Abstract
Quantitative phase imaging (QPI) has emerged as one of the powerful imaging tools for the study of live cells in a non-invasive manner. In particular, multimodal approaches combining QPI and fluorescence microscopic techniques have been recently developed for superior spatiotemporal resolution as well as high molecular specificity. In this review, we briefly summarize recent advances in three-dimensional QPI combined with fluorescence techniques for the correlative study of cell pathophysiology. Through this review, biologists and clinicians can be provided with insights on this rapidly growing field of research and may find broader applications to investigate unrevealed nature in cell physiology and related diseases.
Keywords: quantitative phase imaging, fluorescence imaging, label-free imaging, holotomography, correlative imaging, microscopy
Introduction
Optical imaging of biological cells and tissues has been utilized as an indispensable technique providing invaluable information on the pathophysiology of diseases. Since the observation of cork cells using a microscope by Robert Hooke in the 17th century, various microscopic techniques have been developed to achieve better imaging capabilities. In particular, fluorescence microscopy is one of the important advances that opened a new era in molecular biology and molecular diagnosis. Via specific labeling of target molecules with fluorescence probes, unprecedented molecular specificity and imaging contrast could be achieved. However, the signals from fluorescence probes are qualitative due to varying permeability of fluorescence dyes. Also, repeated measurements are limited due to photobleaching and phototoxicity. More importantly, they require the use of exogenous labeling agents which may prevent from live cell imaging of intact cells, and labeled cells are very limited to in vivo applications.
Quantitative phase imaging (QPI) is an interferometric microscopy technique, which measures the optical phase delay induced by refractive index (RI) difference between a sample and medium [1,2]. Because RI is an intrinsic optical property of a material, no exogenous labeling agent is required to generate an imaging contrast in QPI. Furthermore, from the measured phase delay, the morphological and chemical properties of a sample can be quantitatively retrieved. These advantages make QPI increasingly attractive in studying various biological samples, including blood cells [3-5], bacteria [6-9], neurons [10,11], parasites [12,13], plant cells [14,15], cancer cells [16-19], inflamed tissues [20], and tissue slices [21,22].
Optical diffraction tomography (ODT), one of the three-dimensional (3D) QPI methods, reconstructs the 3D RI distribution of a sample from the measurements of multiple two-dimensional (2D) holograms via inverse scattering principle [23]. Multiple 2D holograms of a sample can be obtained by utilizing illumination angle scanning [24-28] or sample rotation [29-32]. RI distribution of a sample serves as an intrinsic optical imaging contrast, which provides physical and chemical information including protein concentration and cellular dry mass in a quantitative manner [33,34]. In particular, the applicability of ODT to various research area also have been demonstrated, such as the physiology of various biological samples including blood cells [35-37], immune cells [30,38], embryos [39], bacteria, and various eukaryotic cells [40-42].
QPI approaches have provided a new methodology for investigating the pathophysiology of live cells and tissues via label-free and quantitative imaging. Although label-free and high-speed 3D imaging capability of QPI provides the advantage for live cell imaging, the limited molecular specificity strongly restricts broader applications in cell biology and biochemistry.
To overcome the limited molecular specificity in QPI while maintaining the advantages of the method, several multimodal approaches have been recently demonstrated. For example, ODT integrated with multi-spectral light sources [43], Raman spectroscopy [44], and structured illumination microscopy [45,46] have demonstrated the potential for combining molecular specific information and morphological information. In particular, correlative imaging approaches combining fluorescence microscopy and QPI take the advantages of quantitative imaging, superior spatiotemporal resolution, and molecular specificity. Although the exogenous labeling agents are required, synergetic advantages between QPI and fluorescence microscopy suggested new applications.
Here, we review the recent advances in the correlative imaging techniques combining 3D QPI with various fluorescence microscopic techniques. First, we introduce the principle of QPI and ODT. Then, we summarize important demonstrations of the correlative imaging for various biological and medical studies. Prospective applications and futures of the correlative imaging will also be discussed.
Principle of Quantitative Phase Imaging
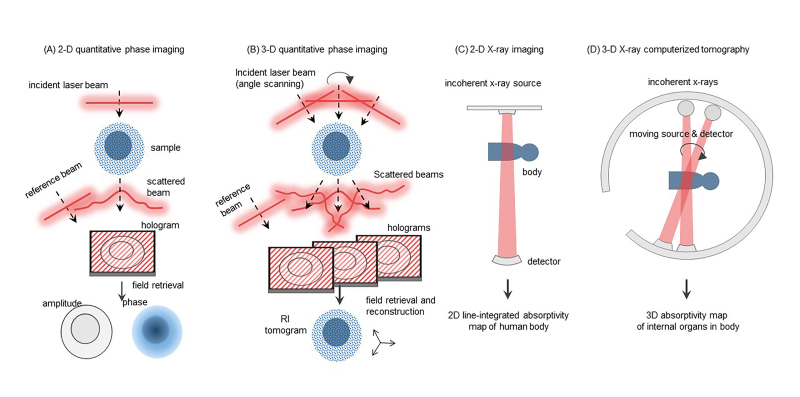
By exploiting the interference nature of light, QPI techniques enable us to retrieve not only the amplitude but also the phase information of scattered light from a sample. Interference between the scattered light and well-defined reference light produces an interference pattern, called a hologram or an interferogram [47,48] (Figure 1A). Several field retrieval algorithms [49,50], utilizing temporally or spatially modulated reference light, have been developed to extract the optical field information, i.e., both the amplitude and phase, from a measured hologram.
Figure 1.
Overview of 2D and 3D imaging. The schematic of (A) 2D quantitative phase imaging, (B) 3D quantitative phase imaging, (C) 2D X-ray imaging, and (D) 3D X-ray computerized tomography.
In an aspect of imaging, the phase information of the light scattered by an object is the optical phase delay map, which is related to the light refraction. Although most biological cells and tissues are transparent under optical wavelengths, light passing through these biological samples exhibits optical phase delays depending on the morphology and distribution of RI values of each sample. In principle, the optical phase delay is calculated as the integration of RI values along the trajectory of light passing through a sample subtracted inside the integration of those passing through surrounding media. Even microscopic cells usually produce significant optical phase delays, which can be precisely measured by QPI techniques without using an exogenous agent. On the contrary, these biological cells are mostly transparent in visible light ranges and thus do not produce enough imaging contrast to be imaged in bright-field microscopy. This aspect makes QPI an especially useful imaging tool for observing live cell morphology and dynamics without disturbing its physiological condition.
In 2D QPI, the measured optical phase delay is simply a coupled value between the thickness of a sample and the mean RI value of the sample. To retrieve one value, the other value should be known. Although 2D QPI can be effectively applied to several applications such as imaging red blood cells (RBCs) or bacteria, the detailed 3D morphology of eukaryotic cells or their internal structures cannot be directly investigated. 3D QPI enables the reconstruction of 3D RI distributions [23,51,52] (Figure 1B).
The principle of ODT is the inverse solving of wave equation (Helmholtz equation for monochromatic wave); from the multiply scattered waves obtained with various illumination angles, scattering potentials or 3D RI tomogram of a sample can be reconstructed. With two pre-assumptions of slightly varying permittivity in a wavelength scale and a weakly scattering condition of a sample, ODT maps the measured 2D optical field onto a 3D scattering potential of the object; a 3D Fourier transformed pair of the object RI tomogram multiplied by a numerical factor. Then, to precisely infer the 3D shape of the object from limited information, the remaining portion of the scattering potential, inaccessible by experiments mainly due to finite aperture sizes of an imaging system, is often supplemented with various image regularization methods [53]. Finally, inverse 3D Fourier transform of the mapped scattering potential gives a 3D complex RI tomogram of a sample.
In ODT imaging systems, the successive measurements of 2D optical fields, necessary for obtaining a tomogram, are usually accomplished either by varying illumination beam angles impinging onto a fixed sample [25,27,54], rotating the sample under a fixed beam illumination [29,55], or varying illumination beam wavelengths [56,57]. Further, the suppression of coherent noises in reconstructed 3D RI tomograms can be achieved in partially coherent [58,59] or incoherent [60] ODT at the expense of an additional sample scanning process in an axial direction. The details on the principle of ODT can be found elsewhere [12,52,61].
ODT can be understood as an optical analogous to X-ray computerized tomography (CT). Whereas 2D X-ray only provides the integrated projection images (Figure 1C), X-ray CT reconstructs 3D X-ray absorptivity tomograms of a human body (Figure 1D). Similarity, 2D QPI provides the projection images of cells, 3D QPI reconstructs 3D optical RI tomograms of a live cell.
Despite the advantages of QPI for observing biological cells, its limited molecular specificity restricts to distinguish different intracellular organelle and protein compositions except for a few cases. In order to overcome this limitation, QPI approaches utilizing hyperspectral wavelengths to address distinctive dispersion spectra of target molecules [43,62,63] have been investigated [43,62,63], discerning cell nuclei using the ultraviolet wavelength [64], and selectively attaching the nanoparticles with high RI values to the target proteins in cells [41,65]. Furthermore, the correlative imaging tools combining QPI with other imaging modalities such as Raman [44], confocal microscopy [66], and structured illumination microscopy [46,67] have also arisen recently, as we will mainly discuss the details in the following sections.
Correlative Imaging Techniques in QPI
Two-dimensional QPI with Correlative Imaging Techniques
As an emerging imaging technique, QPI has shown potential in various fields of study, with its unique advantages of label-free and quantitative imaging capability. However, limited molecular specificity of QPI has prevented from being adopted in many applications of bioimaging. This drawback of QPI can be overcome with the aid of other optical modalities.
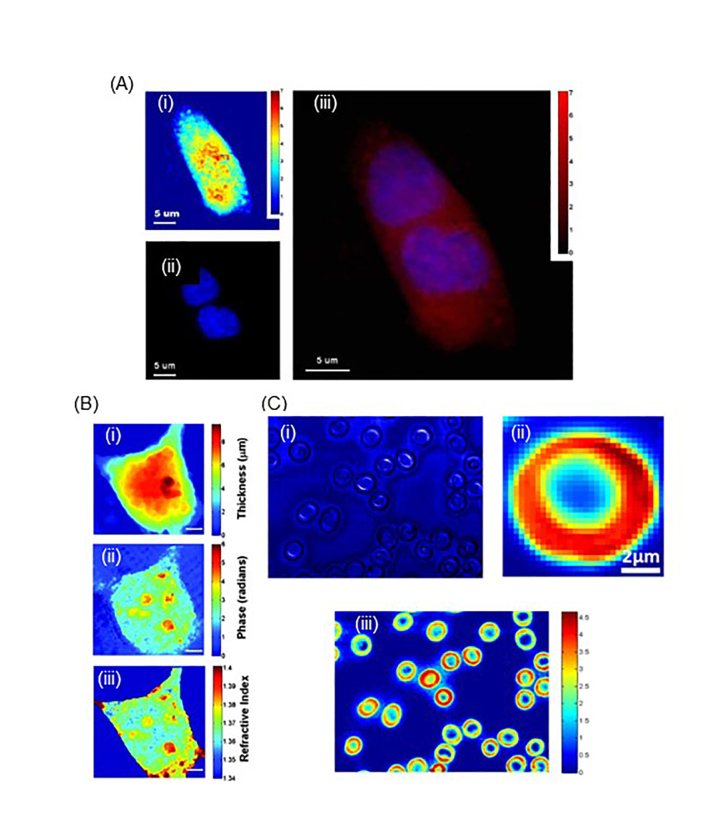
Many other optical techniques can be readily combined with QPI, complementing each other to make an effective method that can address various unanswered problems in biology and medicine (Figure 2). A combination of 2D QPI and 2D fluorescent imaging through diffraction phase and fluorescence microscopy showed quantitative and molecular imaging of living cells (Figure 2A) [68]. Also, the combination of QPI and confocal reflectance microscopy generates 2D quantitative phase images and 3D confocal reflectance images, which determine the optical and physical thicknesses of live cells (Figure 2B) [69]. In addition, correlative imaging through confocal Raman and QPI proved to be useful in obtaining the morphology and chemical compositions of label-free samples by utilizing its high spatial resolution (Figure 2C) [44]. Moreover, QPI and Raman Spectroscopy (RS) were simultaneously used to characterize skin fibroblasts that were exposed to ultraviolet radiation [70]. Under ultra-violet radiation, QPI can be used to retrieve the dry mass and density of skin fibroblasts, while RS detected the changes in biochemical composition, mainly proteins and lipids. Thus, this multimodal optical technique allowed label-free profiling of changes in skin fibroblast due to time-bound ultra-violet radiation. Also, correlative imaging using 2D QPI and 2D fluorescence microscopy was used to monitor the injection of the intracellular injection of glycerol [71], to study nucleus components [72], and to investigate the response of cells to optical tweezers [73].
Figure 2.
2D QPI with other correlative imaging techniques. (A) Through this imaging technique, the mitosis of a kidney cell was accurately visualized both through quantitative phase image, fluorescence image, and their overlaid image. (B) Through this technique, axially averaged refractive index of cells was determined. This was used for the calculation of protein concentrations. (C) Through this multimodal imaging system, normal and P. falciparum-infected red blood cells (RBCs) were analyzed to have different morphology and hemoglobin distribution of the RBCs, which were determined by QPM and confocal Raman microscopy, respectively. (A-C) are modified from refs. [68,69], and [44], respectively, with permissions.
Three-dimensional QPI with Correlative Imaging Techniques
Due to the rapidly emerging demands for more detailed morphological imaging of biological samples, ODT or 3D QPI techniques have become more prominently used in biological studies [74].
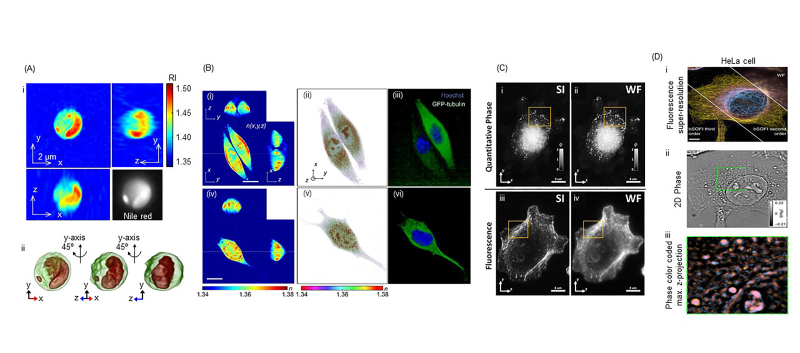
The combination of ODT and other microscopic techniques opens possibilities for superior spatiotemporal resolution, quantitative imaging, and high molecular-specific analysis of live cells (Figure 3). This opens grounds for new findings in cell biology, cellular pathophysiology, and novel diagnosis and treatment of diseases. ODT can be used to visualize the morphology and to quantify subcellular contents of living cells, which can be simultaneously verified by 2D fluorescence imaging (Figure 3A) [75,76]. Jung et al. imaged and quantified lipid contents in label-free microalgal cells through 3D RI tomography, which was validated through 2D fluorescence imaging of Nile red dye. ODT combined with 3D fluorescence images is correlatively used to image live cells for quantitative and precise spatial molecular specificity [77] (Figure 3B). Simultaneous 3D QPI and 3D fluorescence imaging are optimal for cellular dynamics imaging, especially targeting the organelles, as phototoxicity and photobleaching can be minimized through QPI. Recently, ODT and 3D fluorescence correlative imaging have been used in RI and fluorescence tomography with optofluidic rotation (RAFTOR), which analyzed suspended cell quantitatively with molecular specificity [78]. Also, 3D QPI and 3D confocal fluorescence imaging was used together for the study of yeast cells [79].
Figure 3.
3D QPI with other correlative imaging techniques. (A) ODT visualizes and quantifies lipids in algae, which are validated through 2D FL Nile red dye imaging. (B) Through this multimodal optical system, volume of nucleus and cytoplasm of HeLa and NIH-3T3 cells were determined by quantitative analysis. (C) Due to improvements in lateral resolution and depth localization, ODT and 3D SIM visualize clearer sub-diffraction structures of A549 cell, outlined in yellow box, compared to the conventional widefield (WF) imaging system. (D) Through multi-plane phase and SOFI imaging, clear subcellular structures of HeLa cell were imaged, making this system an accurate 4D imaging modality. (A-D) are modified from refs. [76,77,45], and [80], respectively, with permissions.
Furthermore, ODT combined with super-resolution fluorescent microscopic techniques has also been presented (Figure 3C and 3D). By employing 3D structured illumination microscopy, correlative ODT and 3D sub-diffraction fluorescence imaging, which enhanced the visualization of subcellular dynamics due to the sub-diffraction resolution of images (Figure 3C) [45,46]. Also, correlative imaging of 3D phase imaging and 3D super-resolution optical fluctuation imaging ensures high-speed dynamic 4D images with high molecular specificity (Figure 3D) [80]. Multimodal imaging through ODT and 3D structured illumination microscopy makes quantitative and sub-diffraction imaging possible due to the high spatiotemporal resolution of the system, which increases molecular specificity that allows analysis of biochemistry and biophysics of living cells with minimal perturbation [67].
Biological Applications of Correlative QPI Techniques
Infectious Disease
Electron microscopy (EM), scanning electron microscope and transmission electron microscope, has been extensively used to visualize and investigate the mechanism of infectious diseases [81,82]. Due to its high spatial resolution, pathogens and host cells can be visualized using EM. However, EM can only measure a still shot image of a dead cell due to the destructive nature of the imaging process, which requires the measurements metal-coated samples in vacuum condition. Since EM does not allow live-cell imaging, investigating the mechanism of infections is significantly limited. An alternate method is imaging the labeled host and pathogens to visualize and characterize the infectious disease, using fluorescence proteins or dyes. However, labeling is a cumbersome process, exogenous labels may alter the mechanisms of the states of host cells and pathogens, and some parasites are difficult to be labeled with existing methods. Also, this labeling method is only qualitative, having limitations quantifying the biophysical and biochemical properties of the infectious diseases.
On the other hand, QPI allows a unique approach for quantitative imaging of infectious diseases. QPI can visualize the morphology and dynamics of living cells as well as parasites, enabling a quantitative characterization of infectious diseases. RBCs infected by malaria-inducing Plasmodium falciparum (Pf) were characterized by QPI [83]. By measuring RI and membrane fluctuation of parasitized human RBCs using QPI, morphological, biochemical, and biophysical changes were studied. The infected RBCs were identified by using fluorescence signals coming from the nucleus of parasites, and then the infected RBCs were measured using QPI. By measuring 3D RI maps of the infected cells, different stages of Pf infected RBCs were visualized and systematically investigated. The decrease in the volume of cytosol and the concentration of hemoglobin from healthy to different stages of infected RBCs was quantified through RI. Also, a decrease in membrane fluctuation of infected RBCs indicated the loss in cell deformability of parasitized RBCs.
Also, the biophysics of Pf egressing from infected erythrocytes was studied using ODT [84]. To accurately characterize and understand the mechanism of parasitic egress, it is important to study the dynamics of the parasite infecting the host without any perturbation. 3D RI tomograms showed that parasitophorous vacuole plays an important role in RBCs’ morphology and the egress of the parasite from infected RBCs. This study provided new insight into the biochemical and biophysical principles that govern the exit of parasites from infected RBCs. This was done with the structural and mechanistic interpretation of the changes in parasitophorous vacuole. Kim et al. demonstrated high-resolution 3D optical RI tomograms of Pf infected RBCs using ODT [12]. Hemozoin inside Pf-RBC, the crystalline forms of hemoglobin metabolized by Pf, was imaged and analyzed. QPI can be used for not only malaria pathophysiology, but also with other infectious diseases, such as Babesiosis caused by Babesia microti (Bm) [35]. The morphological, biochemical, and biomechanical changes in Bm-RBCs are characterized by optical micro-tomographic technique, as they are important in understanding the pathophysiology of Babesiosis. A combined ODT and 3D fluorescence imaging technique was used to investigate the biophysical properties of live erythrocytes from Pelophylax nigromaculatus. Cell membranes were studied from the measured 3D RI tomograms and the locations and shapes of the nuclei were confirmed using DAPI [85].
Recently, infections of macrophages were researched by QPI [86,87]. Ekpenyong et al. investigated primary murine bone marrow-derived macrophages (BMDM) infected with Salmonella enterica serovar Typhimurium. They reported that there is a decrease in RI of Salmonella-infected BMDM compared to that of normal BMDM. Mendoza-Rodriguez et al. characterized differences in morphology and integral 3D RI of macrophages infected by Leishmania at different stages of infection. The size of macrophage and RI increased due to phagocytosis. Also, viral infections with H3N2 influenza virus on A549 human cells were investigated by correlative imaging with fluorescence confocal and tomographic diffractive microscopy [88]. Molecular specificity of the virus was observed through the confocal, and morphology of A549 cells was visualized through quantitative phase images. Spherical particles were visualized only on the membranes of infected cells, which were postulated to be budding of viral particles.
One of the challenges to applying QPI techniques was the complicated optical system. To obtain QPI images, the samples were usually sent to a physics or engineering laboratory where a QPI instrument was available. Recently, QPI instruments are commercially available. Among them, Tomocube Inc. (Republic of Korea) and Nanolive Ltd. (Switzerland) commercialized ODT techniques. They also provide instruments for correlative QPI; Tomocube’s HT-2 can measure 3D RI tomograms and 3D fluorescence images and Nanolive’s 3D Cell Explorer-fluo provide 3D RI tomograms and 2D fluorescence images.
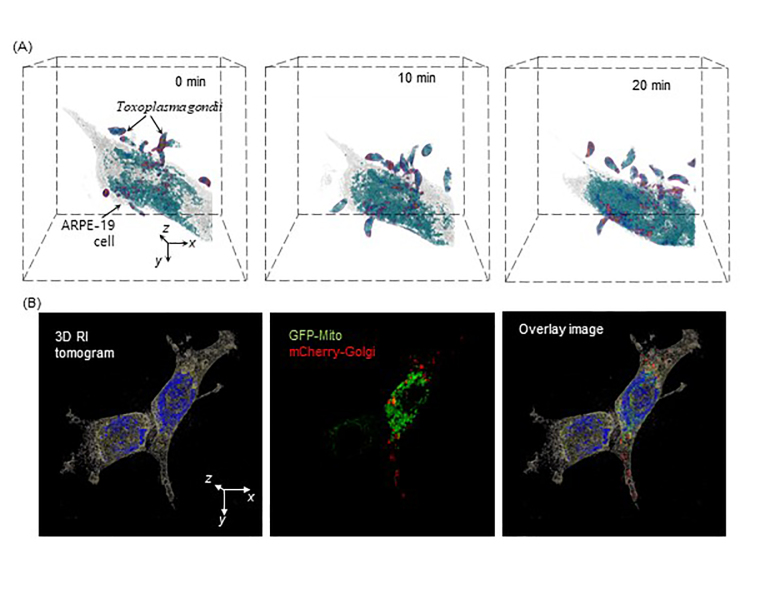
Figure 4A shows the applications of a commercial QPI technique for the study of infectious diseases. The time-lapse 3D images of Toxoplasma gondii infecting ARPE-19 were measured using a commercial ODT system (HT-1H, Tomocube Inc., Republic of Korea). As T. gondii penetrate the ARPE-19, the cell undergoes death. This high-resolution time-lapse 3D images can provide important insights on the mechanism of infection.
Figure 4.
Demonstration of applicability of ODT and multimodal approach combining ODT and 3D fluorescence. (A) Study of Toxoplasma gondii infecting ARPE-19 through timelapse QPI. (B) NIH-3T3 cell research through ODT and 3D FL (GFP-Mito and mCherry-Golgi). Both images are obtained by commercialized ODT setup (HT-2H, Tomocube, Inc., South Korea).
Cell Biology
Studying the structure and understanding related functions in the cells are crucial for the study of biology and various diseases. QPI is a powerful imaging technique for cell biology research as it enables quantification of biophysical and biochemical properties of label-free living cells. Other existing imaging techniques that need labeling through chemicals are not optimal for cell biology research as the labeling might change the metabolism of cells.
One important cell biology is lipid droplets dynamics in cells, as lipid droplets are gaining importance in clinical pathology. Kim et al. studied the cellular dynamics of lipid droplets in human hepatocyte [43]. Previous studies on lipid droplets through fluorescence microscopy made lipid droplets visible with high molecular specificity; however, the cell dynamics of lipid droplets cannot be accurately determined due to phototoxicity and photobleaching, which causes cellular perturbation and loss of fluorescence signal of lipid droplets, respectively. Using ODT, Kim et al. were able to reconstruct 3D RI distribution of lipid droplets in hepatocytes. Lipid droplets were quantified and tracked in 4D. This application has potential in discovering new biochemical metabolism and storage of lipids.
QPI can also be useful in studying gold nanoparticle particles (GNPs) applications. GNPs are widely used in the biological and medical field for imaging, diagnostic, and therapeutic purposes. The dynamics of gold nanoparticles in cells were studied through ODT [76]. Fluorescence is the conventional imaging technique to study the dynamics of GNPs in cells. However, due to phototoxicity and photobleaching, long-term dynamic analysis of GNPs in cells were not possible. Kim et al. used ODT to visualize and analyze the dynamics of GNPs in HeLa cells and murine breast cancer cells (4T1 cells). Through this QPI technique, quantitative analysis of the spatial distribution of aggregate GNPs and time-dependent localization of GNPs were measured. This paper proposes that ODT with GNPs can be used widely in the biomedical field for cancer and tissue imaging. Both lipid droplet and gold nanoparticles research using ODT were validated through correlative spatial comparison of fluorescence images of lipid droplets stained with Nile red dye and Alexa Fluor 555 dyes, respectively to the 3D RI distributions.
Many cell biology studies have been done through NIH-3T3, originating from mouse embryonic fibroblast cells, which can be easily transfected. To accurately view and characterize the effects of transfection in NIH-3T3, ODT and 3D FL are used (Figure 4B). Subcellular structures, mitochondria and Golgi-apparatus are visualized through FL imaging of GFP and mCherry, respectively.
Conclusions and Outlook
Here, we reviewed recent progress in the correlative approaches in 3D QPI. The research work highlighted in this review shows that the multimodal approaches combining QPI and fluorescence microscopy have provided valuable information about various quantitative imaging parameters, and also implies that these correlative approaches may play an important role in enhancing our understanding of the physiology and pathology of cells and tissues. This may make a critical impact on the diagnosis and treatment of various diseases as the combination of PET and CT opened new opportunities in medical diagnosis.
However, the uses of the correlative QPI approaches have not yet been fully explored. There exist several issues to be solved so that the QPI techniques developed in optics laboratories are extensively utilized in biological laboratories and clinical environments. Unlike other existing optical microscopy techniques, QPI methods have relatively complex instrumentation and also requires precise alignments and adjustments because QPI techniques are based on laser interferometry. Recently, various QPI techniques have been commercialized, which will enable wide utilization of QPI techniques by biologists and clinicians.
Furthermore, this emerging field of research can also be effectively assisted by the artificial intelligence (AI) approaches. The multimodal approaches combining QPI and fluorescence microscopy have unique advantages in AI-assisted research and diagnosis because these multimodal approaches provide both the morphological and molecular specific information and the RI can provide highly reproducing and quantitative information about cells and tissues. Recently, various research work presented the combination of QPI and AI, including the classification of individual red blood cells [89], bacterial genus [90], bacterial species [91], lymphocytes [9], macrophage [92], the analysis of unlabelled sperm cells [93], and cancer cells [94]. When the multimodal approaches combining QPI and fluorescence microscopy were powered by AI, it potentially enables diagnosis of various diseases based on both the morphological and molecular information at the individual cell levels and open a new avenue for medical study and diagnosis.
Lastly, to address important biological and medical problems with new approaches in imaging technology, it is crucial to develop interdisciplinary collaborations among optical physicists, engineers, biologists, and clinicians. Considering unique advantages that these multimodal approaches combining QPI and fluorescence image methods provide, we believe that this merging approaches will enhance the research and diagnosis of various diseases.
Acknowledgments
This work was supported by KAIST, BK21+ program, the research fund of Chungnam National University, and National Research Foundation (NRF) of Korea (2017M3C1A3013923, 2015R1A3A2066550, 2014K1A3A1A09063027, 2013R1A1A 2062046).
Glossary
- QPI
quantitative phase imaging
- RI
refractive index
- 2D
two-dimensional
- 3D
three-dimensional
- ODT
optical diffraction tomography
- RBCs
red blood cells
- CT
computerized tomography
- RS
Raman Spectroscopy
- RAFTOR
RI and fluorescence tomography with optofluidic rotation
- EM
electron microscopy
- Pf
Plasmodium falciparum
- Bm
Babesia microti
- BMDM
bone marrow-derived macrophages
- GNPs
gold nanoparticle particles
- AI
artificial intelligence
Disclosures
Mr. Y.S. Kim, Mr. S. Shin, Dr. S. Lee, Dr. W. Park, and Prof. Park have financial interests in Tomocube Inc., a company that commercializes optical diffraction tomography and quantitative phase imaging instruments.
References
- Lee K, Kim K, Jung J, Heo JH, Cho S, Lee S, et al. Quantitative phase imaging techniques for the study of cell pathophysiology: from principles to applications. Sensors (Basel). 2013;13(4):4170–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G. Quantitative phase imaging of cells and tissues. New York: McGraw Hill Professional; 2011. [Google Scholar]
- Park Y, Best CA, Auth T, Gov NS, Safran SA, Popescu G, et al. Metabolic remodeling of the human red blood cell membrane. Proc Natl Acad Sci USA. 2010;107(4):1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Matemba LE, Lee K, Kazyoba PE, Yoon J, Massaga JJ, et al. Optical characterization of red blood cells from individuals with sickle cell trait and disease in Tanzania using quantitative phase imaging. Sci Rep. 2016;6:31698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G, Ikeda T, Dasari RR, Feld MS. Diffraction phase microscopy for quantifying cell structure and dynamics. Opt Lett. 2006;31(6):775–7. [DOI] [PubMed] [Google Scholar]
- Rappaz B, Cano E, Colomb T, Kuhn J, Depeursinge CD, Simanis V, et al. Noninvasive characterization of the fission yeast cell cycle by monitoring dry mass with digital holographic microscopy. J Biomed Opt. 2009;14(3):034049. [DOI] [PubMed] [Google Scholar]
- Molaei M, Sheng J. Imaging bacterial 3D motion using digital in-line holographic microscopy and correlation-based de-noising algorithm. Opt Express. 2014;22(26):32119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet M, Gur D, Yoon J, Park Y, Faivre D. A bacteria‐based remotely tunable photonic device. Adv Opt Mater. 2017;5(1):1600617. [Google Scholar]
- Yoon J, Jo Y, et al. Identification of non-activated lymphocytes using three-dimensional refractive index tomography and machine learning. Sci Rep. 2017:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet P, Rappaz B, Magistretti PJ, Cuche E, Emery Y, Colomb T, et al. Digital holographic microscopy: a noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy. Opt Lett. 2005;30(5):468–70. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Pavillon N, Moratal C, Boss D, Rappaz B, Depeursinge C, et al. Determination of transmembrane water fluxes in neurons elicited by glutamate ionotropic receptors and by the cotransporters KCC2 and NKCC1: a digital holographic microscopy study. J Neurosci. 2011;31(33):11846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Yoon H, Diez-Silva M, Dao M, Dasari RR, Park Y. High-resolution three-dimensional imaging of red blood cells parasitized byPlasmodium falciparum and in situ hemozoin crystals using optical diffraction tomography. J Biomed Opt. 2013;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougan T, Edula JR, Takashima E, Morita M, Shinohara M, Shinohara A, et al. Molecular Camouflage of Plasmodium falciparum Merozoites by Binding of Host Vitronectin to P47 Fragment of SERA5. Sci Rep. 2018;8(1):5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Lee S, Kim G, Lee S, Lee J, Heo T, et al. Three-dimensional refractive index distributions of individual angiosperm pollen grains. bioRxiv. 2018:353243. [Google Scholar]
- Kim G, Lee S, Shin S, Park Y. 3D label-free imaging and analysis of Pinus pollen grains using optical diffraction tomography. bioRxiv. 2017:219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B, Carl DD, Schnekenburger J, Bredebusch I, Schäfer M, Domschke W, et al. Investigation of living pancreas tumor cells by digital holographic microscopy. J Biomed Opt. 2006;11(3):034005. [DOI] [PubMed] [Google Scholar]
- Kostencka J, Kozacki T, Kuś A, Kemper B, Kujawińska M. Holographic tomography with scanning of illumination: space-domain reconstruction for spatially invariant accuracy. Biomed Opt Express. 2016;7(10):4086–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastl L, Isbach M, Dirksen D, Schnekenburger J, Kemper B. Quantitative phase imaging for cell culture quality control. Cytometry A. 2017;91(5):470–81. [DOI] [PubMed] [Google Scholar]
- Kwon S, Lee Y, Jung Y, Kim JH, Baek B, Lim B, et al. Mitochondria-targeting indolizino [3, 2-c] quinolines as novel class of photosensitizers for photodynamic anticancer activity. Eur J Med Chem. 2018;148:116–27. [DOI] [PubMed] [Google Scholar]
- Bettenworth D, Bokemeyer A, Poremba C, Ding NS, Ketelhut S, Lenz P, et al. Quantitative phase microscopy for evaluation of intestinal inflammation and wound healing utilizing label-free biophysical markers. Histol Histopathol. 2017:11937. [DOI] [PubMed] [Google Scholar]
- Lee M, Lee E, Jung J, et al. Label-free optical quantification of structural alterations in Alzheimer’s disease. Sci Rep. 2016;6:31034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Wang Z, Liang X, Boppart SA, Tangella K, Popescu G. Measuring the scattering parameters of tissues from quantitative phase imaging of thin slices. Opt Lett. 2011;36(12):2281–3. [DOI] [PubMed] [Google Scholar]
- Wolf E. Three-dimensional structure determination of semi-transparent objects from holographic data. Opt Commun. 1969;1(4):153–6. [Google Scholar]
- Lauer V. New approach to optical diffraction tomography yielding a vector equation of diffraction tomography and a novel tomographic microscope. J Microsc. 2002;205(2):165–76. [DOI] [PubMed] [Google Scholar]
- Choi W, Fang-Yen C, Badizadegan K, Oh S, Lue N, Dasari RR, et al. Tomographic phase microscopy. Nat Methods. 2007;4(9):717–9. [DOI] [PubMed] [Google Scholar]
- Cotte Y, Toy F, Jourdain P, Pavillon N, Boss D, Magistretti P, et al. Marker-free phase nanoscopy. Nat Photonics. 2013;7(2):113–7. [Google Scholar]
- Kim Y, Shim H, Kim K, Park H, Heo JH, Yoon J, et al. Common-path diffraction optical tomography for investigation of three-dimensional structures and dynamics of biological cells. Opt Express. 2014;22(9):10398–407. [DOI] [PubMed] [Google Scholar]
- Shin S, Kim K, Yoon J, Park Y. Active illumination using a digital micromirror device for quantitative phase imaging. Opt Lett. 2015;40(22):5407–10. [DOI] [PubMed] [Google Scholar]
- Charrière F, Marian A, Montfort F, Kuehn J, Colomb T, Cuche E, et al. Cell refractive index tomography by digital holographic microscopy. Opt Lett. 2006;31(2):178–80. [DOI] [PubMed] [Google Scholar]
- Kuś A, Dudek M, Kemper B, Kujawińska M, Vollmer A. Tomographic phase microscopy of living three-dimensional cell cultures. J Biomed Opt. 2014;19(4):046009. [DOI] [PubMed] [Google Scholar]
- Memmolo P, Miccio L, Merola F, Gennari O, Netti PA, Ferraro P. 3D morphometry of red blood cells by digital holography. Cytometry A. 2014;85(12):1030–6. [DOI] [PubMed] [Google Scholar]
- Barty A, Nugent K, Roberts A, Paganin D. Quantitative phase tomography. Opt Commun. 2000;175(4):329–36. [Google Scholar]
- Barer R. Determination of dry mass, thickness, solid and water concentration in living cells. Nature. 1953;172(4389):1097–8. [DOI] [PubMed] [Google Scholar]
- Barer R, Joseph S. Refractometry of living cells. J Cell Sci. 1954;95(part 4):399–423. [Google Scholar]
- Park H, Hong SH, Kim K, Cho SH, Lee WJ, Kim Y, et al. Characterizations of individual mouse red blood cells parasitized by Babesia microti using 3-D holographic microscopy. Sci Rep. 2015;5:10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Shim H, Kim K, Park H, Jang S, Park Y. Profiling individual human red blood cells using common-path diffraction optical tomography. Sci Rep. 2014;4:6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park H, Kim K, Sohn Y, Jang S, Park Y. Refractive index tomograms and dynamic membrane fluctuations of red blood cells from patients with diabetes mellitus. Sci Rep. 2017;7(1):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Kim K, Park H, Choi C, Jang S, Park Y. Label-free characterization of white blood cells by measuring 3D refractive index maps. Biomed Opt Express. 2015;6(10):3865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Kandel ME, Rubessa M, Wheeler MB, Popescu G. Gradient light interference microscopy for 3D imaging of unlabeled specimens. Nat Commun. 2017;8(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y, Lue N, Hamza B, Martel J, Irimia D, Dasari RR, et al. Three-dimensional holographic refractive-index measurement of continuously flowing cells in a microfluidic channel. Phys Rev Appl. 2014;1(1):014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Oh N, Kim K, Lee S, Pack CG, Park JH, et al. Label-free high-resolution 3-D imaging of gold nanoparticles inside live cells using optical diffraction tomography. Methods. 2018;136:160–7. [DOI] [PubMed] [Google Scholar]
- Isikman SO, Bishara W, Zhu H, Ozcan A. Optofluidic tomography on a chip. Appl Phys Lett. 2011;98(16):161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Kim K, Yoon J, Park Y. Hyperspectral optical diffraction tomography. Opt Express. 2016;24(3):2006–12. [DOI] [PubMed] [Google Scholar]
- Kang JW, Lue N, Kong CR, Barman I, Dingari NC, Goldfless SJ, et al. Combined confocal Raman and quantitative phase microscopy system for biomedical diagnosis. Biomed Opt Express. 2011;2(9):2484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Eldridge WJ, Wax A, Izatt JA. Structured illumination multimodal 3D-resolved quantitative phase and fluorescence sub-diffraction microscopy. Biomed Opt Express. 2017;8(5):2496–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Kim D, Kim K, Park Y. Super-resolution three-dimensional fluorescence and optical diffraction tomography of live cells using structured illumination generated by a digital micromirror device. Sci Rep. 2018;8:9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir M, Bhaduri B, Wang R, Zhu R, Popescu G. Quantitative phase imaging. Prog Opt. 2012;57:133–217. [Google Scholar]
- Lee K, Kim K, Jung J, Heo J, Cho S, Lee S, et al. Quantitative phase imaging techniques for the study of cell pathophysiology: from principles to applications. Sensors (Basel). 2013;13(4):4170–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi I. Kato Ji, Ohta S, Mizuno J. Image formation in phase-shifting digital holography and applications to microscopy. Appl Opt. 2001;40(34):6177–86. [DOI] [PubMed] [Google Scholar]
- Debnath SK, Park Y. Real-time quantitative phase imaging with a spatial phase-shifting algorithm. Opt Lett. 2011;36(23):4677–9. [DOI] [PubMed] [Google Scholar]
- Sung Y, Choi W, Fang-Yen C, Badizadegan K, Dasari RR, Feld MS. Optical diffraction tomography for high resolution live cell imaging. Opt Express. 2009;17(1):266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Yoon J, Shin S, Lee S, Yang SA, Park Y. Optical diffraction tomography techniques for the study of cell pathophysiology. Journal of Biomedical Photonics & Engineering. 2016;2(2). [Google Scholar]
- Lim J, Lee K, Jin KH, Shin S, Lee S, Park Y, et al. Comparative study of iterative reconstruction algorithms for missing cone problems in optical diffraction tomography. Opt Express. 2015;23(13):16933–48. [DOI] [PubMed] [Google Scholar]
- Shin S, Kim K, Kim T, Yoon J, Hong K, Park J, et al. Optical diffraction tomography using a digital micromirror device for stable measurements of 4D refractive index tomography of cells. Quantitative Phase Imaging II. International Society for Optics and Photonics; 2016. [Google Scholar]
- Fauver M, Seibel EJ, Rahn JR, Meyer MG, Patten FW, Neumann T, et al. Three-dimensional imaging of single isolated cell nuclei using optical projection tomography. Opt Express. 2005;13(11):4210–23. [DOI] [PubMed] [Google Scholar]
- Kühn J, Montfort F, Colomb T, Rappaz B, Moratal C, Pavillon N, et al. Submicrometer tomography of cells by multiple-wavelength digital holographic microscopy in reflection. Opt Lett. 2009;34(5):653–5. [DOI] [PubMed] [Google Scholar]
- Yu L, Kim MK. Wavelength-scanning digital interference holography for tomographic three-dimensional imaging by use of the angular spectrum method. Opt Lett. 2005;30(16):2092–4. [DOI] [PubMed] [Google Scholar]
- Soto JM, Rodrigo JA, Alieva T. Partially coherent optical diffraction tomography for label-free quantitative microscopy. Information Optics (WIO), 2017. 16th Workshop on; 2017: IEEE.
- Streibl N. Three-dimensional imaging by a microscope. J Opt Soc Am A. 1985;2(2):121–7. [Google Scholar]
- Kim T, Zhou R, Mir M, Babacan SD, Carney PS, Goddard LL, et al. White-light diffraction tomography of unlabelled live cells. Nat Photonics. 2014;8(3):256–63. [Google Scholar]
- Kim T, Zhou R, Goddard LL, Popescu G. Solving inverse scattering problems in biological samples by quantitative phase imaging. Laser Photonics Rev. 2016;10(1):13–39. [Google Scholar]
- Jung JH, Jang J, Park Y. Spectro-refractometry of individual microscopic objects using swept-source quantitative phase imaging. Anal Chem. 2013;85(21):10519–25. [DOI] [PubMed] [Google Scholar]
- Jung J, Kim K, Yu H, Lee K, Lee S, Nahm S, et al. Biomedical applications of holographic microspectroscopy. Appl Opt. 2014;53(27):G111-G22. [DOI] [PubMed] [Google Scholar]
- Sung Y, Choi W, Lue N, Dasari RR, Yaqoob Z. Stain-free quantification of chromosomes in live cells using regularized tomographic phase microscopy. PLoS One. 2012;7(11):e49502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turko NA, Peled A, Shaked NT. Wide-field interferometric phase microscopy with molecular specificity using plasmonic nanoparticles. J Biomed Opt. 2013;18(11):111414. [DOI] [PubMed] [Google Scholar]
- Curl CL, Bellair CJ, Harris T, Allman BE, Harris PJ, Stewart AG, et al. Refractive index measurement in viable cells using quantitative phase‐amplitude microscopy and confocal microscopy. Cytometry A. 2005;65(1):88–92. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Eldridge WJ, Wax A, Izatt JA. Structured illumination microscopy for dual-modality 3D sub-diffraction resolution fluorescence and refractive-index reconstruction. Biomed Opt Express. 2017;8(12):5776–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Popescu G, Badizadegan K, Dasari RR, Feld MS. Diffraction phase and fluorescence microscopy. Opt Express. 2006;14(18):8263–8. [DOI] [PubMed] [Google Scholar]
- Lue N, Choi W, Popescu G, Yaqoob Z, Badizadegan K, Dasari RR, et al. Live Cell Refractometry Using Hilbert Phase Microscopy and Confocal Reflectance Microscopy. J Phys Chem A. 2009;113(47):13327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Kang S, Kang JW, So PT, Dasari RR, Yaqoob Z, et al. Label-free characterization of ultra violet-radiation-induced changes in skin fibroblasts with Raman spectroscopy and quantitative phase microscopy. Sci Rep. 2017;7(1):10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel CE, Dierker C, Schmidt L, Przibilla S, von Bally G, Kemper B, et al. Contrast-enhanced digital holographic imaging of cellular structures by manipulating the intracellular refractive index. J Biomed Opt. 2010;15(4):041509. [DOI] [PubMed] [Google Scholar]
- Kemper B, Schmidt L, Przibilla S, Rommel C, Vollmer A, Ketelhut S, et al. Influence of sample preparation and identification of subcelluar structures in quantitative holographic phase contrast microscopy. Biophotonics: Photonic Solutions for Better Health Care II. International Society for Optics and Photonics; 2010. [Google Scholar]
- Barroso Á, Woerdemann M, Vollmer A, von Bally G, Kemper B, Denz C. Three‐dimensional exploration and mechano‐biophysical analysis of the inner structure of living cells. Small. 2013;9(6):885–93. [DOI] [PubMed] [Google Scholar]
- Stephens DJ, Allan VJ. Light Microscopy Techniques for Live Cell Imaging. Science. 2003;300(5616):82–6. [DOI] [PubMed] [Google Scholar]
- Gatfield J, Menyhart K, Wanner D, Gnerre C, Monnier L, Morrison K, et al. The selexipag active metabolite ACT-333679 displays strong anti-contractile and anti- remodeling effects, but low β-arrestin recruitment and desensitization potential. J Pharmacol Exp Ther. 2017 [DOI] [PubMed] [Google Scholar]
- Jung J, Hong S-J, Kim H-B, et al. Label-free non-invasive quantitative measurement of lipid contents in individual microalgal cells using refractive index tomography. Sci Rep. 2018;8:6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Park WS, Na S, Kim S, Kim T, Do Heo W, et al. Correlative three-dimensional fluorescence and refractive index tomography: bridging the gap between molecular specificity and quantitative bioimaging. Biomed Opt Express. 2017;8(12):5688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann M, Cojoc G, Girardo S, Ulbricht E, Guck J, Müller P. Three‐dimensional correlative single‐cell imaging utilizing fluorescence and refractive index tomography. J Biophotonics. 2018;11(3):e201700145. [DOI] [PubMed] [Google Scholar]
- Habaza M, Gilboa B, Roichman Y, Shaked NT. Tomographic phase microscopy with 180 rotation of live cells in suspension by holographic optical tweezers. Opt Lett. 2015;40(8):1881–4. [DOI] [PubMed] [Google Scholar]
- Descloux A, Grußmayer KS, Bostan E, Lukes T, Bouwens A, Sharipov A, et al. Combined multi-plane phase retrieval and super-resolution optical fluctuation imaging for 4D cell microscopy. Nat Photonics. 2018;12(3):165–72. [Google Scholar]
- Carter WJ, Yan Z, Cassai ND, Sidhu GS. Detection of Extracellular Forms of Babesia in the Blood by Electron Microscopy: A Diagnostic Method for Differentiation from Plasmodium falciparum. Ultrastruct Pathol. 2003;27(4):211–6. [DOI] [PubMed] [Google Scholar]
- Dorr I. How Striga Parasitizes its Host: a TEM and SEM Study. Ann Bot (Lond). 1997;79(5):463–72. [Google Scholar]
- Park Y, Diez-Silva M, Popescu G, Lykotrafitis G, Choi W, Feld MS, et al. Refractive index maps and membrane dynamics of human red blood cells parasitized by Plasmodium falciparum. Proc Natl Acad Sci USA. 2008;105(37):13730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohanadas R, Park Y, Lui L, Li A, Quinn D, Liew K, et al. Biophysics of Malarial Parasite Exit from Infected Erythrocytes. PLoS One. 2011;6(6):e20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Lee M, Youn S, Lee E, Kwon D, Shin J, et al. Measurements of three-dimensional refractive index tomography and membrane deformability of live erythrocytes from Pelophylax nigromaculatus. Sci Rep. 2018;8:9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpenyong AE, Man SM, Achouri S, Bryant CE, Guck J, Chalut KJ. Bacterial infection of macrophages induces decrease in refractive index. J Biophotonics. 2013;6(5):393–7. [DOI] [PubMed] [Google Scholar]
- Mendoza-Rodríguez E, Organista-Castelblanco C, Camacho M, Monroy-Ramírez F. Digital holographic microscopy as a technique to monitor macrophages infected by leishmania. SPIE Optical Metrology. SPIE; 2017. [Google Scholar]
- Simon B, Debailleul M, Beghin A, Tourneur Y, Haeberlé O. High‐resolution tomographic diffractive microscopy of biological samples. J Biophotonics. 2010;3(7):462–7. [DOI] [PubMed] [Google Scholar]
- Liu R, Dey DK, Boss D, Marquet P, Javidi B. Recognition and classification of red blood cells using digital holographic microscopy and data clustering with discriminant analysis. J Opt Soc Am A Opt Image Sci Vis. 2011;28(6):1204–10. [DOI] [PubMed] [Google Scholar]
- Jo Y. Jung J, Kim M-h, Park H, Kang S-J, Park Y. Label-free identification of individual bacteria using Fourier transform light scattering. Opt Express. 2015;23(12):15792–805. [DOI] [PubMed] [Google Scholar]
- Jo Y, Park S, Jung J, et al. Holographic deep learning for rapid optical screening of anthrax spores. Sci Adv. 2017;3(8):e1700606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavillon N, Hobro AJ, Akira S, Smith NI. Noninvasive detection of macrophage activation with single-cell resolution through machine learning. Proc Natl Acad Sci USA. 2018:201711872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky SK, Barnea I, Levi M, Greenspan H, Shaked NT. Automated analysis of individual sperm cells using stain‐free interferometric phase microscopy and machine learning. Cytometry A. 2017;91(9):893–900. [DOI] [PubMed] [Google Scholar]
- Roitshtain D, Wolbromsky L, Bal E, Greenspan H, Satterwhite LL, Shaked NT. Quantitative phase microscopy spatial signatures of cancer cells. Cytometry A. 2017;91(5):482–93. [DOI] [PubMed] [Google Scholar]