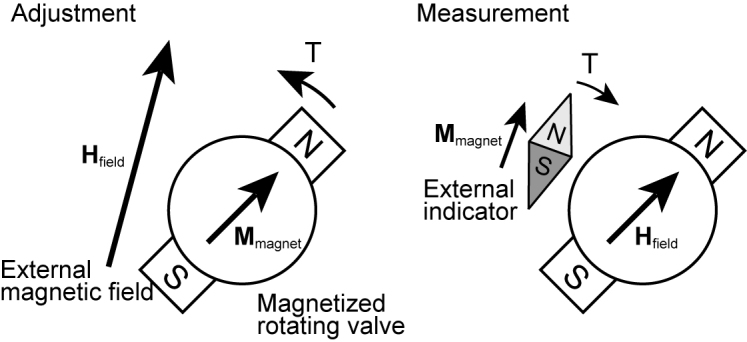
Working principle of a variable pressure valve. An external magnetic tool is used to rotate and adjust the valve. The orientation of the valve is measured by an external indicator.
The Codman Hakim Programmable Valve (CHPV), also known as the programmable Medos shunt, is a double spring ball valve, where the inlet valve controls the total pressure [13]. This specific section consists of a synthetic ruby ball depressed in the valve seat by a 316L flat stainless-steel spring. Opening pressure of the valve is adjusted by raising the spring on a spiral polyethersulfone staircase using the magnetic field from an external handheld apparatus [14]. The CHPV has a range of 30 to 200 mm H2O, with incremental steps of 10 mm H2O. To confirm the valve setting, plain skull radiography may be needed after shunt pressure adjustment. The Codman Hakim valve can withstand up to a 3 Tesla magnetic field, although the valve must be immediately readjusted after exposure to such a field to avoid complications such as a changed shunt setting from the magnetic interference or permanent disability of the valve [13]. In one documented study, the Medos valve behaved very well with one-third of the valves remaining fixed after being exposed to an external magnetic field of 1.5T from an MRI [15]. Despite this, the study conducted still found causes of concern as in 11 of 15 occasions, when valves were set to a starting value of 200 mm H2O, the magnetic field induced by the MRI caused the flat spring of the valve to move across the top of the cam and fall to a level below 30 mm H2O. A drastic reduction in the pressure setting from 200 mm H2O to 30 mm H2O could lead to siphoning of CSF and deleterious effects on the patient, such as subdural hematomas. One of the five Medos valves tested was no longer programmable after four test runs in the MRI. Attempts to reprogram the valve were conducted after 12 hours and 30 days, both of which failed to resolve the issue.
The Strata valve also uses a ball and a magnetic rotor, as has been detailed for the Medos valve. With the Strata, there are only five adjustable pressure settings, and a setting is selected depending on the specific needs of the patient. The Strata solution includes preoperative and postoperative adjustment tools. With the locator tool and the indicator tool, both preoperative and postoperative tools follow the mechanism detailed in Figure 2. The postoperative tools, named StrataVarius, includes a digital display to allow noninvasive measurement of pressure. The Strata is approved for MRI usage, but patients must seek readjustment immediately after imaging to ensure valve pressure invariability.
The Sophy programmable pressure valves were the first programmable valves. They also include spring ball valves [15]. Sophy valves include Mini SM8 and Polaris, with the design consisting of a semicircular spring attached to the end of a pivoting bar. This bar contains two micromagnets of cobalt-samarium that change the force exerted on the ball. The Polaris and Mini SM8 contain pressure settings ranging from 30 to 200 mmH2O. Both valves also support a variety of unique special pressure ranges depending on the needs of the patient. After implantation, the Sophy programmable valve is checked ex-cutaneously by radiography. During a study using a 1.5T MRI, it was found that Sophy valves nearly always changed to extreme positions (“low” or “high” pressure settings) in an unpredictable manner [13]. However, in the study it was always possible to reprogram the Sophy valve, indicating that the valve was not rendered unresponsive. The shunts are currently marketed as being capable of resisting magnet strength up to 3 Teslas.
The ProGAV valve, manufactured by Miethke and Aesculap, also contains a ball in cone valve system for adjustable pressure settings. However, unlike the previous valves this unit consists of two separate compartments that first house an adjustable unit and then a non-adjustable gravitational Shunt Assistant®, which will be discussed below. The variable unit has adjustable settings in the range of 0 to 200 mm H2O. The ProGAV shunt contains a brake system inside the valve that maintains rotor position in order to prevent unwanted re-adjustment, in the instance that the shunt experiences an unexpected magnetic field. Unlike other valves, this valve has a relatively large diameter (18 mm) intended to reduce CSF obstruction. Interestingly, unlike most other valves this valve can be implanted in the chest as opposed to on the skull. The actual position of the valve is not important so long as the Shunt Assistant® is placed parallel to the longitudinal axis of the body to minimize or eliminate siphoning effects. The ProGav Shunt comes with a special compass, which reads the valve’s current setting, and an external adjustment tool. Unlike other valves, force must first be applied onto the superior surface to unlock the braking mechanism. To allow rotation of the shunt, a downward force of 800 to 1600 gram force is applied using the ProGAV adjustment tool, and then the adjustment tool’s magnet alters the pressure setting. This braking system performed well under an external magnetic field and was deemed MRI safe in one study of three ProGAV shunts [16]. Nonetheless, it is still prudent to check the valve setting after MR imaging to ensure correct function. Due to the excessive overlap of these valves, other types of Miethke valves will not be discussed here.
Siphon Resistive Devices
Standard CSF shunt valves control flow typically through the use of ball-in-spring valves. Once opened, the valve provides minimum flow resistance. Often gravitational effects may lead to deleterious effects, particularly to largely negative intracranial pressures (ICPs), which are known as the “siphoning” effect. This negative hydrostatic pressure occurs when the patient stands in an upright position and gravity “sucks” fluid out of the shunt. This causes over-drainage in the shunt and potential complications, such as subdural hematomas. To prevent this, many current shunts come equipped with devices to reduce the tendency to siphon CSF. Three main siphon resistive devices (SRDs) have evolved – a mechanism composed of a subcutaneous varying membrane, a gravitational mechanism, as well as a flow regulating mechanism. The flow regulating mechanism is very similar in design to the gravitational mechanism, and will be described in conjunction with it.
Membranous devices include anti-siphon devices or siphon control devices. As shown in Figure 3, these anti-siphon devices have silicone membranes that close when postural over drainage begins to occur. Membranous compartments are sensitive to negative hydrostatic pressure across each side of the catheter and increase the required valve opening pressure in order to close the valve, as the patient stands in an upright, vertical position. The Delta Valve, currently built into the Medtronic PS Medical and Strata shunts, is based on this design [17]. The Delta Valve is produced in three opening pressure designations termed levels 1, 1.5, and 2, which correspond to 70, 105, and 120 mm H2O, respectively. These shunts have been very successful in reducing the amount of siphoning and are cited as suitable for implementation in pediatric patients [18].
Figure 3.
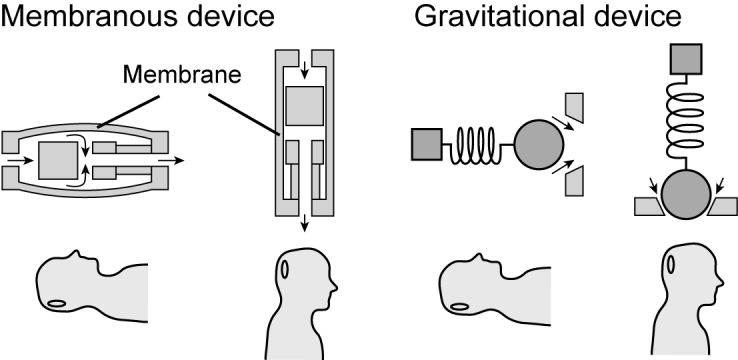
Siphon resistive devices. (a) Membranous devices include a pressure sensitive membranous compartment. (b) Gravitational mechanisms include a mass-spring system that reacts to gravity and regulates the opening of a valve.
Gravitational valves, often termed hydrostatic mechanisms, are another passive SRD that use metal balls that fall within a cone-shaped seat as the patient moves into a standing (vertical) position (see Figure 3). In the horizontal position the ball is away from the CSF path and provides little resistance to the fluid, but as the patient moves to a standing position the gravity assisted ball blocks the path of the fluid and creates an opening pressure approximately equal to the sum of the opening pressure of the adjustable unit and gravitational unit [18]. This compensation mechanism is precisely how the ProGAV Gravitational Shunt Assistant functions with adjustable Aesculap Miethke valves. The main drawback of these types of valves occurs when the compartment is displaced from the vertical position, due to unexpected patient activities (intensive sports, movements, etc.). This displacement results in changing opening pressures due to an angled valve experiencing different gravitational effects.
The SIPHONGUARD® Device, incorporated into Codman Hakim valves, follows similar properties of the aforementioned gravitational valves, but is considered a flow regulating mechanism. The device has two channels for removing CSF, with the central channel including a ball on spring valve that responds to changes in CSF (due to the effect of gravity on the fluid) by closing when flow rate exceeds a specific threshold level and remaining open at all other times [19]. The second-high resistance passageway is used after the first closes, and again drains CSF, helping to lower flow rate. This high hydrodynamic resistance helps to prevent posture related over drainage in patients experiencing such issues. One issue with these SRDs is that even in a high resistance state, the valve may allow flow more than 20 mL/hour, resulting in continuous drainage and decreased ICP [20,21].
Current Pressure Sensing Technology
Measurement of ICP is often used to monitor patients after injury as ICP increase is associated with brain swelling, tumors, or other trauma. Normal ICP tends to range between 5 and 15 mm Hg (68 and 200 mm H2O), although simple acts such as coughing or sneezing can transiently elevate ICP to 50 mm Hg (680 mm H2O) temporarily [22]. All ICP monitoring devices commercially available need a physical connection between the brain and the external environment, and cannot be implanted long term. Externally connecting the sensors stands as a potential source of infection, as well as limiting ICP monitoring time. Implantable telemetric monitoring devices, which transmit data transcutaneously, have been fabricated over the past twenty years, but issues have limited their clinical use [23]. Currently, there are three viable ways of measuring ICP including ventricular catheters, subdural screws, and epidural sensors. Epidural sensors evolved based on the ease of implantation into the epidural space. In addition, there is low incidence of hemorrhage and serious infection [24]. The subdural screw is used for immediate monitoring and contains a hollow bolt that goes into the skull near the dura, allowing CSF to enter into the bolt [1]. These systems provide the benefit of low risk of infection. However, the screws themselves often underestimate ICP and become clogged with debris inside the dura. The most widely utilized method for ICP measurement is the external ventricular catheter, which is a fluid-filled catheter transducer system allowing for calibration and correction of zero drift based on its known position [24]. These catheter tip transducer systems include strain gauges or fiber optic pressure sensors. These multi-purpose devices can measure ICP and drain excess CSF to reduce ICP.
Attempts for CSF Flow Sensing
Currently, none of the four major companies provide solutions to monitor CSF flow noninvasively. An external device called the ShuntCheck employs thermal change as a surrogate for flow in the distal catheter. It requires the use of an ice cube to cool the skin for 1 minute and then measure flow through thermal change. This method has proven 92 percent effective in a test of 26 patients [25]. However, there remains great variability regarding flow detection between patient postures and further research into this developing technology is required. Additionally, patient discomfort from placing ice on the skin for 1 minute is not necessarily desirable, especially with children.
Bork et al. reported on a CMOS thermal anemometer-based sensor [26], where the flow rate is found by measuring changes in the temperature distribution resulting from the heat flux from an integrated source located near the temperature sensors. A system like this has been applied neither clinically nor in vivo, mostly because of the difficulty associated with the integration of electrical sensors and other components in an implantable system.
Our Recent Attempt for CSF Flow Sensing
We have recently developed a device capable of noninvasive measurement of internal shunt fluid flow in real time [27]. The mechanism contains a micromechanical hair sensor responsive to small variations in flow speed, which can be detected externally using an infrared light. This novel development theoretically provides the ability to measure shunt function by measuring flow rate noninvasively.
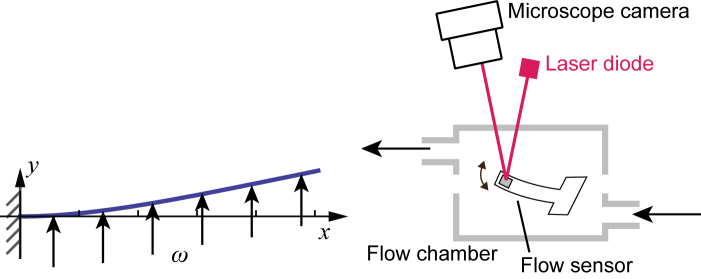
The device includes a two-piece compartment and a flow sensor situated inside the compartmental cavity held by two layers of polydimethylsiloxane (PDMS) film. Figure 4 shows the flow sensing cantilever, or a hair sensor, which provides the key function of the system. As illustrated in Figure 4, in the presence of fluid flow the cantilever deflects due to fluid pressure. The sensor is fabricated by patterning an SU-8 negative photoresist film through photolithography. The fabrication process is similar to that of the micro force sensor we previously reported [28]. In this study, we used a cantilever sized 1500 µm (length) × 200 µm (width) × 2 µm (thickness). A 100 nm-thick titanium layer was deposited at the tip. We varied fluid flow rates and recorded angle deviation through optical detection. A 650 nm laser light was focused onto the sensor through an opening in the compartment. The reflected light captured by a microscopic camera positioned above measures angular variations.
Figure 4.
Design of the flow sensor. Cantilever bending is induced by the flow and is measured optically.
The working principle can be described as a cantilever deflected by a uniformly distributed pressure. When the distributed pressure ω is applied to a cantilever (length l, the area moment of inertia I, and Young’s modulus E), deflection δy at position x is expressed as:
| (1) |
The maximum deflection occurs at the end of the cantilever (x = l):
| (2) |
The sensor was tested with varied flow rates created by a programmable syringe pump. A modulation flow rate resulted in a varying angle of reflection, which was measured externally by the camera. The sensor output at a modulated flow of 60 mL/hr with a duty cycle of 8-sec is shown in Figure 5. The solid line represents the measured light intensity at the sensor tip. The background light intensity measured as a control is shown as the dotted line in the figure. The sensor output amplitudes plotted in Figure 6 demonstrate very good linearity. With regards to the data collected in Figures 5 and 6, the fabricated sensor successfully measured slow flow rates of approximately 20 to 90 mL/hr, which closely correlates to the sensitivity required to assess current shunt functionalities.
Figure 5.
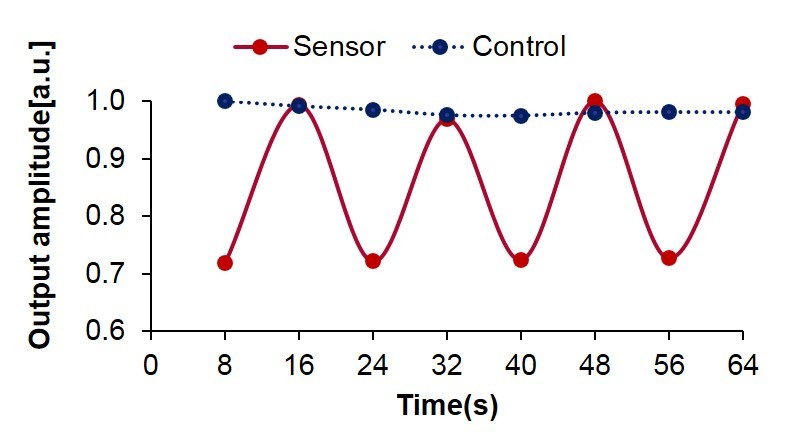
Sensor output at an on-and-off flow of 60 mL/hr.
Figure 6.
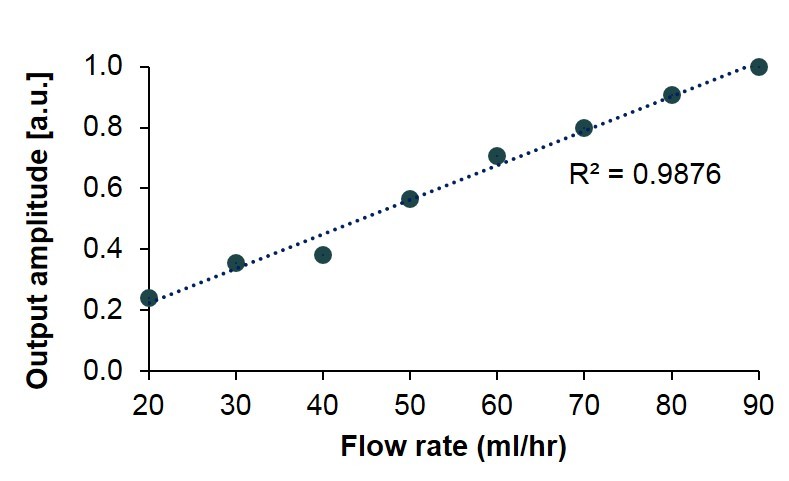
Sensor output for different flow rates.
Discussion
CSF serves not only to provide nutrients to the brain but also acts as a “cushion” in the event of unexpected impact. Therefore, understanding ICP or CSF flow fluctuations throughout the day can provide a window into brain function and help elucidate the brain response to injury. A few ongoing and future efforts seek to develop devices to measure ICP and CSF flow noninvasively. The concept behind the smart sensing technology is to provide a feedback system where data measurements provide information on shunt efficiency, hardware malfunction or damage, and for optimizing shunt usage. With 27,870 patient hospital visits each year, the ability to easily check potential shunt malfunctions excutaneously will improve patient and caretaker peace of mind and reduce unnecessary hospital procedures and visits, especially if the signal can be broadcast to the patient through a smartphone or similar device. Although in vivo animal studies remain to be performed, key elements needed for testing have been verified. Our device design is ready to be replicated using FDA approved biocompatible materials, such as polydimethylsiloxane (PDMS) or polypropylene, which are used in commercially available shunt valves [29].
Conclusion
We have reviewed the current technologies used in shunt valves. The improvement of shunt technology, since their advent, has been limited. Shunt failure rates remain high and even the slightest headache can be cause for alarm. While variable pressure valves and siphon resistive devices have both had large success in dealing with variable ICP, a more sophisticated, continuous monitoring system is needed to ensure shunt viability and patient safety. The micromechanical device presented here may provide such relief. Equipped with an optical monitoring system, the sensor has the ability to track fluid flow and determine flow stagnation, indicating shunt obstruction. We are continuing to work on the “smart” shunt system with the goal of pairing the device with current shunt products for broad use of the device.
Glossary
- CSF
Cerebrospinal Fluid
- MRI
Magnetic Resonance Imaging
- CHPV
Codman Hakim Programmable Valve
- SRDs
Siphon Resistive Devices
- CMOS
Complementary metal–oxide–semiconductor
- PDMS
Polydimethylsiloxane
Author Contributions
GS wrote the paper, and reviewed literature in preparation for writing. He also performed measurements and analysis prior to manuscript preparation. MB performed measurements and analysis. DJ performed measurements and analysis. HPZ provided conceptualization of the project, performed literature review, and performed clinical overview. MLD conducted both literature review and clinical overview. RAG performed conceptualization of the project, conducted review of literature, and provided clinical overview. KH performed conceptualization of the project, wrote the paper, conducted literature review, and measurements and analysis.
References
- Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166–86. [DOI] [PubMed] [Google Scholar]
- Gallia GL, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2(7):375–81. [DOI] [PubMed] [Google Scholar]
- Nassar BR, Lippa CF. Idiopathic normal pressure hydrocephalus: A review for general practitioners. Gerontol Geriatr Med. 2016;2:2333721416643702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng S, Gupta N, Wrensch M, Zhao S, Wu YW. Prevalence of congenital hydrocephalus in california, 1991-2000. Pediatr Neurol. 2011;45(2):67–71. [DOI] [PubMed] [Google Scholar]
- Drake J, Kestle J, Tuli S. CSF shunts 50 years on–past, present and future. Childs Nerv Syst. 2000;16(10):800–4. [DOI] [PubMed] [Google Scholar]
- Lutz BR, Venkataraman P, Browd SR. New and improved ways to treat hydrocephalus: pursuit of a smart shunt. Surg Neurol Int. 2013. March;4 Suppl 1:S38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg. 1995;23(5):254–9. [DOI] [PubMed] [Google Scholar]
- Patwardhan RV, Nanda A. Implanted ventricular shunts in the united states: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56(1):139–45. [DOI] [PubMed] [Google Scholar]
- Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR, et al. Hospital care for children with hydrocephalus in the united states: Utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131–7. [DOI] [PubMed] [Google Scholar]
- Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, LaFleur B, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the united states. J Neurosurg Pediatr. 2009;4(2):156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain H, Sgouros S, Walsh A, Hockley A. The treatment of infantile hydrocephalus:” differential-pressure” or” flow-control” valves. Childs Nerv Syst. 2000;16(4):242–6. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Buck DW, Sciubba D, Woodworth GF, Carson B, Weingart J, et al. Adjustable vs set-pressure valves decrease the risk of proximal shunt obstruction in the treatment of pediatric hydrocephalus. Childs Nerv Syst. 2007;23(3):289–95. [DOI] [PubMed] [Google Scholar]
- Ortler M, Kostron H, Felber S. Transcutaneous pressure-adjustable valves and magnetic resonance imaging: an ex vivo examination of the codman-medos programmable valve and the sophy adjustable pressure valve. Neurosurgery. 1997;40(5):1050–8. [DOI] [PubMed] [Google Scholar]
- Black PM, Hakim R, Bailey NO. The use of the codman-medos programmable hakim valve in the management of patients with hydrocephalus: illustrative cases. Neurosurgery. 1994;34(6):1110–3. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kuzu Y, Ogasawara K, Ogawa A. Effect of 3-tesla magnetic resonance imaging on various pressure programmable shunt valves. J Neurosurg Pediatr. 2005;103(2):163–5. [DOI] [PubMed] [Google Scholar]
- Allin DM, Richards HK, Pickard JD, Czosnyka M, Czosnyka ZH. In vitro hydrodynamic properties of the miethke ProGAV hydrocephalus shunt. Cerebrospinal Fluid Res. 2006;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnyka Z, Czosnyka M, Richards H, Pickard J. Evaluation of three new models of hydrocephalus shunts [Suppl] Acta Neurochir (Wien). 2005;95:223–7. [DOI] [PubMed] [Google Scholar]
- Ahn ES, Bookland M, Carson BS, Weingart JD, Jallo GI. The strata programmable valve for shunt-dependent hydrocephalus: the pediatric experience at a single institution. Childs Nerv Syst. 2007;23(3):297–303. [DOI] [PubMed] [Google Scholar]
- Czosnyka Z, Czosnyka M. D PICKARD J. Hydrodynamic performance of a new siphon preventing device: the SiphonGuard. J Neurol Neurosurg Psychiatry. 1999;66(3):408–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtom KH, Magram G. Siphon regulatory devices: their role in the treatment of hydrocephalus. Neurosurg Focus. 2007;22(4):1–8. [DOI] [PubMed] [Google Scholar]
- Chari A, Czosnyka M, Richards HK, Pickard JD, Czosnyka ZH. Hydrocephalus shunt technology: 20 years of experience from the cambridge shunt evaluation laboratory. J Neurosurg. 2014;120(3):697–707. [DOI] [PubMed] [Google Scholar]
- Ravi R, Morgan R. Intracranial pressure monitoring. Curr Anaesth Crit Care. 2003;14(5):229–35. [Google Scholar]
- Frischholz M, Sarmento L, Wenzel M, Aquilina K, Edwards R, Coakham H. Telemetric implantable pressure sensor for short-and long-term monitoring of intracranial pressure. Proceedings of the 29th Annual International Conference of the IEEE EMBS; 2007: 514. [DOI] [PubMed]
- Zhong J, Dujovny M, Park HK, Perez E, Perlin AR, Diaz FG. Advances in ICP monitoring techniques. Neurol Res. 2003;25(4):339–50. [DOI] [PubMed] [Google Scholar]
- Abazi G, Fleming L, Swoboda M, Anor T, Madsen JR. Evaluation of CSF flow in shunts using a non-invasive thermal technique. Cerebrospinal Fluid Res. 2009;6 S1:S41. [Google Scholar]
- Bork T, Hogg A, Lempen M, Müller D, Joss D, Bardyn T, et al. Development and in-vitro characterization of an implantable flow sensing transducer for hydrocephalus. Biomed Microdevices. 2010;12(4):607–18. [DOI] [PubMed] [Google Scholar]
- Bao M, Soler G, Jaiswal D, Zaveri HP, Grant RA, DiLuna ML, et al. A novel flow sensor for a smart shunt system. Proc. Northeast Bioengineering Conference. 2017:137
- Jaiswal D, Cowley N, Bian Z, Zheng G, Claffey KP, Hoshino K. Stiffness analysis of 3D spheroids using microtweezers. PLoS One. 2017;12(11):e0188346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale ML, Falk GL. In vitro assessment of back pressure on ventriculoperitoneal shunt valves. Surg Endosc. 1999. May;13(5):512–5. [DOI] [PubMed] [Google Scholar]