Abstract
Background
Efficacy of house dust mite (HDM) allergen immunotherapy (AIT) in allergic rhinitis and controlled allergic asthma has been documented in controlled trials with adults and children. However, tolerability comparing clinical development and post marketing data, particularly in different subgroups, is missing.
Methods
We performed an analysis of pooled safety data for subcutaneous AIT (SCIT) with a high-dose house dust mite allergoid from 6 randomized, controlled trials (RCT) in HDM allergic respiratory disease (ARD) and of post marketing safety data from more than 10 years including different subgroups (age, gender, asthma status).
Results
In all, 500 patients with ARD were treated in RCTs: 279 received the marketed dose of 1800 protein nitrogen units (PNU) high-dose HDM allergoid AIT (214 double-blind placebo controlled [HDM-DBPC], 65 children/adolescents usual care controlled [HDM-RCT(UC)]), and 221 placebo (PL). 38.8% adverse events (AEs) were observed with 1800 PNU in HDM-DBPC (31.2% PL, 35.5% HDM-ALL [1800 PNU]); the difference was primarily because of local reactions; there was no difference in systemic reactions (10.9% PL, 11.2% HDM-DBPC, 11.2% HDM-ALL); one out of 279 high-dose HDM allergoid-treated patients had a serious adverse event (SAE).
Children (n = 39)/adolescents (n = 26) had fewer related AEs and local reactions compared to adults; systemic reactions: children 12.8%, adults 11.2% adolescents 7.7%. Females had slightly more AEs. Treatment was well tolerated in asthmatic patients (n = 267; GINA I n = 32, II n = 104, III n = 17, 114 no classification).
In more than 10 years more than 100,000 patients were treated with high-dose HDM allergoid (1800 PNU) under daily practice conditions. Adverse drug reactions (ADRs) were reported in 0.5% of patients. 94.6% of these ADRs were expected.
Conclusion
SCIT with the marketed dose of high-dose HDM allergoid was well tolerated in clinical development and in daily practice. There was no increased risk for the investigated patient subgroups. Tolerability was comparable to HDM sublingual immunotherapy (SLIT) tablets.
Keywords: Tolerability, Subcutaneous allergen immunotherapy, Allergic rhinitis, Asthma, Children
Introduction
Allergen immunotherapy (AIT) is a therapy with disease-modifying effects and the only available treatment to target the disease instead of the symptoms. By administering allergen extracts, specific blocking antibodies, tolerance-inducing cells, and mediators are activated. These prevent further exacerbation of the allergen-triggered immune response, block the specific immune response, and attenuate the inflammatory response in tissue [1, 2].
House dust mite (HDM) allergy is strongly implicated in the pathogenesis of respiratory allergic disease and a large proportion of patients with allergic rhinitis (AR), allergic asthma (AA), or both, are sensitized to HDM, predominantly Dermatophagoides pteronyssinus and Dermatophagoides farinae [3].
Efficacy of AIT in HDM allergy is documented by a number of controlled trials in adults and few controlled trials in children [1, 4]. Subcutaneous immunotherapy (SCIT) has been well investigated for individual preparations in controlled bronchial asthma as defined by the Global Initiative for Asthma (GINA) 2007 as well as in intermittent and mild persistent asthma (GINA 2005). AIT is recommended as a treatment option, in addition to allergen avoidance and pharmacotherapy in GINA 2017, provided there is a clear causal link between respiratory symptoms and the relevant allergen [1, 4, 5].
The high-dose HDM allergoid is a depot formulation with aluminum hydroxide for subcutaneous AIT. Formaldehyde and glutaraldehyde have been used in combination to chemically modify an aqueous extract of purified HDM bodies and to produce a stable allergoid. The allergen extract consists of the HDM Dermatophagoides pteronyssinus or Dermatophagoides farinae alone or in combination. The product is manufactured at two different strengths, which enables a low initial dose and increasing concentration of allergens in the further course of treatment. In maintenance therapy, intervals of up to 8 weeks are very convenient and allow for flexibility. The marketed dose is 3000 protein nitrogen units (PNU)/mL in strength B (strength A: 300 PNU/mL obtained by a 1:10 dilution of strength B) with a maximum injection volume of 0.6 ml (1800 PNU; [8]). The major allergen content of the high-dose HDM allergoid D. pteronyssinus is 12 μq/ml Der p 1 and 10 μg/ml Der p 2; for the high-dose HDM allergoid D. farinae it is 20 μg/ml Der f 1 and 15 μg/ml Der f 2 [9].
This HDM SCIT product has been shown to be effective in patients with mite-induced allergic asthma. Adding the mite allergoid SCIT to pharmacologic treatment was an effective and safe strategy to reduce corticosteroid doses while maintaining disease control and significantly improved bronchial allergen provocation (BAP; [6, 7]). There is however no data available comparing safety information for AIT products from clinical development and spontaneous safety reports from daily practice since launch, particularly not in different patient subgroups [10].
The publication presents safety data of this high-dose HDM allergoid in clinical development and from adverse drug reactions (ADRs) spontaneously reported after launch, including subgroup analysis. SLIT is reported as having ADRs in 40–75% of the cases as temporary local mucosal reactions (pruritus or dysesthesia in the oral cavity, swelling of the oral mucosa, throat irritation) and considered as having a better safety profile than SCIT, especially with respect to systemic reactions, anaphylaxis, and other severe systemic reactions [1]. Therefore, the safety data of this product were compared to the safety of two different SLIT mite tablets as benchmark.
Material and methods
Product
The high-dose HDM allergoid (Acaroid®) consists of the HDM Dermatophagoides pteronyssinus alone (used in Trials AL0104av (EudraCT: 2004-003892-35), 97-09 M, 97-09 UK, AL0106ac (EudraCT: 2006-000934-11), and AL1009ac (EurdraCT: 2011-002248-29)) or in 1:1 combination with Dermatophagoides farinae (used in Trial AL0400av; Table 1). 97-09 M, 97-09 UK, and AL0400av were performed before introduction of the EudraCT database. The drug product is a suspension administered by subcutaneous injection.
Table 1.
Summary of the high-dose HDM allergoid clinical trials included in the pooled safety analysis
| Study | Population | Treatment | n | Included in safety set of | ||||
|---|---|---|---|---|---|---|---|---|
| Indication | Age (years) | HDM-ALL | HDM-DBPC | HDM-RCT(UC) | Placebo | |||
| (n = 279) | (n = 214) | (n = 65) | (n = 221) | |||||
| 97-09 Ma | Allergic rhinitis/rhinoconjunctivitis | 18–58 | 1800 PNU | 20 | Yes | Yes | – | – |
| Placebo | 20 | – | – | – | Yes | |||
| 97-09 UKa | Allergic rhinitis/rhinoconjunctivitis | 22–54 | 1800 PNU | 15 | Yes | Yes | – | – |
| Placebo | 15 | – | – | – | Yes | |||
| AL0106ac EudraCT: 2006-000934-11 |
Allergic rhinitis/rhinoconjunctivitis | 19–46 | 1800 PNU | 51 | Yes | Yes | – | – |
| Placebo | 57 | – | – | – | Yes | |||
| Al0400ava | Allergic rhinitis/rhinoconjunctivitis | 18–54 | 1800 PNU | 69 | Yes | Yes | – | – |
| Placebo | 66 | – | – | – | Yes | |||
| Al1009ac EurdraCT: 2011-002248-29 |
Controlled allergic bronchial asthma and rhinitis/rhinoconjunctivitis |
18–40 | 600 PNU | 24 | – | – | – | – |
| 1800 PNU | 31 | Yes | Yes | – | – | |||
| 3000 PNU | 28 | – | – | – | – | |||
| 5400 PNU | 31 | – | – | – | – | |||
| Placebo | 32 | – | – | – | Yes | |||
| AL0104av EudraCT: 2004-003892-35 |
Allergic asthma with or without allergic rhinitis/rhinoconjunctivitis |
6–406–40 | – | – | – | – | – | – |
| ≥18 | 1800 PNU | 28 | Yes | Yes | – | – | ||
| ≥18 | Placebo | 31 | – | – | – | Yes | ||
| <12 | 1800 PNU | 39 | Yes | – | Yes | – | ||
| 12–17 | 1800 PNU | 26 | Yes | – | Yes | – | ||
| 6–17 | Usual care | 32 | – | – | – | – | ||
aPerformed before introduction of the EudraCT database
HDM house dust mites, HDM-DBPC high-dose HDM allergoid AIT double-blind placebo controlled, HDM-RCT(UC) high-dose HDM allergoid AIT usual care controlled, HDM-ALL HDM-DBPC plus HDM-RCT(UC), PNU protein nitrogen units
The following strengths were used in Trials AL0104av, 97-09 M, 97-09 UK, AL0400av, and AL0106ac: strength B: 3000 PNU/mL; strength A: 300 PNU/mL (obtained by a 1:10 dilution of strength B). In the dose range finding trial AL1009ac 4 different doses were tested: 600 PNU/mL, 1800 PNU/mL, 3000 PNU/mL and 5400 PNU/mL.
Study population
This publication presents safety information from 6 completed, randomized, controlled clinical trials conducted between 2000 and 2015 in patients with allergic rhinitis/rhinoconjunctivitis (97-09 M, 97-09 UK, AL0400av, and AL0106ac), patients with allergic asthma and allergic rhinitis/rhinoconjunctivitis (Trial AL1009ac), or subjects with allergic asthma with or without allergic rhinitis/rhinoconjunctivitis (Trial AL0104av). Demographic details are shown in Table 2. We present safety information from patients treated with the marketed dose of 1800 PNU (n = 279) as maintenance dose (100% D. pteronyssinus or 50%/50% D. pteronyssinus/D. farinae allergens/mixtures). The maximum treatment duration was 3 years and 2 years with placebo, respectively; the maximum double-blind, placebo-controlled treatment phase was 2 years (97-09 M, 97-09 UK, AL0400av, and AL0106ac, AL0104av adults). In study AL0104av, children and adolescents (n = 65; 6–17 years) with asthma were treated for up to 3 years with this HDM AIT (Group: HDM-RCT(UC)).
Table 2.
Demographics and baseline characteristics
| Statistics | 97-09 M | 97-09 UK | AL0104av | AL0106ac | AL0400av | AL1009ac | Total | |
|---|---|---|---|---|---|---|---|---|
| (n = 20) | (n = 15) | (n = 93) | (n = 51) | (n = 69) | (n = 114) | (n = 362) | ||
| Age (years) | Mean (SD) | 29.4 (10.0) | 36.9 (10.4) | 15.2 (8.9) | 29.6 (8.0) | 27.6 (9.3) | 27.3 (6.6) | 25.1 (10.3) |
| Min–Max | 18.0–58.0 | 22.0–54.0 | 6.0–40.0 | 19.0–46.0 | 18.0–54.0 | 18.0–40.0 | 6.0–58.0 | |
| Gender (n [%]) | Female | 5 (25.0) | 10 (66.7) | 33 (35.5) | 24 (47.1) | 29 (42.0) | 49 (43.0) | 150 (41.4) |
| Male | 15 (75.0) | 5 (33.3) | 60 (64.5) | 27 (52.9) | 40 (58.0) | 65 (57.0) | 212 (58.6) | |
| Age group (n [%]) | <12 | 0 (0.0) | 0 (0.0) | 39 (41.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 39 (10.8) |
| 12–17 | 0 (0.0) | 0 (0.0) | 26 (28.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 26 (7.2) | |
| ≥18 | 20 (100.0) | 15 (100.0) | 28 (30.1) | 51 (100.0) | 69 (100.0) | 114 (100.0) | 297 (82.0) | |
| Smoking status (n [%]) | Smoker | 4 (20.0) | 0 (0.0) | 0 (0.0) | 5 (9.8) | 18 (26.1) | 1 (0.9) | 28 (7.7) |
| Nonsmoker | 16 (80.0) | 15 (100.0) | 93 (100.0) | 46 (90.2) | 51 (73.9) | 113 (99.1) | 334 (92.3) | |
| Asthma (n [%]) | Yes | – | – | 93 (100.0) | 26 (51.0) | 34 (49.3) | 114 (100.0) | 267 (73.8) |
| No | – | – | 0 (0.0) | 25 (49.0) | 35 (50.7) | 0 (0.0) | 60 (16.6) | |
| GINA classification (n [%]) | GINA I | – | – | 0 (0.0) | 14 (27.5) | 18 (26.1) | – | 32 (8.8) |
| GINA II | – | – | 76 (81.7) | 12 (23.5) | 16 (23.2) | – | 104 (28.7) | |
| GINA III | – | – | 17 (18.3) | 0 (0.0) | 0 (0.0) | – | 17 (4.7) | |
| GINA IV | – | – | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | |
| No asthma | – | – | 0 (0.0) | 25 (49.0) | 35 (50.7) | – | 60 (16.6) | |
| FEV1 or PEF (% of predicted value) |
n | – | – | 93 | 51 | 69 | 61 | 274 |
| Mean (SD) | – | – | 93.1 (10.6) | 101.8 (15.2) | 104.2 (14.2) | 98.7 (9.8) | 98.8 (13.1) | |
| Min–Max | – | – | 80.0–139.0 | 80.1–135.7 | 75.0–147.0 | 82.2–123.8 | 75.0–147.0 | |
| Duration of allergic rhinoconjunctivitis (years) | n | 20 | 15 | 77 | 51 | 69 | 114 | 346 |
| Mean (SD) | 5.8 (3.2) | 21.1 (10.1) | 5.7 (5.1) | 12.5 (8.6) | 8.6 (7.8) | 8.2 (5.8) | 8.8 (7.4) | |
| Min–Max | 2.0–13.0 | 2.0–40.0 | 1.0–20.0 | 2.0–39.0 | 1.0–45.0 | 0.0–24.0 | 0.0–45.0 |
GINA Global Initiative for Asthma, FEV1 Forced Expiratory Flow in 1 sec, PEF Peak Expiratory Flow
Safety sets
Subjects of the double-blind placebo controlled (DBPC) safety set who received at least 1 dose of the trial drug during the double-blind treatment period with 1800 PNU or placebo were combined (Trials AL1009ac, AL0104av, 97-09 M, 97-09 UK, AL0106ac, and AL0400av) in group HDM-DBPC (n = 214). The safety set HDM-ALL consists of patients from the DBPC phase plus patients from the asthma versus usual care trial (n = 279; Table 1).
Subgroups: The following subgroups were performed:
Age group (<12, 12–17, ≥18 years)
Gender (female/male)
Subjects with asthmatic symptoms (Global Initiative for Asthma classification >0, GINA, 2006). Classification was assessed in Trials AL1009ac, AL0104av, AL0106ac, and AL0400av.
Spontaneous safety reports: For the period after launch of the product (01 July 2005) to 31 March 2016, ADRs were calculated in relation to the number of products sold and patients treated and classified according to expectedness. For unexpected ADRs, the system organ classes (SOCs) were reported.
Coding of treatment-related AEs (TRAEs):
AEs were coded using the MedDRA version 15.0. AEs with onset during or after the first administration of the trial drug were defined as TRAEs. An AE was defined as being related to the trial drug if the causal relationship of the AE was assessed as “at least possible”.
Results
Clinical development
In all, 362 subjects (children, adolescents, adults) have received at least 1 dose of active treatment in the completed trials of the high-dose HDM allergoid clinical development program (Table 1)—114 subjects in the dose finding trial, 93 subjects in the allergic asthma trial, and 155 subjects in the allergic rhinitis/rhinoconjunctivitis trials. A total of 297 patients were treated with this HDM AIT during the DBPC period of the trials, 221 subjects with placebo. In children/adolescents, the trial drug was administered in an open-label design and usual care was used as control instead of placebo (n = 65).
Marketed dose in clinical development
A total of 279 subjects received the marketed dose of 1800 PNU (0.6 ml strength B). The median number of injections administered in the 1800 PNU group was 15 injections during the 1st treatment year (placebo: 15 injections), 12 injections during the 2nd treatment year (placebo: 12 injections), and 8 injections during the 3rd treatment year. The reasons for the difference in injection numbers are the up-dosing phase in year one and the possible prolongation of treatment intervals up to 8 weeks during maintenance treatment. In all, 148 patients received 1800 PNU for >2 years. Overall, 267 patients in the clinical development program of this HDM AIT had asthma: GINA I n = 32, GINA II n = 104, and GINA III n = 17 (114 patients without GINA classification). Patients had rhinoconjunctivitis for a mean of 8.8 (SD 7.4) years, min. 2–max. 45 years. A total of 65 patients were less than 18 years of age (only included in the asthma trial, Trial AL0104av): <12 years (n = 39), 12–17 years (n = 26; Table 1).
Safety of marketed dose in clinical development
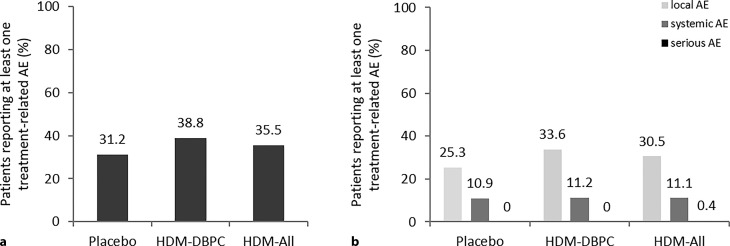
In all, 214 patients received the 1800 PNU during the DBPC phase of the different trials and 65 in the asthma versus usual care trial (RCT(UC); Table 1), whereby 38.8% of patients treated with HDM AIT had an adverse event related to the trial drug, compared to 31.2% with placebo and 35.5% of the HDM AIT patients in the HDM-ALL group (Fig. 1). The difference in adverse events related to the study drug was primarily because of local reactions; there was no difference in the share of patients with systemic reactions (10.9% placebo, 11.2% HDM-DBPC, 11.1% HDM-All). One out of 279 patients in the HDM-All group experienced a serious adverse event (SAE): hospitalization because of erythema, conjunctivitis, asthma, cough, cyanosis, urticaria, and wheezing 15 min after administration of the trial drug during the 3rd treatment year. The event resolved on the same day. Exacerbations of asthma were mentioned in the medical history. The patient was treated with an inhaled corticosteroid dose of 200 µg fluticasone as asthma controller medication that was reduced to 100 µg 4 weeks before the SAE. No SAEs were observed in placebo or HDM-DBPC group.
Fig. 1.
Percentage of patients reporting treatment-related adverse events related to study drug: a in the different treatment groups: placebo, HDM-DBPC, and HDM-All. b Distribution according to local, systemic and serious adverse event (AE). (DBPC Phase: placebo n = 221, HDM DBPC n = 214; HDM-All n = 279). HDM-DBPC high-dose HDM allergoid AIT double-blind placebo controlled, HDM-RCT(UC) high-dose HDM allergoid AIT usual care controlled, HDM-ALL HDM-DBPC plus HDM-RCT(UC), AE adverse event
An overview about the TRAEs related to the trial drug and reported in >2% of patients is presented in Table 3.
Table 3.
Treatment-related adverse events related to the trial drug reported in >2% of subjects by PT in decreasing frequency
| Number (%) subjects | |||
|---|---|---|---|
| DBPC Phase | DBPC plus RCT(UC) | ||
| Placebo | HDM DBPC | HDM-All | |
| (N = 221) | (N = 214) | (N = 279) | |
| Subjects with related treatment-emergent AE | 69 (31.2) | 83 (38.8) | 99 (35.5) |
| Injection site swelling | 51 (23.1) | 61 (28.5) | 71 (25.4) |
| Injection site pruritus | 11 (5.0) | 25 (11.7) | 31 (11.1) |
| Injection site erythema | 4 (1.8) | 16 (7.5) | 17 (6.1) |
| Injection site pain | 5 (2.3) | 9 (4.2) | 15 (5.4) |
| Rhinitis | 5 (2.3) | 7 (3.3) | 8 (2.9) |
| Pruritus | 0 (0.0) | 7 (3.3) | 7 (2.5) |
| Cough | 2 (0.9) | 6 (2.8) | 8 (2.9) |
| Sneezing | 6 (2.7) | 1 (0.5) | 1 (0.4) |
| Rhinitis allergic | 3 (1.4) | 2 (0.9) | 6 (2.2) |
| Fatigue | 1 (0.5) | 5 (2.3) | 5 (1.8) |
DBPC double-blind placebo controlled, RCT(UC) usual care controlled, HDM-DBPC high-dose HDM allergoid AIT double-blind placebo controlled, HDM-RCT(UC) high-dose HDM allergoid AIT usual care controlled, HDM-ALL HDM-DBPC plus HDM-RCT(UC), AE adverse event
Clinical laboratory measurements (biochemistry, hematology, and urinalysis) were analyzed in most of the trials. A shift of normal at baseline to abnormal after treatment was reported for >5% of subjects in any group only for eosinophils and neutrophils (14 subjects with HDM AIT). There were no other noteworthy differences between the groups.
Subgroup analysis
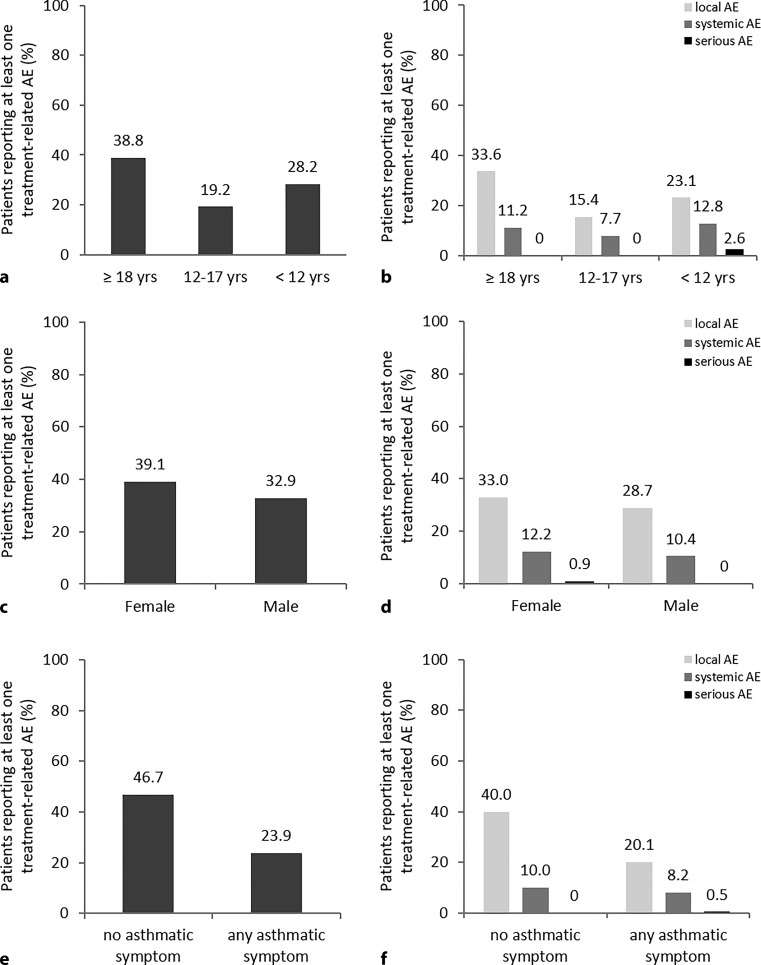
A summary of TRAEs by subgroups—age (a, b), gender (c, d), asthma status (e, f)—is presented in Fig. 2. Children and adolescents had fewer related adverse events and local reactions compared to adults; systemic reactions were slightly more frequent in children (12.8% versus 11.2% in adults), but much lower in adolescents (7.7%). Females had more adverse events in all 4 groups compared to males (see Fig. 2c, d). Treatment was well tolerated in asthmatic patients and the number of related adverse event, local reactions, and systemic reactions were low (see Fig. 2e, f), but one SAE occurred.
Fig. 2.
Percentage of patients reporting treatment-related adverse events related to study drug analyzed in different subgroups: age (a, b), gender (c, d), asthma status (e, f). a Data are shown for HDM-DBPC (≥18 years) and HDM-RCT(UC) (12–17 years and <12 years). b Distribution according to local, systemic and serious AE. c Percentage of female and male patients in HDM-All reporting a treatment-related AE. d Distribution according to local, systemic and serious AE. e Percentage of patients with and without asthma reporting a treatment-related AE and f Distribution according to local, systemic and serious AE. (Age (a, b): ≥18 years n = 214, 12–17 years n = 26, <12 years n = 39; Gender (c, d): female n = 115, male n = 164; Asthma status (e, f): no asthma symptom n = 60, any asthma symptom n = 184). HDM-DBPC high-dose HDM allergoid AIT double-blind placebo controlled, HDM-RCT(UC) high-dose HDM allergoid AIT usual care controlled, HDM-ALL HDM-DBPC plus HDM-RCT(UC), AE adverse event, yrs years
Spontaneous safety reports
In the period from the first market launch (01 July 2005) to 31 March 2016 more than hundred thousand patients were treated with HDM AIT 1800 PNU as maintenance dose. ADRs were reported in 0.5% of patients compared to 38.8% (HDM-DBPC) and 35.5% (HDM-ALL) in clinical development; 94.6% of these ADRs were expected. The unexpected events were primarily reported in the SOCs: nervous system disorders (headache, hypoesthesia, paresthesia); musculoskeletal and connective tissue disorders (arthralgia, myalgia, pain in extremity); general disorders and administration site conditions (pyrexia); gastrointestinal disorders (oral mucosal blistering, nausea, gastrointestinal pain); and skin and subcutaneous tissue disorders (alopecia, eczema). No special patient group could be identified to be at higher risk, and no safety concern has arisen from this received post marketing safety data.
Subgroup analysis of spontaneous safety reports
In the period from the first market launch to 31 March 2016 several ten thousand children and adolescents were also treated with HDM AIT 1800 PNU. ADRs were reported in 0.9% of children (0–11 years of age), 0.6% of adolescents (12–17 years) compared to 0.3% in adults (≥18 years).
If we assume that 50% female and 50% male patients were treated after launch, the number of ADRs were comparable irrespective of gender (female 0.4%, male 0.5%). The same was true for asthma. Asthma is found in up to 38% of patients with allergic rhinitis [11]. With this HDM AIT SCIT 0.4% of ADR reports were in patients with asthma and 0.5% in non-asthmatics.
Discussion
In recent years, publications on HDM immunotherapy focused on sublingual treatment. Several multinational, randomized, placebo-controlled clinical trials of high quality standards in different patient populations and subgroups are available. In addition, this treatment route is known to be safe, which is why we compared our data with relevant data of sublingual products recently published in international literature.
Rhinoconjunctivitis
In our observation of patients with rhinoconjunctivitis with/without asthma, adverse events with at least possible relationship to the high-dose HDM allergoid were 38.8% compared to 31.2% with placebo. This is lower than the, at least possibly, related adverse events seen in allergic rhinitis patients treated with HDM SLIT tablet (48.0% 6 SQ-HDM, 53.0% 12 SQ-HDM, 15.0% placebo; [12]; Table 4). The related AEs were primarily local reactions (33.6%) in HDM SCIT (injection site swelling 28.5%, -pruritus 11.7%, -erythema 7.5%, -pain 4.2%). This is comparable to the safety profile of HDM SLIT where the most common AEs are reported as being related to the investigational medicinal product (IMP) by the investigator were oral pruritus, throat irritation, and mouth edema (20.0, 14.0, and 8.0% of subjects on active treatment, respectively; [12]). In a recently published double-blind trial, evaluating the efficacy and safety of HDM tablets in 968 adolescent and adult patients (aged 12–64 years) with HDM-induced AR with or without intermittent asthma over 52 weeks, the number of subjects with any adverse drug reaction was 66.8% with the recommended dose 300 IR (73.1% with 500 IR), and 18.6% with placebo [13]. In two pooled RC clinical trials with 1215 subjects (12 SQ HDM tablet n = 600, 615 placebo) with a clinical history of HDM allergic rhinitis, and 863 (71%) with additional HDM allergic asthma 50% of subjects on active treatment reported TRAEs compared to 16% of subjects on placebo [14].
Table 4.
Safety overview of the high-dose HDM allergoid versus SLIT mite tablets
| Product | ADRs with at least possible relationship to study drug (%) | ||
|---|---|---|---|
| Rhinoconjunctivitis ± Asthma | Asthma | Pediatrics with asthma (12–17 years) |
|
| HDM allergoid | |||
| 1800 PNU high-dose HDM allergoid | 38.8 | 23.9 | 19.2 |
| Placebo | 31.2 | 18.0 | – |
| HDM SLIT tablet [12, 14–16] | |||
| 6 SQ-HDM | 48 | 39 | 55 |
| 12 SQ-HDM | 53/50 | 46 | 50 |
| Placebo | 15/16 | 17 | 32 |
| HDM SLIT tablet [13] | |||
| 300 IR | 66.8 | – | – |
| 500 IR | 73.1 | – | – |
| Placebo | 18.6 | – | – |
ADR adverse drug reactions, PNU protein nitrogen units, HDM house dust mites, SLIT sublingual immunotherapy
Asthma
HDM is the most common allergen associated with asthma and more than 40% of adult asthmatics are atopic with a positive skin prick test result for the HDM allergens [15]. Adverse events related to the high-dose HDM allergoid were 23.9% in the group of patients with asthma compared to 46.7% in patients without asthma, placebo 18%; subjects with TRAEs were 47.9% compared to 51.6% with placebo. In a recently published study of asthma patients with a sublingual HDM tablet, AIT TRAEs occurred in 39.0% in the 6 SQ-HDM group, 46.0% in the 12 SQ-HDM group, and 17.0% with placebo [14]. A possibly treatment-related serious adverse event was reported for the 12 SQ-HDM tablet: asthma (moderate, alternative etiology was “recently viral infection”; [15]). With the high-dose HDM allergoid a serious adverse event (erythema, conjunctivitis, asthma, cough, cyanosis, urticaria, wheezing) was reported in an asthmatic child 15 min after administration of trial drug. Exacerbations of asthma were mentioned in the medical history and the corticosteroid dose was reduced from 200 µg fluticasone to 100 µg 4 weeks before the SAE. These examples show how important it is that asthma is controlled, especially when an AIT is performed and guidelines are followed.
Pediatrics
There is little data available about the safety of HDM AIT in children and adolescents. In a recently published multicenter, double-blinded, randomized trial in adolescents (12–17 years old) with HDM allergic rhinitis with and without conjunctivitis and with or without asthma, patients received placebo, HDM SLIT tablet 6 SQ or 12 SQ once daily for 28 days. The proportion of subjects who experienced TRAEs was 25.0%, 45.0%, and 52.0% for the placebo, 6 SQ-HDM, and 12 SQ-HDM groups, respectively [16]. With the high-dose HDM allergoid, TRAEs were observed in 19.2% of adolescents (12–17 years old). There was a greater incidence of TRAEs in adolescents with asthma receiving the HDM SLIT tablet compared with placebo (asthma: 32.0%, 55.0%, 50.0%, placebo, 6‑SQ, 12-SQ respectively; without asthma: 21.0%, 40.0%, 54.0%; [16]). In the high-dose HDM allergoid group of adolescents, all had asthma and received the trial drug for up to two years (28 days for HDM SLIT).
Gender
No literature is available about safety differences of HDM immunotherapy in females versus males. In the clinical development program of the HDM SCIT allergoid we observed a few more related AEs in females (39.1%) compared to males (32.9%), but more data are needed.
Spontaneous safety reports
The safety of a medicinal product after launch in daily practice with much higher numbers of treated patients and a broad range of different patients treated adds to the safety information of a product. Very rare side effects might be seen for the first time and unexpected side effects might be reported. With the HDM SCIT product presented in this publication, adverse drug reactions were reported in 0.5% of patients compared to 38.8% (HDM-DBPC) and 35.5% (HDM-ALL) in clinical development, whereby 94.6% of these ADRs were expected, and there was no major concern about the unexpected ADRs related to the safety of this SCIT product in daily routine.
Pediatric data analyzed from spontanous safety reports
Compared to adults slightly more children and adolescents (0.9% children, 0.6% adolescents, 0.3% adults) experienced an ADR. This is based on a great database of several thousand treated patients and possibly a higher reporting rate of ADRs in younger age groups. No new safety information has arisen concerning system organ classes or preferred terms of the reported ADRs.
Gender data analyzed from spontanous safety reports
According to post marketing safety data no gender specific safety difference could be identified (female 0.4%, male 0.5%), and the high-dose HDM allergoid is well tolerated in asthmatic as well as non-asthmatic patients (0.4% asthma, 0.5% non-asthma patients with ADR).
On the basis of more than hundred thousand treated patients with the high-dose HDM allergoid in daily routine and a broad range of different patient groups, no special patient group could be identified to be at higher risk, no safety concern has arisen from the unexpected ADRs and the safety was comparable to what was already known from clinical development.
Conclusion
Subcutaneous treatment with the high-dose HDM allergoid was well tolerated in HDM respiratory allergic disease in clinical development. The observed difference to placebo was primarily driven by local reactions; there was no difference in systemic reactions. The high-dose HDM allergoid was also well tolerated in daily practice. As 95% of ADRs were expected, no safety concern has arisen from the post marketing safety data. The product was well tolerated in all age groups (children, adolescents, and adults), in asthmatics and non-asthmatics. Furthermore, the safety profile was comparable to HDM SLIT tablets.
Acknowledgments
Acknowledgements
The authors like to thank Annemie Narkus, MC Narkus Medical Consulting + Services, Bleckede, Germany for medical writing and Jacqueline Alig, Allergopharma GmbH & Co. KG, for intensive discussions on the results and the way of their presentation.
Funding
Financial support for the development of this article was provided by Allergopharma GmbH & Co. KG. Allergopharma GmbH & Co. KG designed and provided funding for the studies described in this article.
Abbreviations
- AA
Allergic asthma
- ADR
Adverse drug reaction
- AE
Adverse event
- AIT
Allergen immunotherapy
- AR
Allergic rhinitis
- ARD
Allergic respiratory disease
- DBPC
Double-blind placebo controlled
- FEV1
Forced expiratory flow in 1 sec
- GINA
Global Initiative for Asthma
- HDM
House dust mite
- HDM-ALL
HDM-DBPC plus HDM-RCT(UC)
- PEF
Peak expiratory flow
- PL
Placebo
- PNU
Protein nitrogen units
- RCT
Randomized, controlled trials
- SAE
Serious adverse event
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- SOC
System organ class
- TRAE
Treatment-related adverse event
- UC
Usual care
Conflict of interest
L. Klimek has received research grants from ALK-Abelló, Denmark; Allergopharma, Germany; Bionorica, Germany; Biomay, Austria, Boehringer Ingelheim, Germany, Circassia, USA; Stallergenes, France; HAL, Netherlands; Allergy Therapeutics/Bencard, Great Britain/Germany; Hartington, Spain; Lofarma, Italy; MEDA, Sweden; Novartis, Switzerland, Leti, Spain; ROXALL, Germany; Glaxo-Smith-Kline (GSK), Great Britain; Cytos, Switzerland; Curalogic, Denmark; and/or has served on the speaker’s bureau or was consulting for the above mentioned pharmaceutical companies. G.-C. Fox and S. Thum-Oltmer are employees of Allergopharma GmbH & Co. KG, Reinbek, Germany.
Footnotes
All authors concur with the submission.
Author contributions
G.-C. Fox, S. Thum-Oltmer, L. Klimek were involved in manuscript conception and interpretation of the data. G.-C. Fox performed the data analysis. S. Thum-Oltmer prepared figures and substantially contributed to the manuscript from the first draft stage. L. Klimek supervised and participated in writing the manuscript. G.-C. Fox, S. Thum-Oltmer, L. Klimek reviewed, commented and approved the final manuscript.
References
- 1.Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD) Allergo J Int. 2014;23:282–319. doi: 10.1007/s40629-014-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2017 doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 3.Calderon MA, Kleine-Tebbe J, Linneberg A, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. House dust mite respiratory allergy: an overview of current therapeutic strategies. J Allergy Clin Immunol Pract. 2015;3:843–855. doi: 10.1016/j.jaip.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Global strategy for asthma management and prevention. https://www.ginasthma.org. 2017. Accessed 02/01/2018
- 5.Dhami S, Nurmatov U, Agache I, Lau S, Muraro A, Jutel M, et al. Allergen immunotherapy for allergic asthma: protocol for a systematic review. Clin Transl Allergy. 2015;6:5. doi: 10.1186/s13601-016-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010;126(5):942–949. doi: 10.1016/j.jaci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Rosewich M, Arendt S, El Moussaoui S, Schulze J, Schubert R, Zielen S. Bronchial allergen provocation: a useful method to assess the efficacy of specific immunotherapy in children. Pediatr Allergy Immunol. 2013;24:434–440. doi: 10.1111/pai.12068. [DOI] [PubMed] [Google Scholar]
- 8.Allergopharma, editor. Summary of product characteristics, Acaroid Milbenpräparate. 2017. [Google Scholar]
- 9.Brehler R, Kahlert H, Thum-Oltmer S. Hypoallergene Präparate in der SCIT. Allergo J Int. 2010;19:477–484. doi: 10.1007/BF03370748. [DOI] [Google Scholar]
- 10.van der Valk JP, de Jong NW, Gerth van Wijk R. Review on immunotherapy in airway allergen sensitised patients. Neth J Med. 2015;73:263–269. [PubMed] [Google Scholar]
- 11.Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine-Tebbe J. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2016;137:444–451.e8. doi: 10.1016/j.jaci.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto Y, Fujieda S, Okano M, Yoshida Y, Kakudo S, Masuyama K. House dust mite sublingual tablet is effective and safe in patients with allergic rhinitis. Allergy. 2017;72:435–443. doi: 10.1111/all.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emminger W, Hernandez MD, Cardona V, Smeenk F, Fogh BS, Calderon MA, et al. The SQ house dust mite SLIT-tablet is well tolerated in patients with house dust mite respiratory allergic disease. Int Arch Allergy Immunol. 2017;174:35–44. doi: 10.1159/000478699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virchow JC, Backer V, Kuna P, Prieto L, Nolte H, Villesen HH, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715–1725. doi: 10.1001/jama.2016.3964. [DOI] [PubMed] [Google Scholar]
- 16.Maloney J, Prenner BM, Bernstein DI, Lu S, Gawchik S, Berman G, et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol. 2016;116:59–65. doi: 10.1016/j.anai.2015.10.024. [DOI] [PubMed] [Google Scholar]