Abstract
Conjunctival goblet cell loss in ocular surface diseases is accompanied by increased number of interleukin-12 (IL-12)-producing antigen-presenting cells (APCs) and increased interferon-γ (IFN-γ) expression. This study tested the hypothesis that mouse conjunctival goblet cells produce biologically active retinoic acid (RA) that suppresses CD86 expression and IL-12 production by myeloid cells. We found that conditioned media from cultured conjunctival goblet cells (CjCM) suppressed stimulated CD86 expression, NF-κB p65 activation and IL-12 and IFN-γ production in unstimulated and lipopolysaccharide-stimulated cultured bone marrow-derived cells (BMDCs) containing a mixed population of APCs. Goblet cell-conditioned, ovalbumin-loaded APCs suppressed IFN-γ production and increased IL-13 production in co-cultured OTII cells. The goblet cell suppressive activity is due in part to their ability to synthesize RA from retinol. Conjunctival goblet cells had greater expression of aldehyde dehydrogenases Aldh1a1 and a3 and ALDEFLUOR activity than cornea epithelium lacking goblet cells. The conditioning activity was lost in goblet cells treated with an ALDH inhibitor, and a retinoid receptor alpha antagonist blocked the suppressive effects of CjCM on IL-12 production. Similar to RA, CjCM increased expression of suppressor of cytokine signaling 3 (SOCS3) in BMDCs. SOCS3 silencing reversed the IL-12-suppressive effects of CjCM. Our findings indicate that conjunctival goblet cells are capable of synthesizing RA from retinol secreted by the lacrimal gland into tears that can condition APCs. Evidence suggests goblet cell RA may function in maintaining conjunctival immune tolerance and loss of conjunctival goblet cells may contribute to increased Th1 priming in dry eye.
Keywords: dendritic cell, goblet cells, IL-12, monocyte, retinoic acid
Conjunctival goblet cells suppress APCs via retinoic acid
Introduction
The ocular surface is the most exposed mucosa in the body that is subject to environmental, mechanical, microbial and inflammatory insults (1). The conjunctiva covers two-thirds of the ocular surface and contains epithelial goblet cells and immune cells in the epithelium and stroma. Goblet cell density decreases in aqueous-deficient dry eye and certain ocular surface inflammatory conditions, such as Stevens–Johnson syndrome and graft versus host disease (2–6). Infiltration of the conjunctiva with CD4+ T cells accompanied by increased interferon-γ (IFN-γ) expression has been reported in aqueous deficiency (6–8). IFN-γ amplifies dry eye severity by promoting secretory dysfunction, unfolded protein response and apoptosis in goblet cells (9, 10). We reported bidirectional secretion of conjunctival goblet cells onto the ocular surface and into the stroma following cholinergic stimulation (11). Similar to the intestine, there are goblet cell-associated passages in the conjunctiva that can function as conduits for tear and surface antigens to pass into antigen-presenting cells (APCs) in the basal epithelium and stroma (11). A mixed population of APCs consisting of monocytes, macrophages and dendritic cells (DCs) has been identified in the conjunctiva and these APCs express activation markers (e.g. CD86) and migrate to the draining lymph nodes in response to desiccating stress-induced dry eye (11–16). Loss of tolerance to topically applied antigen has been noted in mice with experimentally induced dry eye with accompanying goblet cell loss and in Spdef (SAM pointed domain containing ETS transcription factor) knockout (KO) mice that are devoid of goblet cells (11, 17, 18). Spdef KO mice had a greater number of interleukin-12-positive (IL-12+) macrophage and dendritic APCs in the conjunctiva and treatment of these mice with conditioned media from cultured wild-type conjunctival goblet cells suppressed lipopolysaccharide (LPS)-stimulated IL-12 production (16). The suppressive effect of goblet cell conditioned media on IL-12 production by conjunctival APCs was prevented by pretreatment with an retinoid receptor alpha (RARα) inhibitor, suggesting that conjunctival goblet cells produce retinoic acid (RA) (16).
It is well recognized that vitamin A is essential to maintain a healthy ocular surface. Systemic vitamin A deficiency is associated with loss of conjunctival goblet cells, hyperkeratinization of the ocular surface epithelium, inflammation, severe dry eye and increased risk of cornea ulceration (19). The lacrimal gland produces and secretes vitamin A, in the form of retinol into the tears (20, 21). Expression of alcohol dehydrogenase (ADH) and retinaldehyde dehydrogenase, the enzymes that are required for the two-step oxidation of vitamin A (retinol) into the active metabolite RA, was previously reported in whole conjunctiva lysates (22). This study investigated if conjunctival goblet cells modulate differentiation and activation of cultured bone marrow-derived cells (BMDCs) containing a mixed population of monocyte-derived APCs and if they produce biologically active RA that suppresses CD86 expression and IL-12 production by the BMDCs.
Methods
Mice
Female C57BL/6J and B6.Cg-Tg(TcraTcrb)425Cbn/J (OTII) mice 6–8 weeks old were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Spdef KO mice on a B6 background were obtained from Drs Jeffrey Whitsett (Cincinnati Children’s Hospital, Cincinnati, OH, USA) and Hans Clevers (Hubrecht Institute, Utrecht, the Netherlands), were backcrossed with C57BL/6J for six generations and were bred in a conventional specific pathogen-free vivarium. The Institutional Animal Care and Use Committees at Baylor College of Medicine approved all animal experiments. All studies adhered to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
RA and retinoid receptor antagonist
All-trans RA (10 nM; Millipore Sigma, St Louis, MO, USA) was diluted in dimethyl sulfoxide (DMSO), aliquoted and stored separately in separate dark airtight plastic bags at −80°C. Each aliquot was used once and discarded. Ro41-5253 (RO, RARα antagonist, 1µM; Enzo Life Sciences, Farmingdale, NY, USA) and DEAB (N,N-diethylaminobenzaldehyde, 10 nM; Millipore Sigma) were diluted in 0.35% ethanol; retinyl palmitate (1 nM; Millipore Sigma) was diluted in ethanol and stored in dark airtight plastic bags.
Histology, histochemical and immunofluorescent staining
Eyes and adnexae from 6- to 8-week-old female C57BL/6J mice were surgically excised and immediately placed in optimal cutting temperature compound (OCT; VWR, Suwanee, GA, USA) and flash frozen in liquid nitrogen or were fixed in 10% formalin. Tissue sections (5 µm) were cut at the center of the eye, where the lens has its maximum diameter. Immunofluorescent staining was performed with anti-ALDH1a3 (aldehyde dehydrogenase 1a3; ab129815, 1:100; Abcam, Cambridge, UK), rabbit anti-ADH4 (HPA020525, 1:100; Millipore Sigma), anti-RBP1 (HPA007338, 1:100; Millipore Sigma) or anti-major histocompatibility complex class II (MHCII) (I-A/I-E, clone M5/114.15.2, 1:50; BD Biosciences, San Diego, CA), using a species-specific AlexaFluor 594-conjugated secondary antibody (ThermoFisher). After washing with phosphate-buffered saline (PBS), sections were incubated with Alexa Fluor 488-conjugated Wheat Germ Agglutinin (WGA; ThermoFisher) diluted 1:200 in PBS for 60 min at room temperature in a dark room. After 1 h, they were washed in PBS and counterstained with DAPI (4,6-diamidino-2-phenylindole) DNA-binding dye (1:500) for 2 min and washed, and square coverslips (Electron Microscopy Sciences, Hatfield, PA, USA) were applied with ~30 µl of Gel Mount (ThermoFisher). Digital confocal images were captured with a laser scanning confocal microscope LSM800 (Carl Zeiss AG, Oberkochen, Germany) wavelength 400–750 nm and 1-µm z-step. The images were processed using ZEN 2.3 (Carl Zeiss AG).
Epithelial cell culture
To initiate ocular surface epithelial cell cultures, cornea or conjunctiva explants were taken from 6- to 8-week-old female C57BL/6J or Spdef KO mice. Conjunctiva explants were cultured by previously described methods (23). Conjunctiva explants were excised from the forniceal conjunctiva of C57BL/6 mice or Spdef KO mice and placed in keratinocyte serum-free medium (KSFM; ThermoFisher) supplemented with 3% fetal bovine serum (FBS), 1.25 µg ml−1 amphotericin B (product 15290-018; ThermoFisher), 0.5 µl ml−1 gentamicin (product 15750-060; ThermoFisher) and 5 µg ml−1 dispase II (product 04942078001; Roche) for 15 min at 37°C. For cornea epithelial cultures, explants were placed in supplemented hormone epithelial medium (SHEM) containing Dulbecco modified Eagle medium/F12 medium (product D8437; Millipore Sigma) supplemented with 5 ng ml−1 epidermal growth factor (EGF), 5 µg ml−1 insulin, 5 µg ml−1 transferrin, 5 ng ml−1 sodium selenite, 0.5 µg ml−1 hydrocortisone, 30 ng ml−1 cholera toxin A, 0.5% DMSO, 50 µg ml−1 gentamicin, 1.25 µg ml−1 amphotericin B, 3% FBS and 5 µg ml−1 dispase II (Roche) left for 30 min. Vitamin A (retinol) palmitate (Millipore Sigma) was added to the media of some epithelial cultures to determine their ability to metabolize vitamin A to RA. Conjunctival and corneal explants were separately plated in 24-well and 48-well plates. Conjunctiva cultures received 200 µl per well of KSFM with 80 ng ml−1 mouse EGF (BD Biosciences, San Jose, CA, USA) and cornea cultures received 500 µl per well SHEM. Culture plates were then placed in a 37°C incubator with 5% CO2. After 3 days, cornea cultures received 500 µl of fresh SHEM per well for a total of 1 ml medium per well; medium was changed every 3 days. On day 7, media was exchanged with 200 and 500 µl of Iscove’s modified Dulbecco media with 3% FBS (IMDM) in conjunctiva and cornea cultures, respectively, and cells were protected from light. On day 11, supernatants were harvested as conditioned media, called CjCM (conjunctival conditioned media) and KCM (cornea conditioned media). Cultures established from four to six mice yielded sufficient conditioned media to treat all cultured BMDCs (1 × 106 per well). Conditioned media were frozen at −80°C and protected from light until use.
To inhibit RA synthesis, conjunctival epithelium was cultured as above, but the aldehyde dehydrogenase (ALDH) inhibitor, DEAB (1 nM), was added from day 4 to day 7, then media was exchanged to IMDM and the supernatants harvested on day 11.
Bone marrow cell culture
Bone marrow cells were obtained by flushing cells from femurs of female C57BL/6J mice with ice-cold PBS. After red blood cell lysis, marrow cells were cultured for 9–10 days at 3 × 106 cells per well in 10-cm-diameter plates in complete RPMI 1640 medium (10% FBS, 50 µg ml−1 gentamicin, 1.25 µg ml−1 amphotericin B) supplemented with mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng ml−1) and IL-4 (5 ng ml−1; PeproTech, Rocky Hill, NJ, USA). On day 3, fresh medium containing GM-CSF and IL-4 was added. CjCM, KCM or RA (10 nM), with or without 2-h pretreatment with RARα antagonist Ro41-5253 (1 µM; Millipore Sigma), was added to the cultures on day 6. On day 8, BMDCs were stimulated with LPS (1 µg ml−1; Millipore Sigma) for 4 h for PCR and 24 h for flow cytometry. CD11c+ cells were sorted from the entire population of cultured cells on day 6 using a CD11c positive selection kit II (StemCell, Cambridge, MA, USA) and the identity of these cells was confirmed by flow cytometry.
RNA isolation
RNA was extracted from the cultured bone marrow cells on day 8 or epithelial cultures on day 11 using the RNeasy Plus Micro kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The RNA concentration was measured using a Nanodrop 2000 spectrophotometer (ThermoFisher). cDNA was synthesized using Ready-To-Go You-Prime First-Strand beads (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), as previously reported (10).
Quantitative reverse transcriptase–PCR
PCR was run on cDNA using a Step One Plus real-time PCR system (Applied Biosystems, Grand Island, NY, USA). Gene expression was analyzed by the comparative threshold cycle (ΔΔCT) method. CT values for each gene were normalized to the CT values of the housekeeping gene, hypoxanthine guanine phosphoribosyl transferase (HPRT), for each sample using untreated cultures as the calibrator. Fold differences in expression were calculated after comparing values for each gene to the those in the untreated group. Each experiment in this study was completed using untreated control from the same batch of mice. Taqman probes (Life Technologies, Grand Island, NY, USA) used in this study included Aldh1a1 (ABI assay ID Mm00657317_m1), Aldh1a2 (ABI assay ID Mm00501306_m1), Aldh1a3 (ABI assay ID Mm00474049_m1), Adh (ABI assay ID Mn00478838_m1), Rbp1 (ABI assay ID Mn00441119_m1), IFN-γ (ABI assay ID Mm00801778_m1), IL-1β (ABI assay ID Mm00434228_m1), IL-6 (ABI assay ID Mm00446190_m1), IL-10a (ABI assay ID Mm00439616_m1), IL-12a (ABI assay ID Mm00434165_m1), IL-23A (ABI assay ID Mm00518984_m1), Socs3 (suppressor of cytokine signaling 3; ABI assay ID Mm00545913_s1), TGF-β1 (ABI assay ID Mm00436952_m1), TGF-β2 (Mm00436952_m1) and Hprt-1 (ABI assay ID Mm00446968_m1). There were at least four biological replicates in each treatment group/experiment.
Protein isolation and analysis
BMDCs (4 × 106) were placed in sterile 1.5 ml tubes, centrifuged at 250 × g for 8 min, the supernatant was discarded and 250 µl of radioimmunoprecipitation assay buffer (Millipore Sigma) treated with a complete, ethylenediaminetetraacetic acid-free protease inhibitor cocktail tablet (Roche, Basel, Switzerland) was added. After pipetting 10 times, the sample was placed on ice for 30 min, then stored at −80°C. Protein concentrations were measured using a Pierce BCA protein assay kit (Life Technologies). Western blot was performed as previously reported (10) using anti-SOCS3 (1 µg ml−1; catalog #ab16030, Abcam) overnight at four degrees. Membranes were washed in Tris-buffered saline with Tween 20 (TBST) and incubated in secondary horseradish peroxidase (HRP)-rabbit-anti-goat (1:5000; ThermoFisher) washed 3× with TBST and developed with Clarity western ECL blotting substrate (Bio-Rad, Hercules, CA, USA). Gels were stripped and restained with anti-β-actin (0.2 µg ml−1; catalog #SC-47778, Santa Cruz). Band densities were measured on a ChemiDoc™ Touch Imaging System (Bio-Rad).
Detection of NF-κB p65 activation
NF-κB p65 activation was quantitatively measured by a Fast-activated cell-based ELISA (FACE™) NF-κB p65 Profiler Kit (Active Motif, Carlsbad, CA, USA) that specifically measures phosphorylated and total NF-κB p65. Briefly, BMDCs were cultured in 96-well plates coated with poly-lysine (Millipore Sigma) and stimulated with LPS with or without NF-κB inhibitor (NF-κB-I, 10 µM; Millipore). Following treatment, the cells were rapidly fixed to preserve activation-specific protein modifications. After incubation with HRP-conjugated secondary antibody and colorimetric developing solution, the absorbance in each well was read at 450 nm with a reference wavelength of 655 nm by an Infinite 200 Pro microplate reader (Tecan, Mannedorf, Switzerland). The plate was then washed and crystal violet added to count cells. The measured OD450 readings were corrected for cell number by dividing the OD450 reading for a given well by the OD595 reading for that well.
RA bioassay
Sil-15 F9-RARE-lacZ reporter cell line, kindly provided by Dr Michael Wagner, State University of New York, Brooklyn, NY, USA, was used to assess RA production by cultured epithelial cells and BMDCs. Sil-15 cells were grown on gelatin-coated 96-well plates (BD Labware, Bedford, MA, USA) in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1% G418 (Life Technologies). Culture supernatants or control IMDM +3% FBS were added to confluent monolayers of Sil-15 cells. After overnight incubation, supernatants were removed, and Sil-15 cells were lysed by three freeze-thaw cycles in PBS. β-Galactosidase activity in Sil-15 lysates was then determined using X-Gal (1mg ml−1; Thermofisher) in developer solution made of 5mm K3[Fe(CN)6], 5mm K4[Fe(CN)6] and 2mm MgCl2 in PBS, and color development was measured at 630nm. RA production was calculated as RA equivalents based on a standard curve generated with known amounts of RA (detection range 50pM–100nM).
Ovalbumin antigen presentation assay
BMDCs harvested on day 9 (with or without conditioning with CjCM or RA or stimulation with LPS) serving as APCs were loaded with ovalbumin (OVA) peptide for 2 h, then co-cultured with CD4+ T cells isolated from OTII mice (1:5 ratio). After 3 days of co-culture, cells were harvested for intra-cellular staining (CD4, IL-13, IFN-γ and Foxp3). This experiment was repeated three times with n = 3 each time. CD4+ T cells were isolated from spleens of OTII mice using an EasySep™ Mouse CD4+ T Cell Isolation Kit (Stem Cell Technologies, Vancouver, British Columbia, Canada). T-cell proliferation was determined using WST-1 assay (Abcam, Cambridge, MA). After co-culture for 3–4 days, the cells were harvested for intra-cellular staining and flow cytometry.
Flow cytometry
Antibodies directed against the following APC surface antigens were used: CD11c_FITC (clone HL3, catalog #553801, BD Biosciences), CD11b_Cy7 (clone M1/70, catalog #561039, BD Biosciences), MHCII_PE (clone M5/114.15.2, catalog #55700, BD Biosciences), CD86_pacific blue (clone GL1, catalog #105022, Biolegend, San Diego, CA, USA), Ly6C_allophycocyanin (APC) (clone AL-21, catalog #560595, BD Biosciences), F4/80_Cy5 (clone BM8, catalog #123112, Biolegend) and CD45-PE_BV510 (clone 30-F11, catalog #103138, Biolegend). A violet live/dead fixable dye (Life Technologies) was used to exclude dead cells. A Canto II flow cytometer (BD Biosciences) and FlowJo 7.6.5 software (TreeStar, Ashland, OR, USA) were used for analysis. This experiment was performed three times and results were averaged.
The capacity of live cells to convert retinaldehyde to RA was measured using the flow cytometry-based ALDEFLUOR assay according to the manufacturer’s protocol (Stem Cell). The ALDEFLUOR assay uses a fluorescent non-toxic aminoacetaldehyde, which freely diffuses into intact and viable cells and is converted by ALDH into an aminoacetate that is retained inside the cells. This experiment was performed three times and results were averaged.
For intra-cellular staining, primed OTII single cell suspensions were washed with 1× permeabilization solution and incubated with anti-CD16/32, followed by staining with anti-CD4_APC (clone GK1.5, catalog #552051, BD Bioscience), IFN-γ_Pacific Blue (clone XMG1.2, catalog #505818, Biolegend) or IL-13_efluor660 (clone eBio13A, catalog #50-7133-82, ThermoFisher). Cells were washed, resuspended and kept on ice until flow cytometry was performed. The gating strategy was as follows: dead cells were excluded by gating infra-red dye negative cells, subsequently gated on the basis of forward scatter height versus forward scatter area (singlets 1), then gated on side scatter height versus side scatter area (singlets 2). Cells were then gated on CD4+ cells and the frequency of CD4+IFN-γ+ or CD4+IL-13+ cells was recorded. Negative controls consisted of fluorescence minus one splenocytes. A Canto II cytometer was used and data were analyzed with BD Diva software version 6.7 (BD Bioscience) and FlowJo software version 10 (BD Bioscience). This experiment was performed three times with n = 3 per well per group each time and results were averaged.
For IL-12 intra-cellular staining, single cell suspensions of treated BMDCs were obtained and 2 × 106 cells were incubated for 5 h with 1 µl Golgi Stop (BD Bioscience) and 1 µl Golgi Plug (BD Bioscience) in 1 ml in complete RPMI. Cells were stained with LIVE/DEAD™ fixable near-IR dead cell stain (Thermofisher) for 30 min, prior to fixation. Cells were then stained with CD16/CD32, followed by anti-IL-12PE (p40/p70 clone C15.6, catalog #554477, BD Biosciences). The gating strategy used in this study was as follows: dead cells were excluded by gating live dye versus FSC-A, subsequently gated on the basis of forward scatter height versus forward scatter area (singlets 1), then gated on side scatter height versus side scatter area (singlets 2). The IL-12 percentage and mean fluorescent intensity (MFI) were then calculated in each treatment group. A BD FACS CANTO II cytometer (Becton Dickinson, San Jose, CA, USA) was used.
Small interfering RNA treatment
Knockdown experiments were performed using predesigned and validated siRNA for SOCS3 (s17191; ThermoFisher) or a non-targeting siRNA sequence control (4390844; ThermoFisher) using Lipofectamine RNAiMAX (ThermoFisher) in Opti-MEM (31985062; ThermoFisher), according to the manufacturer’s instructions. At day 6 of differentiation, BMDCs were plated in poly-l-lysine-coated 24-well plates in antibiotic-free medium and transfected. After overnight incubation, the medium was supplemented with antibiotics and cells incubated at 37°C for 48 h before treatment. SOCS siRNA transfection efficiency was 89 ± 9%, as determined by counting the number of cells containing fluorescently tagged siRNA. Knockdown efficacy after 24 h was confirmed by RT–PCR and western blotting.
Statistical analysis
Data were analyzed using Microsoft Excel 2003 and Analyse-it for Excel, version 1.73 (Leeds, UK), or GraphPad Prism 7 (La Jolla, CA, USA). Results are presented as mean ± SEM. Statistical comparisons between two groups were made with the two-tailed Student’s t-test, and between three groups with analysis of variance with Tukey post hoc testing. Differences were considered significant at P ≤0.05. Multiple comparison tests were corrected for repeated measures.
Results
Goblet cell factors suppress DC differentiation and IL-12 production in cultured bone marrow-derived (myeloid) cells
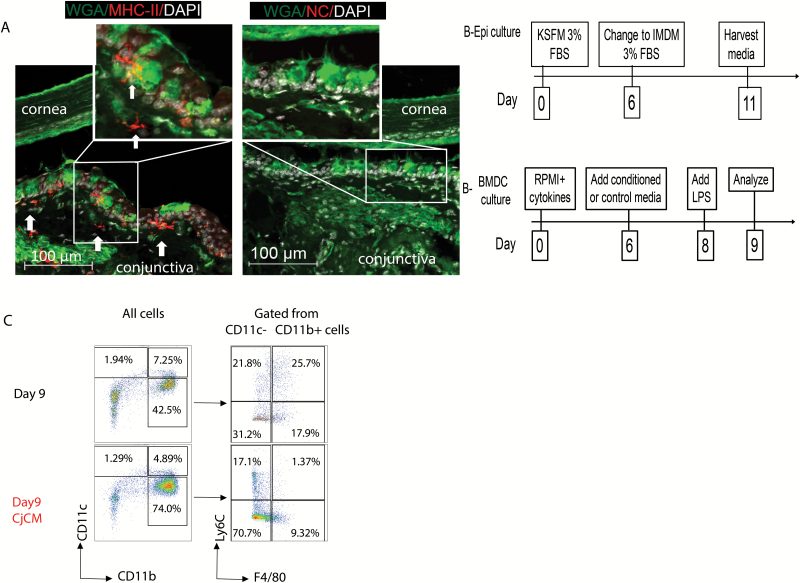
The conjunctiva has a stratified columnar epithelium with numerous goblet cells. We have reported that the conjunctiva contains a mixed population of APCs, including CD11c+ and CD11c+CD11b+ DCs in the epithelium and stroma, and macrophages and monocytes in the stroma (11–13, 15, 16). Certain APCs are in contact with the basolateral aspect of WGA lectin-positive conjunctival goblet cells (Fig. 1A) and can phagocytose topically applied antigen (11). Conjunctival APCs have been found to migrate to the draining cervical lymph nodes in response to desiccating stress where they can prime autoreactive Th1 and Th17 cells (12, 13). Increased expression of the Th1-associated cytokine IFN-γ has been noted in the conjunctiva of experimentally induced mouse and human dry eye that causes goblet cell loss (6, 8, 16). To test our hypothesis that conjunctival goblet cells can modulate differentiation and suppress activation of cultured BMDCs, we evaluated the effects of conditioned media from primary cultured mouse conjunctival epithelium (CjCM) on cultured BMDCs that contain a mixed population of monocyte-derived APCs (24). We have previously reported that explant cultures initiated from the inferior conjunctival fornix differentiate primarily into goblet cells expressing goblet cell-specific markers cytokeratin 7 and mucins MUC2 and MUC5AC (23). The scheme for preparing goblet cell conditioned media is shown in Fig. 1(B). Goblet cells were cultured in KSFM for 6 days, then their medium was changed to IMDM which supports growth of both goblet and bone marrow cells. The BMDCs were initially cultured in RPMI for 6 days, then their medium was replaced by CjCM or control IMDM (Fig. 1B). On day 6, the identity of the BMDCs analyzed by flow cytometry consisted of CD11c+CD11b− and CD11c+CD11b+ DCs and a CD11c−CD11b+ population consisted of Ly6C+ monocytes (36%), F4/80 macrophages (12%) and cells positive for both markers (38%). The effects of CjCM on these cell populations on day 9 were evaluated by flow cytometry (Fig. 1C). The percentages of CD11c+CD11b− and CD11c+CD11b+ DCs decreased, while there was a >30% increase in CD11c−CD11b+ cells. Among the gated CD11b+ populations, CjCM treatment decreased the percentage of Ly6C+F4/80+ and Ly6C+F4/80− cells and increased the percentage of Ly6C−F4/80− cells (Fig. 1C).
Fig. 1.
BMDC culture phenotype and suppressive effects of CjCM. (A) Representative laser confocal images of conjunctival section co-stained with anti-MHCII (red), WGA lectin (green) that binds to goblet cell glycoproteins and DAPI for nuclear DNA (white). Arrows point to MHCII+ dendritic-shaped APCs below the epithelium and in the superficial stroma. Inset in left image of MHCII+ DC adjacent to WGA+ goblet cell in basal conjunctival epithelium. NC = negative control with second antibody only. (B) Experimental design for preparation of conjunctival (goblet cell) epithelial conditioned media (Epi culture, upper) and BMDC culture (lower) treatment groups. (C) Representative density plots of BMDCs cultured in control IMDM media (top) or in conjunctival (goblet cell) epithelial conditioned media (CjCM, bottom) from days 6 to 9. The plots on the right were gated from the CD11c−CD11b+ cells. Day 9 = control BMDCs; Day 9 CjCM = BMDCs treated with conjunctival (goblet cell) epithelial conditioned media; Ly6C = monocyte marker; F4/80 = macrophage marker.
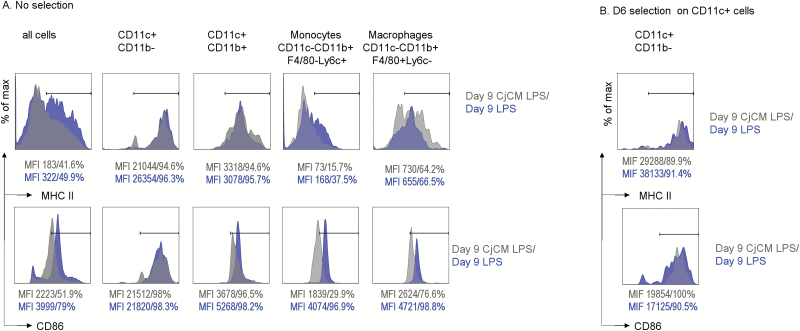
The effects of CjCM on expression of MHCII and the APC activation marker CD86 were evaluated in the entire population of day 9 LPS-stimulated BMDCs and in defined APC populations. CjCM decreased the percentage of MHCII+ cells in the entire population of BMDCs and in the monocyte group (Fig. 2A, top), but not in any of the other cell populations or in the bead-sorted CD11c+ cells (Fig. 2B, top).
Fig. 2.
MHCII and CD86 expression in defined populations of cultured bone marrow-derived (myeloid) cells. (A) MHC (top) and CD86 (bottom) MFI histograms in the entire population of cultured bone marrow-derived (myeloid) cells (all cells) and in defined gated populations (CD11c+CD11b−, CD11c+CD11b+, CD11c−CD11b+F4/80−Ly6c+ monocytes, CD11c−CD11b+F4/80+Ly6c+ macrophages) in LPS or CjCM plus LPS-treated day 9 cultured BMDCs (no bead selection). MFI values and the percentage of positive cells are provided below each histogram. (B) MHCII (top) and CD86 (bottom) MFI histograms in LPS or CjCM plus LPS-treated CD11c+ cells bead sorted from day 9 cultured BMDCs. MFI values and the percentage of positive cells are provided below each histogram.
CjCM also decreased CD86 in the entire population and in CD11c+CD11b+ DC, monocyte (CD11b+Ly6C+) and macrophage (CD11b+F4/80+) populations (Fig. 2A, bottom). The suppressive effect of CjCM was not observed in bead-sorted CD11c+ cells. Unlike the unsorted population, CjCM slightly increased the CD86 percentage and MFI in the sorted CD11c+ cells (Fig. 2B, bottom). These findings suggest that CjCM inhibits DC differentiation and suppresses CD86 expression in monocyte, macrophage and CD11c+CD11b+ APC populations, but not in differentiated CD11c+ DCs.
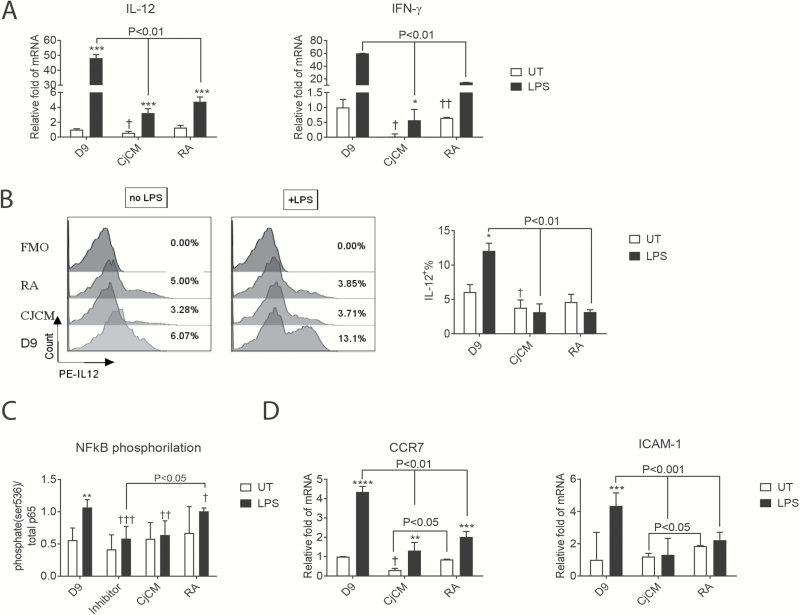
IFN-γ expression increases in the conjunctiva of aqueous-deficient dry eyes, and Spdef KO mice lacking goblet cells have increased IL-12 expression by conjunctival APCs that also produce greater Th1 priming of naive antigen-specific CD4+ T cells than those from WT mice (6, 16). RA produced by mucosal epithelia in the mouth and gastrointestinal tract has been found to be a tolerizing factor on cultured blood or bone marrow-derived monocytes (25–28). Retinol was noted to significantly suppress IL-12 production by stimulated splenic DCs (29). Therefore, we compared the effects of CjCM and RA on expression of Th1 family cytokines. Similar to RA, CjCM decreased expression of IL-12 at the RNA and protein levels in the cultured BMDCs (Fig. 3A and B). CjCM and RA also decreased expression of IFN-γ in these cells (Fig. 3A).
Fig. 3.
Expression of inflammatory mediators by BMDCs. (A) Effects of goblet cell conditioned media (CjCM) or RA compared to control day 9 untreated group on relative fold expression of Th1 cytokines IL-12 and IFN-γ in cultured BMDCs with or without LPS treatment, measured by RT–PCR. Results expressed as mean ± SEM, n = 4, ***P < 0.005. (B) IL-12 intra-cellular staining of BMDCs detected by flow cytometry (mean ± SEM, n = 3). Untreated (UT; i.e. no LPS) versus LPS treated within group, *P < 0.05; †P < 0.05 comparison between day 9 UT versus CjCM UT. (C) Level of NF-kB p65 phosphorylation in DCs with/without CjCM or RA treatment. Day 9 BMDCs were seeded in 96-well plates treated without or with LPS for 45 min and phospho-p65 (ser536) and total p65 were measured by cell-based enzyme-linked immunosorbent assay. Results expressed as %phospho-p65/total p65 are mean ± SEM of three independent experiments. (D) Effects of CjCM or RA compared to control group on relative fold expression of chemokine receptor CCR7 and ICAM-1 in BMDCs (mean ± SEM, n = 4), ****P < 0.001. UT versus LPS within groups: *P < 0.05, **P < 0.01; between-group comparisons: †P < 0.05, ††P < 0.01, †††P < 0.005.
Because LPS-stimulated IL-12 production is mediated through NF-κB activation (30), the effects of CjCM and RA on NF-κB p65 activation were evaluated by FACE assay (Fig. 3C). Both CjCM and RA suppressed LPS-induced p65 activation and the magnitude of CjCM was similar to the NF-κB inhibitor. As further evidence that CjCM suppresses NF-κB, we also evaluated expression of CCR7 and inter-cellular adhesion molecule 1 (ICAM-1), two NF-κB inducible factors involved in APC migration and adhesion. Both CjCM and RA suppressed the LPS-induced increase of CCR7 and ICAM-1 in the cultured bone marrow cells (Fig. 3D).
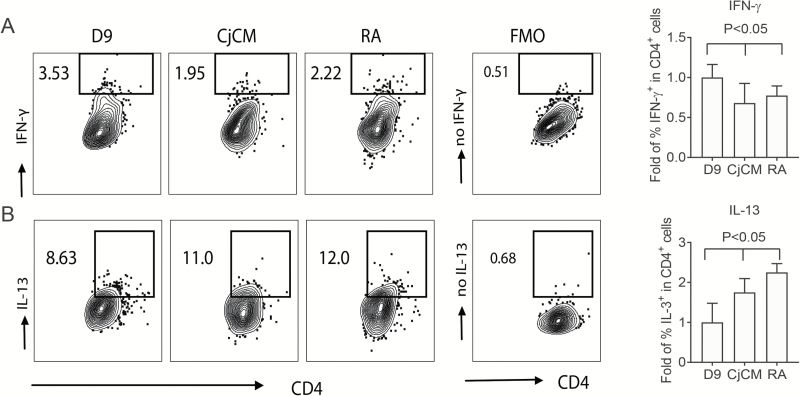
Goblet cell-conditioned APCs suppress Th1 polarization
OVA presentation assays were performed to compare the effects of conditioning BMDCs with CjCM or RA on Th cell polarization by co-culturing OVA peptide-loaded LPS-treated APCs with OTII CD4+ cells. Compared to OTII cells primed with control day 9 APCs, those primed with CjCM- or RA-conditioned APCs showed a lower percentage positive for the Th1 cytokine IFN-γ and higher percentage of cells positive for the Th2 cytokine IL-13 (Fig. 4A and B). Compared to the control group, a 30% reduction of IFN-γ+ (Fig. 4A) and 75% increase in IL-13+ (Fig. 4B) CD4+ T cells were noted in the population primed with CjCM-conditioned APCs. On the basis of these findings, we measured production of IL-6, a cytokine produced by APCs that has been found to promote Th2 polarization by activating nuclear factor of activated T cells (NFAT) and stimulating IL-4Rα expression (31, 32). CjCM increased IL-6 mRNA and released protein in cultured BMDCs compared to control (Supplementary Figure 1).
Fig. 4.
Antigen presentation assay. LPS-treated bone marrow-derived myeloid APCs conditioned with CjCM or RA as described in Fig. 1(B) were harvested on day 9, loaded with OVA peptide for 2 h, then co-cultured with OTII CD4+T cells (1:5 ratio). After 3 days of co-culture, cells were harvested for IFN-γ and IL-13 intra-cellular staining. This experiment was performed three times with n = 3 each time. D9 = control APCs; CjCM = APCs treated with conjunctival conditioned media; RA = APCs treated with 10 nM RA; FMO = fluorescence minus one negative control. (A) Representative contour plots of IFN-γ+CD4+ T cells in different treatment groups shown on the left, and fold change in the percentage of IFN-γ+CD4+ cells relative to day 9 control cells in the bar graph on the right. (B) Representative contour plots of IL-13+CD4+ cells in different treatment groups shown on the left, and fold change in the percentage of IL-13+CD4+ cells relative to day 9 control cells in the bar graph on the right.
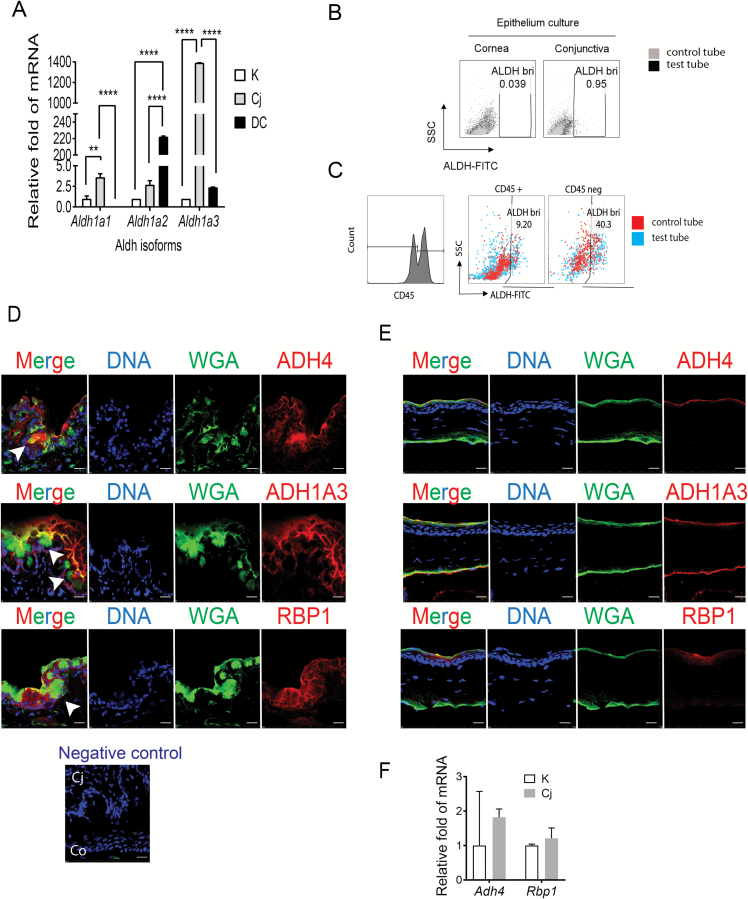
Conjunctival goblet cells produce retinoid acid-metabolizing enzymes
On the basis of the similarities between CjCM and RA on suppressing IL-12 production, NF-κB activation and T-cell priming activity by BMDCs, we hypothesized that goblet cells produce bioactive RA. To test this, we evaluated the expression of the ALDH subfamily of isozymes (Aldh1a1/a2/a3) that catalyze the synthesis of retinal (aldehyde vitamin A metabolite) to RA (33) in cultured conjunctival goblet cell epithelium, cultured corneal (K) epithelium (an ocular surface epithelial lineage that lacks goblet cells) and cultured BMDCs. The conjunctival epithelium had the highest expression of ALDH1a1 and ALDH1a3, while expression of ALDH1a2 which has been found to be highly expressed in lamina propria DCs (34) was highest in the BMDCs (Fig. 5A). The ALDEFLUOR assay was used to measure ALDH activity (ALDHact) in the conjunctival and corneal epithelium. Consistent with the increased expression level, ALDHact was over 20-fold higher in the conjunctival epithelium compared to the cornea epithelium (Fig. 5B). We also evaluated ALDHact in ex vivo suspensions of mouse conjunctival cells that were prepared immediately after euthanasia. ALDHact was over 4-fold higher in the CD45− population consisting primarily of epithelial cells, compared to the CD45+ BMDC population (Fig. 5C). Immunofluorescent staining of mouse conjunctival and corneal tissue sections showed expression of ALDH1a3 protein in the conjunctival epithelium, particularly in the WGA-positive goblet cells (Fig. 5D, arrows) and in the apical layers of the corneal epithelium (Fig. 5E). Tear fluid contains vitamin A in the form of retinol bound to retinol-binding protein (RBP) that is taken up by cells and metabolized to RA by two oxidation steps, an initial conversion from retinol to retinal by ADH and subsequent conversion of retinal to RA by ALDH (21, 35). We immunodetected ADH4, the most abundant form of ADH found in whole conjunctival lysates (22), as well as RBP1 in conjunctival goblet cells (Fig. 5D, arrows). In contrast, staining for these factors was limited to a few apical layers of the corneal epithelium (Fig. 5E). ADH4 and RBP1 were expressed by cultured corneal and conjunctival epithelia and the level of expression was higher in the conjunctiva (Fig. 5F).
Fig. 5.
Expression of RA-metabolizing enzymes by conjunctival goblet cells. (A) RT–PCR to measure the expression of ALDH1 isotypes was performed on RNA harvested from cornea and conjunctival epithelial cultures on day 11 and BMDCs on day 9. K = corneal cells, Cj = conjunctival cells, DC = bone marrow derived cells. (B) ALDH activity (ALDHact) was measured in cultured corneal and conjunctival epithelium in vitro. Single cell suspensions were prepared from cornea and conjunctival epithelial cultures on day 11. DEAB, an ALDH inhibitor, was used as a negative control for ALDH staining (control tube, gray dots). In the absence of DEAB, ALDH+ cells (black dots), which are characterized by high ALDH activity and low SSC, could be detected and distinguished from the ALDH− subset. (C) ALDHact was evaluated in ex vivo suspensions of mouse conjunctival cells that were prepared immediately after euthanasia. ALDHact was over 4-fold higher in the CD45− population consisting primarily of epithelial cells (right), compared to the CD45+ BMDC population (left). (D) Immunofluorescent staining for ADH4, ALDH1a3 and RBP1 in mouse conjunctival tissue sections. WGA lectin stains glycoproteins, including goblet cells mucins. The negative control is secondary antibody only. (E) Immunofluorescent staining for ADH4, ALDH1a3 and RBP1 in mouse cornea tissue sections. (F) RT–PCR to measure expression of ADH4 and RBP1 genes in RNA harvested from cornea and conjunctival epithelial cultures on day 11. **P < 0.01, ****P < 0.001.
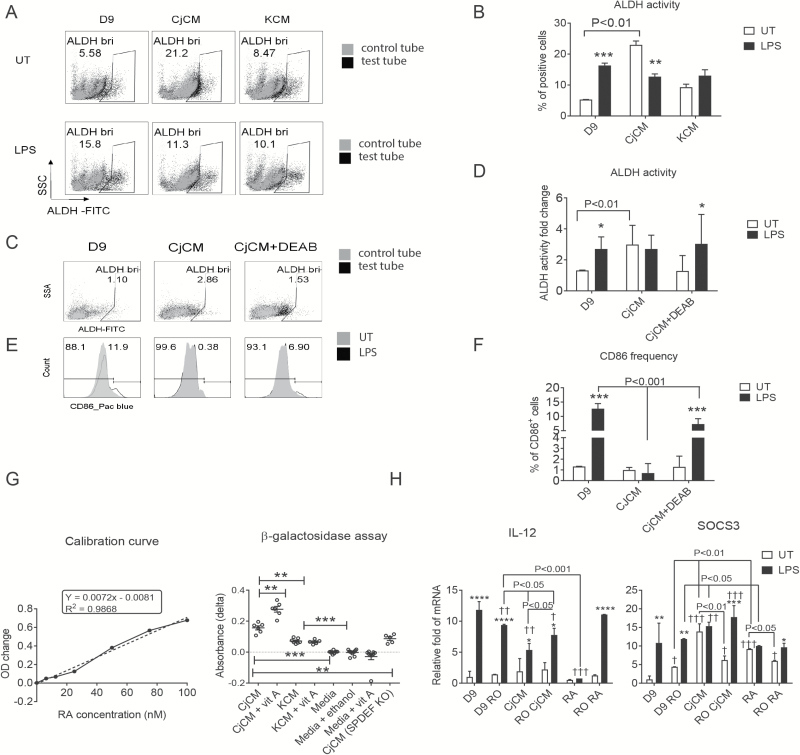
Goblet cells increase ALDH activity in BMDCs
RA produced by gastric and intestinal epithelium has been found to increase RA metabolizing activity in bone marrow- and blood-derived monocytes (25, 28). On the basis of these findings, we evaluated the effects of CjCM and corneal conditioned media (KCM) on ALDHact in unstimulated and LPS-stimulated BMDCs (Fig. 6A). Compared to day 9 control, CjCM increased ALDHact in unstimulated cells and did not change activity in LPS-stimulated cells (Fig. 6B). The stimulatory effects of CjCM on ALDHact in cultured bone marrow cells was lost in goblet cells that were cultured in the presence of the ALDH inhibitor DEAB (Fig. 6C and D). Additionally, the suppressive effects of CjCM on expression of the APC activation marker CD86 was lost in DEAB-treated conjunctival cultures (Fig. 6E and F).
Fig. 6.
Conjunctival goblet cells condition ALDH activity in BMDCs and produce RA. (A) ALDH activity assayed in BMDC treatment groups on day 9, with DEAB-treated cells (gray dots) serving as a negative control for ALDH staining, revealed that CjCM increased the percentage of ALDH+ cells (ALDHbri), n = 3 and experiment performed three times. (B) Statistical comparison of Aldefluor activity using two-way analysis of variance, *P < 0.05, **P < 0.01, ***P < 0.005 means UT versus LPS within groups. (C–F) The supernatant from the conjunctival culture treated with DEAB from day 5 to day 7 was harvested on day 11, then used to condition BMDCs on day 6 (CjCM + DEAB), with CjCM as positive control (CjCM) and media only as negative control (D9). LPS was added to each of the three groups of cultured BMDCs on day 8. After 24 h, the cell suspension was stained with the Aldefluor reagent and CD86. These studies were performed three times with n = 3 per group. (C, D) Representative dot plots (C) and statistical comparisons (D) of ALDH activity assayed in these three groups. (E, F) CD86+ cells displayed as histogram – UT in gray and LPS-treated in black line (E) and the statistical comparisons (F). (G) The F9-RARE-lacZ reporter cell cells were seeded into wells of a 96-well plate, then media (KSFM + 3% FBS), media + vitamin A palmitate (1 nM), conjunctival conditioned media (CjCM) from C57BL/6J or Spdef KO or cornea (KCM) epithelial cultures with or without vitamin A (retinol) palmitate (1 µM), or RA in different concentrations were added. After 16 h, the supernatants were removed and a β-galactosidase assay was performed. Each group was repeated three times with n = 4 per run. The absorbance (OD) values from different concentrations of RA were used to generate the calibration curve (left), and the OD values obtained with different treatment groups are shown on the right. (H) The RARα antagonist, Ro41-5253 (RO), was added to cultured BMDCs 2 h before CjCM or RA on day 6, and RNA harvested for PCR for IL-12 (left) or SOCS3 (right) after 4-h LPS stimulation on day 8. D9 = control BMDCs; D9 RO = BMDCs with 1 µM RO added; CjCM = BMDCs treated with conjunctival conditioned media; RO CjCM= BMDCs with both RO and CjCM added; RA = BMDCs cells treated with 10 nM RA; RO RA = BMDCs with both 1 µM RO and 10 nM RA added; UT = untreated (without LPS stimulation); LPS = BMDCs treated with LPS. UT versus LPS within groups: *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001; between-group comparisons: †P < 0.05, ††P < 0.01, †††P < 0.005.
Goblet cells produce biologically active RA that suppresses IL-12 production by BMDCs
We used a bioassay with Sil-15 F9-RARE-lacZ reporter cells to detect RA activity in conditioned media from cultured conjunctival and corneal epithelial cells. A linear increase in absorbance was observed following treatment of the reporter cells with increasing concentrations of RA from 0 to 100 mM (Fig. 6G, left). CjCM contained a mean RA concentration of 2.5 nM which was significantly higher than control KSFM media containing 3% FBS (media), KCM or media from conjunctival cultures established from the SPDEF KO strain that lacks goblet cells. The RA concentration in CjCM increased significantly when vitamin A palmitate was added to the conjunctival epithelial media (CjCM + vit A), indicating the conjunctival epithelia have the ability to synthesize RA from vitamin A. In contrast, an increase in RA activity was not observed in vitamin A-treated corneal epithelia (KCM + vit A) or when vitamin A was directly added to the Sil-15 culture media (Media + vit A; Fig. 6G, right).
Cultured BMDCs were treated with an RAR-α inhibitor (RO) to determine the contribution of RA on the suppressive effect of CjCM on LPS-stimulated IL-12 production. RO inhibited the suppressive effects of both CjCM and RA on IL-12 expression in LPS-stimulated cells (Fig. 6H, left). SOCS3 is a RA-inducible gene in myeloid lineage APCs that has been reported to suppress NF-κB activation, IL-12 production and capacity to induce Th1 differentiation (36–38). LPS, CjCM and RA all increased SOCS3 expression in day 9 DCs and this effect was reversed by RO (Fig. 6H, right).
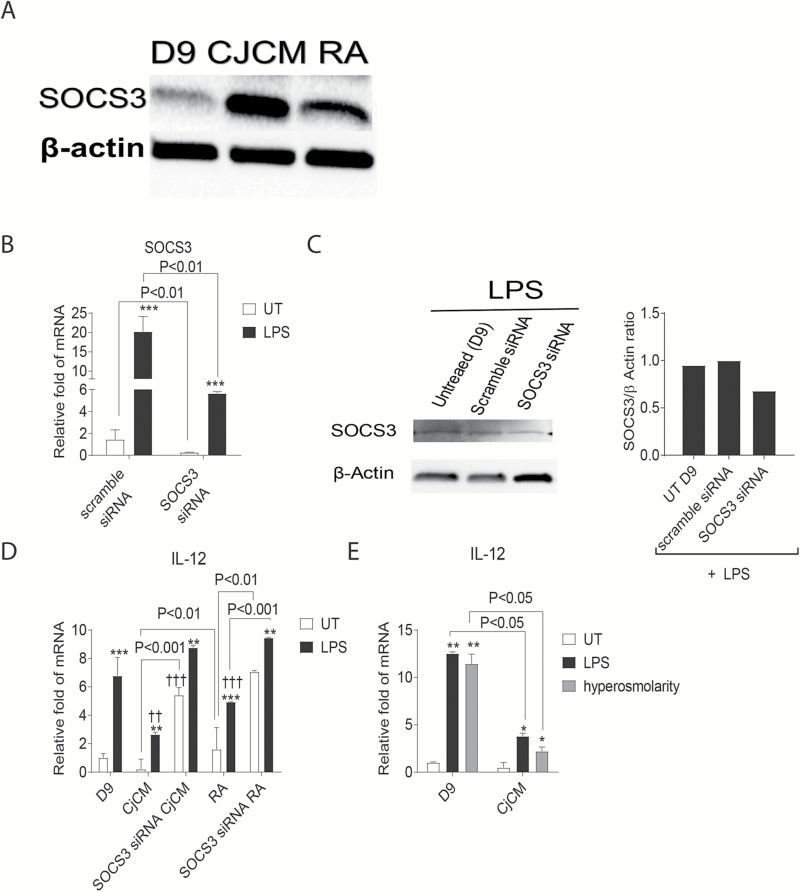
SOCS3 contributes to goblet cell suppression of IL-12
To determine the relative contribution of increased SOCS3 expression on the suppressive effects of CjCM on IL-12 expression, we silenced the SOCS3 gene in BMDCs using SOCS3 siRNA. Both CjCM and RA increased SOCS3 mRNA (Fig. 6H) and protein (Fig. 7A) in the bone marrow cells. Compared to a scrambled RNA sequence, SOCS3 siRNA decreased levels of SOCS3 expression in unstimulated and LPS-stimulated cells on culture day 9 (Fig. 7B) and SOCS3 protein in LPS-stimulated cultures (Fig. 7C). SOCS3 silencing reversed the suppressive effects of CjCM on IL-12 production in unstimulated cells and reversed the effects of RA in both unstimulated and LPS-stimulated cells (Fig. 7D).
Fig. 7.
SOCS3 neutralization. (A) SOCS3 and β-actin western blot of day 9 (D9) cultured BMDCs and D9 BMDCs treated with conjunctival conditioned media (CjCM) and RA. (B) Compared to a control scrambled sequence, SOCS3 siRNA inhibited expression of SOCS3 transcripts at 24 h in BMDCs with or without LPS stimulation measured by RT–PCR. (C) SOCS3 western blot of LPS-stimulated BMDCs after 24-h treatment with control scrambled sequence or SOCS3 siRNA LPS (left) and the SOCS3/β-actin ratio measured from the western blot (right). (D) SOCS3 gene silencing reversed the suppressive effects of CjCM and RA on IL-12 expression in BMDCs. Untreated (UT) versus LPS within groups: *P < 0.05, **P < 0.01, ***P < 0.005; between-group (D9) comparisons: ††P < 0.01, †††P < 0.005. (E) CjCM suppressed expression of IL-12 in BMDCs stimulated with LPS or hyperosmolar (450mOsm) culture media.
The ocular surface has a paucibacterial microbiome (39) and the APCs may be infrequently subjected to microbial danger signals like LPS in vivo; however, they are often subjected to osmotic stress from hyperosmolar tears when the eye becomes dry through desiccation or inadequate tear production (40, 41). Osmotic stress has been found to activate MAPK and NF-κB signaling pathways (17, 42). Therefore, we compared the effects of LPS and osmotic stress with 400mOsm media on IL-12 expression in BMDCs. We found both stresses increased IL-12 over unstimulated control to a similar magnitude and CjCM suppressed the stimulatory effects of both LPS and osmotic stress (Fig. 7E).
Discussion
The ocular surface has a complex lacrimal functional unit consisting of the lacrimal gland, ocular surface epithelia and their neural connections that functions to maintain homeostasis and suppress inflammation (43). The lacrimal gland secretes immunomodulatory cytokines, such as TGF-β, as well as vitamin A in the form of retinol into tears (20, 44). Dry eye resulting from disease or dysfunction of the lacrimal gland leads to conjunctival inflammation with recruitment of pathogenic CD4+ T cells and increased IFN-γ expression (6–8). IFN-γ causes secretory dysfunction, an unfolded protein response, apoptosis and loss of the conjunctival goblet cells leading to mucin tear deficiency and worse ocular surface inflammation and epithelial disease (6, 9, 10).
On the basis of these immunopathogenic changes in dry eye, we hypothesized that the goblet cells suppress inflammation and condition tolerogenic properties in cultured bone marrow cells containing a mixed population of monocyte-derived APCs consisting of monocytes, macrophages and DCs. A similar array of APC phenotypes is also present in the conjunctiva in vivo (11–13, 15, 16). We found that conjunctival goblet cells produce factors that suppress DC differentiation, expression of the activation marker CD86 and production of Th1 family cytokines IL-12 and IFN-γ in the cultured bone marrow cells. Furthermore, goblet cell conditioned media suppressed NF-κB p65 activation and expression of NF-κB inducible genes, CCR7 and ICAM-1 in these cells. Goblet cell-conditioned, OVA-loaded APCs suppressed IFN-γ production and increased IL-13 in co-cultured OTII cells. The goblet cell suppressive activity appears to be due in part to their ability to synthesize RA.
Compared to the stratified cornea epithelium that lacks goblet cells, conjunctival goblet cell epithelium had greater expression and activity of the ALDH subfamily 1a genes (ALDH1a1 and a3) that encode enzymes that metabolize RA from vitamin A (35). Goblet cells also expressed ADH AD4, another key enzyme for RA metabolism. The conditioning effect on cultured BMDCs was lost in goblet cells treated with the ALDH inhibitor DEAB and an RARα antagonist blocked the effects of CjCM on activation and IL-12 production by APCs. We found the modulatory activity of CjCM on the bone marrow cells was similar to RA. RA has been previously reported to inhibit IL-12 in human monocyte-derived DCs (45, 46) and mouse splenic CD11b+ DCs (47). SOCS3 is a suppressor of cytokine signaling in innate and adaptive immunity (48). It is up-regulated in APCs by a variety of cytokine and microbial signals, including LPS (48). SOCS3 has been noted to be a key suppressor of NF-κB activity and IL-12 production in macrophages (38, 49). We found that both CjCM and RA increased expression of SOCS3 in BMDCs and that SOCS3 silencing reduced the suppressive effects of CjCM and RA on DC IL-12 expression, indicating that some of the goblet cell RA activity is mediated through SOCS3. This effect was best observed in unstimulated cells, since SOCS3 may have been maximally expressed in LPS-stimulated cells.
Intestinal epithelium (50, 51), intestinal epithelial cell lines (50, 52) and gastric (28) epithelia have also been found to synthesize RA that can confer the ability to synthesize RA in bone marrow- and blood-derived APCs (28, 52, 53). In the gut, retinol in the diet or secreted by the liver in bile is metabolized by RALDH1a1 in the small intestinal epithelium to RA that can be delivered to and condition tolerogenic properties in substantia propria APCs (36, 53–56). We found the conjunctival goblet cells appear to have properties similar to the intestinal epithelium, in that they are capable of metabolizing vitamin A (retinol) that is secreted by the lacrimal gland into the tear fluid that bathes the ocular mucosa. Additionally, the conjunctiva goblet cells are similar to intestinal goblet cells in their ability to serve as passages for antigens from the mucosal surface to the underlying APCs where they can be delivered along with tolerizing factors, such as RA (11).
In addition to RA, conjunctival goblet cells produce other immunomodulatory factors such as TGF-β1 and -β2 and MUC2 that could also contribute to their conditioning activity (23, 57). Conjunctival goblet cell-produced TGF-β2, activated by thrombospondin-1 (TSP-1) in these cells, was found to suppress MHCII and CD86 expression by BMDCs (57).
APCs conditioned with conjunctival goblet cells were noted to suppress generation of IFN-γ+ T cells, while increasing the percentage of IL-13+ cells in primed OTII cells. This is consistent with findings of KO and associates that APCs from the eye draining cervical nodes of Spdef mice that lack conjunctival goblet cells show greater Th1 priming than those from wild-type mice (16). While the mechanisms by which CjCM promotes Th2 differentiation remain to be determined, it may be due to its ability to decrease CD86 expression and increase IL-6 expression in BMDCs. Antibody-mediated CD86 blockade in mice was noted to promote Th2 polarization and fetal tolerance in abortion prone mice (58). IL-6 has been reported to suppress Th1 and stimulate Th2 differentiation by activating NFAT and increasing IL-4Ra in naive CD4 T cells (31, 32).
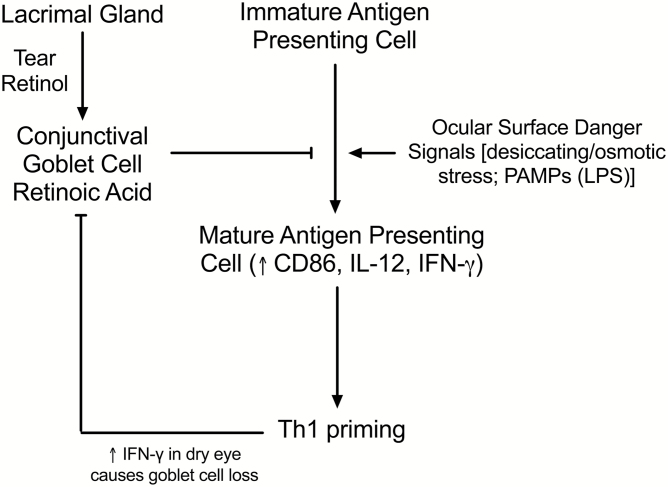
These findings have potential implications for the conjunctiva by linking vitamin A secreted by the lacrimal gland to conjunctival goblet cells that are capable of conditioning adjacent APCs to maintain conjunctival immune tolerance and prevent damaging ocular surface inflammation, particularly in the context of disease related danger signals, like hyperosmolarity in dry eye (Fig. 8). Studies have found that ocular surface immune tolerance is disrupted in three different mouse models of dry eye in mice and this is can be prevented by topical treatment with an NF-κB inhibitor (17, 18, 59, 60). All of these models are associated with conjunctival goblet cell dysfunction or loss that could result in reduced conditioning of APCs leading to increased activation, IL-12 production and priming of Th1 cells that have been shown to be pathogenic to the ocular surface. These findings suggest that conjunctival goblet cells have unique properties for maintaining ocular mucosal tolerance and it is likely goblet cells in other mucosal tissues have similar immunomodulatory activity.
Fig. 8.
Conjunctival goblet cells are capable of metabolizing vitamin A secreted by the lacrimal gland as retinol into tears into RA that can suppress maturation and Th1 cytokine production by APCs that are stimulated by danger signals [desiccating/osmotic stress or pattern associated microbial products (PAMPs)]. IFN-γ produced by Th1 cells in the dry eye conjunctiva can cause goblet cell loss/dysfunction resulting in reduced APC conditioning.
Funding
This work was supported by the National Institutes of Health (NIH) grant EY11915 (S.C.P.), NIH Core grants EY002520 and EY020799, NIH funding to Cytometry and Cell Sorting Core at Baylor College of Medicine (NIAID P30AI036211, NCI P30CA125123 and NCRR S10RR024574), Biology of Inflammation Center Baylor College of Medicine, an unrestricted grant from Research to Prevent Blindness, New York, NY (S.C.P.), the Oshman Foundation, Houston, TX (S.C.P.), the William Stamps Farish Fund, Houston, TX (S.C.P.), Hamill Foundation, Houston, TX (S.C.P.), Sid W. Richardson Foundation, Ft Worth, TX (S.C.P.) and Chinese Scholarship Council and National Natural Science Foundation of China, Bejing (81700809, Y.X.).
Supplementary Material
Acknowledgements
We thank Dr Michael Wagner (State University of New York, Brooklyn, NY) for the F9-RARE-lacZ reporter cell line, and Hans Clevers, PhD (Hubrecht Institute, Utrecht, the Netherlands) and Jeffrey Whitsett, MD, PhD (Cincinnati Children’s Hospital, Cincinnati, OH) for providing the Spdef KO mice. All the authors contributed to this study and performed one or more experiments. Y.X., C.S.D.P. and S.C.P. drafted the manuscript that was reviewed and approved by all authors.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Galletti, J. G., Guzmán, M. and Giordano, M. N. 2017. Mucosal immune tolerance at the ocular surface in health and disease. Immunology 150:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson, J. D. and Wright, J. C. 1984. Conjunctival goblet cell densities in ocular surface disease. Arch. Ophthalmol. 102:1049. [DOI] [PubMed] [Google Scholar]
- 3. Pflugfelder, S. C., Tseng, S. C., Yoshino, K., Monroy, D., Felix, C. and Reis, B. L. 1997. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology 104:223. [DOI] [PubMed] [Google Scholar]
- 4. Tatematsu, Y., Ogawa, Y., Shimmura, S., et al. 2012. Mucosal microvilli in dry eye patients with chronic GVHD. Bone Marrow Transplant. 47:416. [DOI] [PubMed] [Google Scholar]
- 5. Ralph, R. A. 1975. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest. Ophthalmol. 14:299. [PubMed] [Google Scholar]
- 6. Pflugfelder, S. C., De Paiva, C. S., Moore, Q. L., et al. 2015. Aqueous tear deficiency increases conjunctival interferon-γ (IFN-γ) expression and goblet cell loss. Invest. Ophthalmol. Vis. Sci. 56:7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kunert, K. S., Tisdale, A. S., Stern, M. E., Smith, J. A. and Gipson, I. K. 2000. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch. Ophthalmol. 118:1489. [DOI] [PubMed] [Google Scholar]
- 8. De Paiva, C. S., Villarreal, A. L., Corrales, R. M., et al. 2007. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest. Ophthalmol. Vis. Sci. 48:2553. [DOI] [PubMed] [Google Scholar]
- 9. García-Posadas, L., Hodges, R. R., Li, D., et al. 2016. Interaction of IFN-γ with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 9:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coursey, T. G., Tukler Henriksson, J., Barbosa, F. L., de Paiva, C. S. and Pflugfelder, S. C. 2016. Interferon-γ-induced unfolded protein response in conjunctival goblet cells as a cause of mucin deficiency in Sjögren syndrome. Am. J. Pathol. 186:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbosa, F. L., Xiao, Y., Bian, F., et al. 2017. Goblet cells contribute to ocular surface immune tolerance-implications for dry eye disease. Int. J. Mol. Sci. 18. doi: 10.3390/ijms18050978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang, X., Volpe, E. A., Gandhi, N. B., et al. 2012. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One 7:e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaumburg, C. S., Siemasko, K. F., De Paiva, C. S., et al. 2011. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J. Immunol. 187:3653. [DOI] [PubMed] [Google Scholar]
- 14. Khandelwal, P., Blanco-Mezquita, T., Emami, P., et al. 2013. Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PLoS One 8:e64193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. You, I. C., Coursey, T. G., Bian, F., Barbosa, F. L., de Paiva, C. S. and Pflugfelder, S. C. 2015. Macrophage phenotype in the ocular surface of experimental murine dry eye disease. Arch. Immunol. Ther. Exp. (Warsz) 63:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ko, B. Y., Xiao, Y., Barbosa, F. L., de Paiva, C. S., and Pflugfelder, S. C. 2018. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight 3. doi: 10.1172/jci.insight.98222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guzmán, M., Keitelman, I., Sabbione, F., Trevani, A. S., Giordano, M. N. and Galletti, J. G. 2016. Desiccating stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clin. Exp. Immunol. 184:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guzmán, M., Keitelman, I., Sabbione, F., Trevani, A. S., Giordano, M. N. and Galletti, J. G. 2016. Mucosal tolerance disruption favors disease progression in an extraorbital lacrimal gland excision model of murine dry eye. Exp. Eye Res. 151:19. [DOI] [PubMed] [Google Scholar]
- 19. Samarawickrama, C., Chew, S. and Watson, S. 2015. Retinoic acid and the ocular surface. Surv. Ophthalmol. 60:183. [DOI] [PubMed] [Google Scholar]
- 20. Ubels, J. L. and MacRae, S. M. 1984. Vitamin A is present as retinol in the tears of humans and rabbits. Curr. Eye Res. 3:815. [DOI] [PubMed] [Google Scholar]
- 21. Lee, S. Y., Ubels, J. L. and Soprano, D. R. 1992. The lacrimal gland synthesizes retinol-binding protein. Exp. Eye Res. 55:163. [DOI] [PubMed] [Google Scholar]
- 22. Nezzar, H., Chiambaretta, F., Marceau, G., et al. 2007. Molecular and metabolic retinoid pathways in the human ocular surface. Mol. Vis. 13:1641. [PubMed] [Google Scholar]
- 23. Tuckler Henricksson, J., Coursey, T. G., Corry, D. B., DePaiva, C. S. and Pflugfelder, S. C. 2015. IL-13 stimulates proliferation and expression of mucins and immunomodulatory gene in cultured conjunctival goblet cells. Invest. Ophthalmol. Vis. Sci. 56:4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price, J. D. and Tarbell, K. V. 2015. The role of dendritic cell subsets and innate immunity in the pathogenesis of type 1 diabetes and other autoimmune diseases. Front. Immunol. 6:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iliev, I. D., Spadoni, I., Mileti, E., et al. 2009. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58:1481. [DOI] [PubMed] [Google Scholar]
- 26. Rescigno, M. 2014. Dendritic cell-epithelial cell crosstalk in the gut. Immunol. Rev. 260:118. [DOI] [PubMed] [Google Scholar]
- 27. Kato, H., Izumi, K., Saito, T., et al. 2013. Distinct expression patterns and roles of aldehyde dehydrogenases in normal oral mucosa keratinocytes: differential inhibitory effects of a pharmacological inhibitor and RNAi-mediated knockdown on cellular phenotype and epithelial morphology. Histochem. Cell Biol. 139:847. [DOI] [PubMed] [Google Scholar]
- 28. Bimczok, D., Kao, J. Y., Zhang, M., et al. 2015. Human gastric epithelial cells contribute to gastric immune regulation by providing retinoic acid to dendritic cells. Mucosal Immunol. 8:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manicassamy, S., Ravindran, R., Deng, J., et al. 2009. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 15:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dev, A., Iyer, S., Razani, B. and Cheng, G. 2011. NF-κB and innate immunity. Curr. Top. Microbiol. Immunol. 349:115. [DOI] [PubMed] [Google Scholar]
- 31. Diehl, S. and Rincón, M. 2002. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 39:531. [DOI] [PubMed] [Google Scholar]
- 32. Braune, J., Weyer, U., Hobusch, C., et al. 2017. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J. Immunol. 198:2927. [DOI] [PubMed] [Google Scholar]
- 33. Andrýs, C., Krejsek, J., Slezák, R., Drahosová, M. and Kopecký, O. 1999. Serum soluble adhesion molecules (sICAM-1, sVCAM-1, sE-selectin) and neopterin in patients with Sjögren’s syndrome. Acta Medica (Hradec Kralove) 42:97. [PubMed] [Google Scholar]
- 34. Molenaar, R., Knippenberg, M., Goverse, G., et al. 2011. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J. Immunol. 186:1934. [DOI] [PubMed] [Google Scholar]
- 35. Koppaka, V., Thompson, D. C., Chen, Y., et al. 2012. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 64:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manicassamy, S. and Pulendran, B. 2009. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin. Immunol. 21:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin, H., Holdbrooks, A. T., Liu, Y., Reynolds, S. L., Yanagisawa, L. L. and Benveniste, E. N. 2012. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 189:3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qin, H., Yeh, W. I., De Sarno, P., et al. 2012. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc. Natl Acad. Sci. USA 109:5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Paiva, C. S., Jones, D. B., Stern, M. E., et al. 2016. Altered mucosal microbiome diversity and disease severity in Sjögren syndrome. Sci. Rep. 6:23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stewart, P., Chen, Z., Farley, W., Olmos, L. and Pflugfelder, S. C. 2005. Effect of experimental dry eye on tear sodium concentration in the mouse. Eye Contact Lens 31:175. [DOI] [PubMed] [Google Scholar]
- 41. Lemp, M. A., Bron, A. J., Baudouin, C., et al. 2011. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 151:792.e1. [DOI] [PubMed] [Google Scholar]
- 42. Luo, L., Li, D. Q., Corrales, R. M. and Pflugfelder, S. C. 2005. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 31:186. [DOI] [PubMed] [Google Scholar]
- 43. Stern, M. E., Schaumburg, C. S., Dana, R., Calonge, M., Niederkorn, J. Y. and Pflugfelder, S. C. 2010. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 3:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshino, K., Garg, R., Monroy, D., Ji, Z. and Pflugfelder, S. C. 1996. Production and secretion of transforming growth factor beta (TGF-beta) by the human lacrimal gland. Curr. Eye Res. 15:615. [DOI] [PubMed] [Google Scholar]
- 45. Tao, Y., Yang, Y. and Wang, W. 2006. Effect of all-trans-retinoic acid on the differentiation, maturation and functions of dendritic cells derived from cord blood monocytes. FEMS Immunol. Med. Microbiol. 47:444. [DOI] [PubMed] [Google Scholar]
- 46. Wada, Y., Hisamatsu, T., Kamada, N., Okamoto, S. and Hibi, T. 2009. Retinoic acid contributes to the induction of IL-12-hypoproducing dendritic cells. Inflamm. Bowel Dis. 15:1548. [DOI] [PubMed] [Google Scholar]
- 47. Zhu, B., Buttrick, T., Bassil, R., et al. 2013. IL-4 and retinoic acid synergistically induce regulatory dendritic cells expressing Aldh1a2. J. Immunol. 191:3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoshimura, A., Naka, T. and Kubo, M. 2007. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7:454. [DOI] [PubMed] [Google Scholar]
- 49. Zhang, X., Wang, Y., Yuan, J., et al. 2018. Macrophage/microglial Ezh2 facilitates autoimmune inflammation through inhibition of Socs3. J Exp Med. 215:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lampen, A., Meyer, S., Arnhold, T. and Nau, H. 2000. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J. Pharmacol. Exp. Ther. 295:979. [PubMed] [Google Scholar]
- 51. Thomas, S., Prabhu, R. and Balasubramanian, K. A. 2005. Retinoid metabolism in the rat small intestine. Br. J. Nutr. 93:59. [DOI] [PubMed] [Google Scholar]
- 52. Iliev, I. D., Mileti, E., Matteoli, G., Chieppa, M. and Rescigno, M. 2009. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2:340. [DOI] [PubMed] [Google Scholar]
- 53. McDonald, K. G., Leach, M. R., Brooke, K. W., et al. 2012. Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids. Am. J. Pathol. 180:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jaensson-Gyllenbäck, E., Kotarsky, K., Zapata, F., et al. 2011. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 4:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Agace, W. W. and Persson, E. K. 2012. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 33:42. [DOI] [PubMed] [Google Scholar]
- 56. Gottesman, M. E., Quadro, L. and Blaner, W. S. 2001. Studies of vitamin A metabolism in mouse model systems. Bioessays 23:409. [DOI] [PubMed] [Google Scholar]
- 57. Contreras-Ruiz, L. and Masli, S. 2015. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One 10:e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu, X. Y., Zhou, Y. H., Wang, M. Y., Jin, L. P., Yuan, M. M. and Li, D. J. 2005. Blockade of CD86 signaling facilitates a Th2 bias at the maternal-fetal interface and expands peripheral CD4+CD25+ regulatory T cells to rescue abortion-prone fetuses. Biol. Reprod. 72:338. [DOI] [PubMed] [Google Scholar]
- 59. Galletti, J. G., Gabelloni, M. L., Morande, P. E., et al. 2013. Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol. 6:24. [DOI] [PubMed] [Google Scholar]
- 60. Guzmán, M., Sabbione, F., Gabelloni, M. L., et al. 2014. Restoring conjunctival tolerance by topical nuclear factor-κB inhibitors reduces preservative-facilitated allergic conjunctivitis in mice. Invest. Ophthalmol. Vis. Sci. 55:6116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.