Artemether-lumefantrine is often coadministered with efavirenz-based antiretroviral therapy for malaria treatment in HIV-infected women during pregnancy. Previous studies showed changes in lumefantrine pharmacokinetics due to interaction with efavirenz in nonpregnant adults.
KEYWORDS: antimalarial agents, antiretroviral agents, drug interactions, efavirenz, human immunodeficiency virus, lumefantrine, pharmacokinetics
ABSTRACT
Artemether-lumefantrine is often coadministered with efavirenz-based antiretroviral therapy for malaria treatment in HIV-infected women during pregnancy. Previous studies showed changes in lumefantrine pharmacokinetics due to interaction with efavirenz in nonpregnant adults. The influence of pregnancy on this interaction has not been reported. This pharmacokinetic study involved 35 pregnant and 34 nonpregnant HIV-malaria-coinfected women receiving efavirenz-based antiretroviral therapy and was conducted in four health facilities in Nigeria. Participants received a 3-day standard regimen of artemether-lumefantrine for malaria treatment, and intensive pharmacokinetic sampling was conducted from 0.5 to 96 h after the last dose. Plasma efavirenz, lumefantrine, and desbutyl-lumefantrine were quantified using validated assays, and pharmacokinetic parameters were derived using noncompartmental analysis. The median middose plasma concentrations of efavirenz were significantly lower in pregnant women (n = 32) than in nonpregnant women (n = 32) at 1,820 ng/ml (interquartile range, 1,300 to 2,610 ng/ml) versus 2,760 ng/ml (interquartile range, 2,020 to 5,640 ng/ml), respectively (P = 0.006). The lumefantrine area under the concentration-time curve from 0 to 96 h was significantly higher in pregnant women (n = 27) at 155,832 ng · h/ml (interquartile range, 102,400 to 214,011 ng · h/ml) than nonpregnant women at 90,594 ng · h/ml (interquartile range, 58,869 to 149,775 ng · h/ml) (P = 0.03). A similar trend was observed for the lumefantrine concentration at 12 h after the last dose of lumefantrine, which was 2,870 ng/ml (interquartile range, 2,180 to 4,880 ng/ml) versus 2,080 ng/ml (interquartile range, 1,190 to 2,970 ng/ml) in pregnant and nonpregnant women, respectively (P = 0.02). The lumefantrine-to-desbutyl-lumefantrine ratio also tended to be lower in pregnant women than in nonpregnant women (P = 0.076). Overall, pregnancy tempered the extent of efavirenz-lumefantrine interactions, resulting in increased lumefantrine exposure. However, any consideration of dosage adjustment for artemether-lumefantrine to enhance exposure in this population needs to be based on data from a prospective study with safety and efficacy endpoints.
INTRODUCTION
The geographical overlap in the prevalence of human immunodeficiency virus (HIV) and malaria infections often necessitates comedication of patients with antiretroviral and antimalarial drugs. Malaria in pregnancy is associated with an increased risk of maternal anemia, a low-birth-weight infant, fetal loss, and maternal and fetal mortalities (1). This is exacerbated in the presence of HIV infection, where pregnant women are more likely to experience serious complications and, without adequate interventions, exposed babies may become infected with HIV and/or malaria. This can be prevented by effective management using first-line antiretroviral therapy (ART) for HIV and artemisinin-based combination therapy (ACT) for malaria in line with current World Health Organization (WHO) guidelines (2, 3). The preferred first-line ART during pregnancy in sub-Saharan Africa consists of efavirenz at 600 mg in a fixed-dose combination with tenofovir (TDF) at 300 mg and lamivudine at 300 mg or emtricitabine at 200 mg (3). Regarding antimalarial medicines, artemether-lumefantrine is one of the most popular artemisinin-based combination therapies as a result of its excellent safety and efficacy during pregnancy (4, 5). The artemether component is short acting and clears both the asexual and sexual forms of the malaria parasite rapidly, while the lumefantrine component is longer acting and serves the dual role of protecting from the development of resistance to the artemisinin moiety and being responsible for the prophylactic efficacy (1).
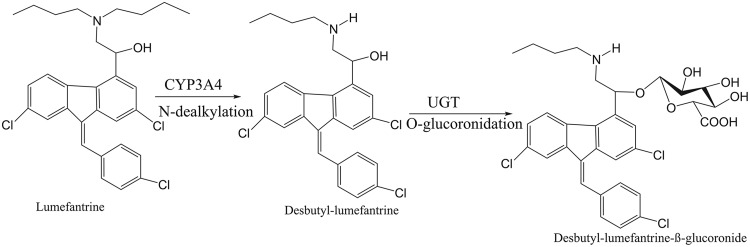
Efavirenz is principally metabolized to 8-hydroxyefavirenz by cytochrome P450 (CYP) 2B6 (CYP2B6), with minimum contributions from CYP3A4, CYP2A6, and CYP1A2 (6). Efavirenz as well as nevirapine, another nonnucleoside reverse transcriptase inhibitor, is known to significantly mediate drug-drug interactions through the induction of CYP isoforms, particularly CYP2B6 and CYP3A4 (7, 8). Other ART previously reported to be perpetrators of the drug-drug interaction through CYP inhibition include protease inhibitors, such as lopinavir-ritonavir (9, 10). However, both CYP3A4 and CYP2B6 metabolize artemether through demethylation to dihydroartemisinin, while lumefantrine is metabolized to desbutyl-lumefantrine through dealkylation by CYP3A4 (11). Thus, both components of ACTs might be the victims of a phase I metabolic drug-drug interaction mediated by efavirenz. The products of phase I metabolisms are subsequently conjugated by the microsomal enzyme (Fig. 1) uridine-glucuronosyltransferase to more polar compounds prior to renal excretion (12), and it has been reported that efavirenz may also influence the activity of uridine-glucuronosyltransferase isoforms (13). Such interactions were confirmed in nonpregnant adults (7, 8, 14); however, the dynamic might be different in pregnant women.
FIG 1.
Phase I and II metabolism of lumefantrine shows the conversion of lumefantrine to desbutyl-lumefantrine by CYP3A4, a cytochrome P450, and the subsequent conversion of desbutyl-lumefantrine to a more polar metabolite by a uridine-glucuronosyltransferase (UGT) isoform.
Some enzymes of the hepatic CYP system, such as CYP3A4 and CYP2B6, are upregulated during pregnancy, resulting in a higher rate of metabolism, while others, such as CYP1A2 and CYP2C19, are downregulated and, hence, impair metabolic clearance (15). The role of estrogens and progesterone in the regulation of CYP expression during pregnancy is supported by contraceptives, which have effects on CYP activity similar to those observed during pregnancy (16, 17). Considering this, induction of CYP3A4- and CYP2B6-mediated metabolisms of lumefantrine and efavirenz, respectively, may result in a low plasma exposure and a high metabolic ratio of each drug. Since pregnancy upregulates both CYP3A4 and CYP2B6 activities, modifications of efavirenz pharmacokinetics at steady state are imminent (18, 19), which may partially explain the difference in the plasma concentrations of efavirenz in a cohort of HIV-infected pregnant women compared with that in a cohort of nonpregnant women who are receiving the same daily dose of efavirenz. We hypothesized that different efavirenz concentrations would result in varied degrees of efavirenz-mediated CYP3A4 induction, which might subsequently alter the magnitude of the interaction with lumefantrine.
The objective of the present study was to investigate the influence of pregnancy on the dynamics of the drug-drug interaction between efavirenz and lumefantrine in the context of HIV and malaria coinfection.
RESULTS
Patients characteristics.
Out of 308 patients, all of whom had already been receiving the regimen of tenofovir (TDF) at 300 mg, lamivudine at 300 mg, and efavirenz at 600 mg for ≥2 weeks, 39 pregnant women and 61 nonpregnant women were positive for the malaria parasite. However, only 73 subjects (38 pregnant women and 35 nonpregnant women) gave written consent to participate in the study, and of these, 69 subjects participated in the sampling for pharmacokinetic and pharmacogenetic purposes. The 69 subjects included 35 pregnant women (mean age, 30 years; mean weight, 61.8 kg) and 34 nonpregnant women (mean age, 35.6 years; mean weight, 57.3 kg). Of these, 27 HIV-infected pregnant women and 25 HIV-infected nonpregnant women provided lumefantrine pharmacokinetic results. The characteristics of all participants are presented in Table 1.
TABLE 1.
Basic characteristics of study participants
| Characteristic | Value(s) fora: |
|
|---|---|---|
| Pregnant women (n = 35) | Nonpregnant women (n = 34) | |
| Age (yr) | 30.0 (5.4) | 35.6 (5.5) |
| Ht (m) | 1.62 (0.10) | 1.59 (0.07) |
| Wt (kg) | 61.8 (11.5) | 57.3 (11.1) |
| BMIc (kg m−2) | 23.7 (4.0) | 22.7 (3.5) |
| No. of women in the following stage of pregnancy: | ||
| Second trimester | 7 | NAb |
| Third trimester | 28 | NA |
| Gestational age (wk) | 27.7 (6.1) | NA |
| No. of women with the following malaria diagnosis: | ||
| Symptomatic | 15 | 21 |
| Asymptomatic | 20 | 13 |
| Hematocrit (%) | 32.2 (3.3) | 33.2 (4) |
| Hemoglobin concn (g/dl) | 10.4 (1.3) | 10.6 (1.3) |
| Platelet count (no. of cells/liter) | 215.9 (74.1) | 222.8 (85.38) |
| CD4 count (no. of cells/μl) | 469.4 (210.5) | 406 (308.5) |
Values are expressed as the mean (standard deviation). All women were treated with efavirenz at 600 mg, tenofovir at 300 mg, and lamivudine at 300 mg once daily.
NA, not applicable.
BMI, body mass index.
Influence of pregnancy on efavirenz and lumefantrine interaction.
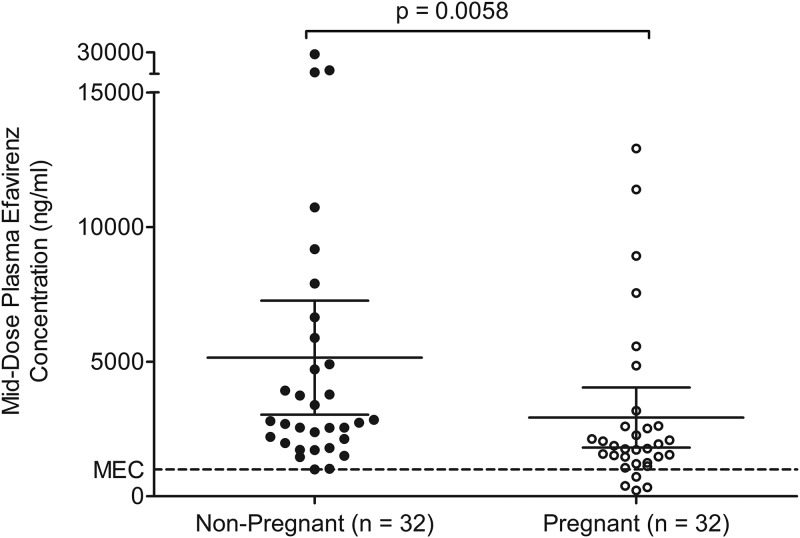
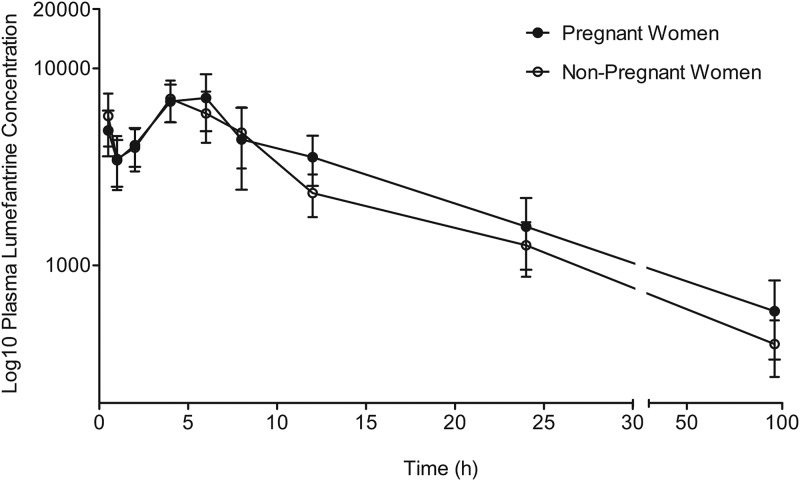
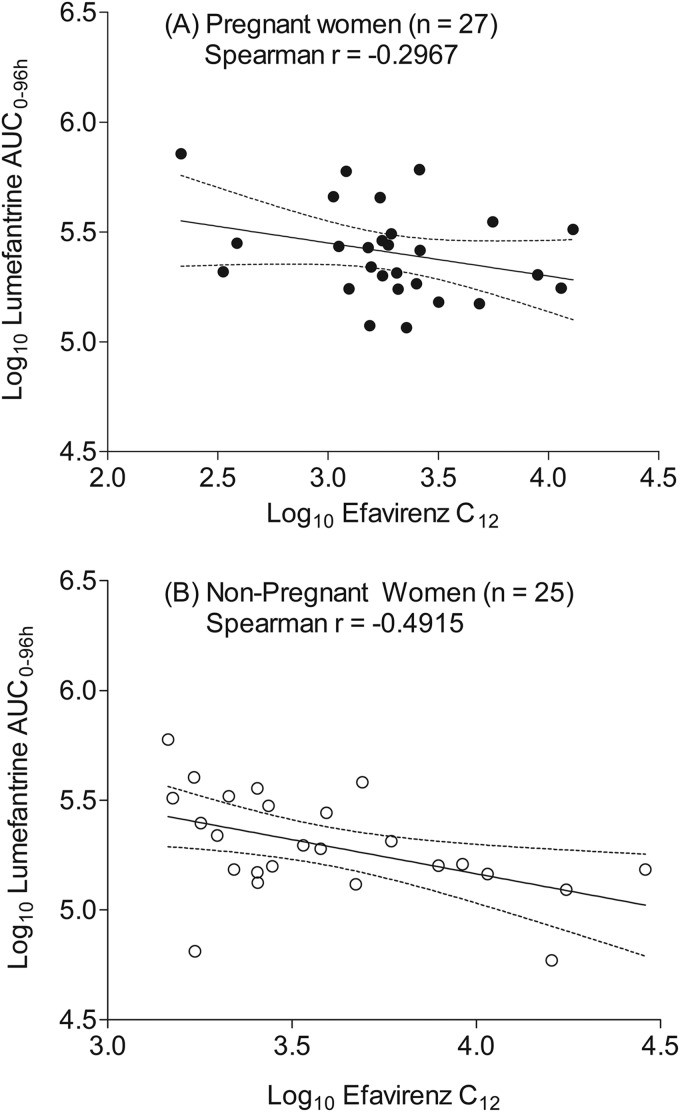
The median middose (at 12 h postdose) plasma efavirenz concentration was significantly lower in pregnant women at 1,820 ng/ml (interquartile range [IQR], 1,300 to 2,610 ng/ml) than in nonpregnant women at 2,760 (IQR, 2,020 to 5,640 ng/ml) by the Mann-Whitney U test (P = 0.006) (Fig. 2). The lumefantrine concentration-time profiles for the pregnant women and those who were nonpregnant are presented in Fig. 3, while the area under the concentration-time curve (AUC) and the other lumefantrine pharmacokinetic parameters generated are presented in Table 2. A moderate negative correlation between efavirenz and lumefantrine plasma exposure was observed, with the Spearman correlation coefficient (r) being −0.4021 (95% confidence interval [CI], −0.6082 to −0.1642) when data from pregnant and nonpregnant women were combined (n = 52) and the P value being 0.002 (Fig. 4).
FIG 2.
Middose (12 h postdose) plasma efavirenz concentrations in pregnant versus nonpregnant women. Dotted lines show the recommended 1,000-ng/ml minimum effective concentration (MEC) of efavirenz. Data are presented as the mean (95% confidence interval).
FIG 3.
Plasma concentration-time profile of lumefantrine following oral coadministration of artemether-lumefantrine with efavirenz-based antiretroviral therapy in pregnant women and nonpregnant women. Data are presented as the mean (95% confidence interval).
TABLE 2.
Lumefantrine and desbutyl-lumefantrine pharmacokinetics in pregnant compared with nonpregnant HIV-positive subjects with ongoing efavirenz medication
| Drug and pharmacokinetic parameterb | Values fora: |
% difference | GMR (95% CI) | P valuec | |
|---|---|---|---|---|---|
| Pregnant women (n = 27) | Nonpregnant women (n = 25) | ||||
| Lumefantrine | |||||
| Cmax (ng/ml) | 6,750 (4,820–9,620) | 7,860 (4,450–9,400) | −14.07 | 1.03 (1.01–1.05) | 0.89 |
| C12 (ng/ml) | 2,870 (2,180–4,880) | 2,080 (1,190–2,970) | 37.87 | 1.75 (1.7–1.80) | 0.017 |
| C7 (ng/ml) | 279 (120–610) | 212 (133–400) | 31.64 | 1.16 (1.15–1.16) | 0.66 |
| CL (h) | 2.66 (1.73–4.32) | 4.23 (2.71–6.6) | −37.12 | 0.07 | |
| AUC0–96 (ng · h/ml) | 155,832 (102,400–214,011) | 90,594 (58,869–149,775) | 72.01 | 1.44 (1.41–1.46) | 0.03 |
| AUCt (ng · h/ml) | 191,441 (111,190–277,538) | 112,086 (70,865–177,053) | 70.80 | 1.62 (1.59–1.70) | 0.02 |
| Desbutyl-lumefantrine | |||||
| Cmax (ng/ml) | 162 (141–238) | 154 (92–207) | 5.56 | 0.33 | |
| C7 (ng/ml) | 13.2 (6.6–28.5) | 15.92 (7.99–24.1) | −17.21 | 0.42 | |
| AUC0–96 (ng · h/ml) | 3,824 (1,383–5,084) | 2,780 (1,360–5,214) | 37.53 | 0.79 | |
| MRd | 0.021 (0.013–0.034) | 0.036 (0.016–0.049) | −41.67 | 0.08 | |
Values are presented as the median (interquartile range). The values of the pharmacokinetic parameters were determined following the 6th dose of artemether-lumefantrine. Significant differences are in boldface.
Cmax, maximum plasma concentration; C12, plasma concentration at 12 h postdose; CL, clearance; AUC0–96, area under the concentration-time curve from 0 to 96 h; AUCt, area under the concentration-time curve at time t; MR, metabolic ratio, which is calculated from the ratio of the AUC for desbutyl-lumefantrine to the AUC for lumefantrine.
P values were determined using the Mann-Whitney U test.
FIG 4.
Correlations between plasma lumefantrine expose (log AUC0–96) and middose plasma efavirenz concentration (log10 C12) in pregnant women (A) and nonpregnant women (B). The solid and dotted lines represent the mean and 95% confidence intervals, respectively.
The AUC from 0 to 96 h (AUC0–96), the AUC from 0 h to infinity (AUC0–∞), and the concentration at 12 h after the last dose of lumefantrine (C12) were statistically significantly higher in the pregnant group than in the nonpregnant group. The lumefantrine AUC was 72% higher in the pregnant group (P = 0.03) than in the nonpregnant group (Table 2). The median value for C12 after the last dose on day 3 and the day 7 concentrations were higher in women who were pregnant than in those who were not pregnant. The median plasma concentration was 2,870 ng/ml (IQR, 2,180 to 4,880 ng/ml) in the pregnant women, whereas it was 2,080 ng/ml (IQR, 1,190 to 2,970 ng/ml) in the nonpregnant women (Mann-Whitney U test, P = 0.0170). On day 7, the median plasma concentration in pregnant women was 279.15 ng/ml (IQR, 119.9 to 605.5 ng/ml), whereas that in nonpregnant women was 212.05 ng/ml (IQR, 133.4 to 404.1 ng/ml) (P = 0.53). The metabolic ratio was higher in the nonpregnant group than in the pregnant group (0.036 versus 0.024).
DISCUSSION
This study demonstrates changes in lumefantrine pharmacokinetics during coadministration with efavirenz in pregnant women. The presented data indicate that the pharmacokinetic parameters of lumefantrine (AUC0–96 and the plasma concentrations at 12 h postdose) were higher in the pregnant group than in the nonpregnant group. Previous studies on artemether and efavirenz drug-drug interactions conducted among healthy volunteers or people living with HIV have already shown interactions which often result in significant reductions in the plasma exposure of lumefantrine, artemether, and dihydroartemisinin (7). In the present study, lumefantrine exposure was also inversely correlated with the efavirenz plasma concentrations in both the pregnant and the nonpregnant groups. The correlation plots obtained from the log efavirenz concentrations versus the log lumefantrine plasma exposures suggest that the reduction in lumefantrine plasma exposure is directly related to efavirenz exposure in the two study groups. However, the effect was more in those who were not pregnant than in those who were pregnant. This observation aligns with previously published data, where a high plasma concentration of efavirenz was associated with a lower day 7 lumefantrine plasma concentration (20). The day 7 lumefantrine plasma concentration is considered a surrogate of lumefantrine efficacy and posttreatment prophylaxis (particularly the day 28 recurrence of parasitemia) (20).
Both lumefantrine and efavirenz undergo CYP3A4 metabolism, and induction of this pathway by efavirenz may lead to a reduction in lumefantrine plasma exposure, which may lead to an increase in the risk of lumefantrine treatment failure. This drug-drug interaction therefore warrants further investigation, particularly given that HIV infection increases the risk of malaria infection and clinical malaria in adults, especially in pregnant women and in those with advanced immunosuppression (21). HIV-infected adults are also at increased risk of complicated and severe malaria and death in settings with unstable malaria transmission (3). This study and other similar studies indicate that efavirenz-based ART may inversely influence the malaria treatment outcome. This clearly requires further study and may warrant dose adjustment of the ACT to ensure adequate lumefantrine plasma exposure and adequate posttreatment prophylaxis in malaria-HIV-coinfected patients.
Both CYP3A4 and CYP2B6 are affected by pregnancy-related physiological changes, such as an increase in estradiol concentrations. The upregulation of CYP2B6 and CYP3A4 often results in enhanced metabolic function, leading to a reduction in the plasma exposure of their substrates (22, 23). The data presented here show that pregnancy is associated with reduced efavirenz plasma concentrations, in line with previous observations (19, 24). Since the efavirenz-mediated induction of CYP3A4 and CYP2B6 is concentration dependent, low efavirenz concentrations may be associated with a reduction in the degree of induction, as shown previously for levonorgestrel implants (25). This is also evident in the data presented in this study, as a significant increase in the lumefantrine plasma exposure during pregnancy was noted. Thus, the suggested dose adjustment for ACTs following coadministration with efavirenz-based ART in a cohort of nonpregnant women (26) may need further refinement during pregnancy. Efavirenz might have induced CYP3A4, while pregnancy further upregulated the isoform, which may have further reduced the plasma exposure of lumefantrine. However, it is noted that pregnancy also upregulates CYP2B6, a CYP isoform mainly responsible for the metabolism of efavirenz, resulting in a low plasma concentration of efavirenz during pregnancy. Thus, the degree of CYP3A4 induction depends on efavirenz plasma concentrations, and the lower efavirenz plasma concentrations reported during pregnancy might be associated with a decline in CYP3A4 induction. This might have led to the observed reduction in lumefantrine clearance (i.e., reduced metabolic ratio) among the women in the pregnant group compared to that among the women in the nonpregnant group.
At present, the WHO recommends a standard six doses of artemether-lumefantrine (80/480 mg) within 60 h as a first-line regimen for the treatment of uncomplicated malaria in adult populations, including pregnant women. Several studies have previously convincingly described that efavirenz interacts with the longer-acting components of ACTs (7, 26) in nonpregnant adult populations. Our findings demonstrate significant pregnancy-induced changes in lumefantrine pharmacokinetics. The most notable changes were both AUC0–96 (geometric mean ratio [GMR], 1.44; interquartile range, 1.41 to 1.46) and the plasma concentration at 12 h postdose on day 3 (GMR, 1.75; interquartile range, 1.70 to 1.80), while the differences seen in the other pharmacokinetic parameters, such as the lumefantrine concentration at day 7, clearance, the maximum plasma concentration (Cmax), and the time to the peak concentration (Tmax), were not significant. The lumefantrine AUC serves as a predictor of the therapeutic outcome of artemether-lumefantrine treatment, while day 7 and day 3 concentrations are surrogate makers (27). The lumefantrine plasma exposures in the pregnant and nonpregnant groups were not bioequivalent, considering the GMR of the lumefantrine AUC0–96 between the two groups. The estimated GMR was 1.44, or 144% (Mann-Whitney test, P = 0.031), which exceeded the U.S. Food and Drug Administration (FDA)-recommended bioequivalence range of 0.8 to 1.25 (28).
Further exploratory analysis showed that a large proportion of the study subjects recorded low plasma concentrations on day 7, below the 280-ng/ml cutoff previously considered for lumefantrine therapeutic efficacy (20). This finding is important, considering that a dose adjustment has been advised in populations where day 7 plasma concentrations of lumefantrine are substantially low (8, 29). A previous study also evaluated the predictive value of the lumefantrine day 3 concentration and reported that the day 3 concentration was a stronger predictor of a 28-day recurrence than the day 7 concentration (27). Again, considering logistic feasibility, sampling on day 3 may be preferable to sampling on day 7, as many study participants are usually lost to follow-up when the pharmacokinetic sampling period is prolonged (27, 30). Therefore, the lumefantrine C12 obtained on day 3 was considered in the present study. In comparing the pregnant group with the nonpregnant group, this parameter differed significantly (median C12, 2,870 ng/ml [IQR, 2,180 to 4,880 ng/ml] versus 2,080 ng/ml [1,190 to 2,970 ng/ml]), with a Mann-Whitney U test P value of 0.017. Moreover, the data in this study align with the data presented by Tchaparian et al. (27), where a median day 3 concentration of 2,777 ng/ml in a Ugandan cohort of 105 children was reported (27). In a setting where artemether-lumefantrine is administered without efavirenz-based ART, gestation-induced physiological changes often determine the overall differences in the pharmacokinetics of lumefantrine during pregnancy. For instance, the lumefantrine AUC and day 7 concentration values were significantly lower in pregnant women than in nonpregnant patient populations with uncomplicated malaria, leading to high failure rates among the pregnant women in some studies (31–34).
In conclusion, our study showed low plasma concentrations of efavirenz during pregnancy and an inverse correlation between the efavirenz plasma exposure and the lumefantrine plasma exposure. Overall, pregnancy was associated with a higher lumefantrine AUC in the context of the efavirenz-lumefantrine drug-drug interaction. However, this value was significantly lower (an approximately 40% reduction) than the AUC of lumefantrine in those malaria patients who were not pregnant and HIV treatment naive (14). Thus, further adjustment to artemether-lumefantrine dosing to achieve a higher exposure may be warranted in pregnant women receiving efavirenz-based ART. The data presented further highlight the need for drug-drug interaction studies with newer anti-HIV drugs and other drugs commonly considered for use in the pregnant population.
MATERIALS AND METHODS
Study design and participants.
This was a two-period, single-sequence crossover study conducted in two stages. This was a two-arm, observational study to assess the influence of pregnancy on the pharmacokinetics of lumefantrine in ongoing efavirenz (600 mg)-based ART. The participants were eligible if they met the following inclusion criteria: they provided written informed consent, they were 18 to 45 years old, they were HIV infected and had been receiving an efavirenz-based regimen for at least 2 weeks, and they were diagnosed with uncomplicated malaria and prescribed artemether-lumefantrine. Patients who were receiving other drugs or products with a known or suspected interaction with the study drugs (e.g., antituberculosis drugs, caffeine, grapefruit juice) and who had a history of renal or hepatic diseases, severe illness, a hemoglobin concentration of less than 8 mg/dl, and a record of QT interval prolongation or symptomatic cardiac dysrhythmia and electrolyte disturbances were excluded.
Ethics and patient recruitment.
Ethics approvals were obtained from the Health Research and Ethics Committees of the Obafemi Awolowo University Teaching Hospital, Ile Ife, Nigeria (NHREC/27/02/2009a), and the Ladoke Akintola University of Technology Teaching Hospital (LTH), Osogbo, Nigeria (LTH/REC/2017/03/291). A material transfer agreement between the principal investigator and the host supervisor at the University of Liverpool was completed and was approved by the National Health Research and Ethics Committee, Abuja, Nigeria (NHREC number NHREC/01/01/2007). Patients were required to sign a written informed consent before enrollment. Between September 2016 and April 2017, eligible patients were recruited from four hospitals: LTH, Osogbo, Nigeria; the State Specialist Hospital, Ilesa, Nigeria; the State Specialist Hospital, Iwo, Nigeria; and the State Specialist Hospital, Asubiaro, Nigeria. Relevant information on the medical, gestational, and social history of the patients was abstracted from hospital records. The laboratory tests for the following were conducted prior to enrollment: malaria parasite diagnosis, CD4 count, packed cell volume, hemoglobin concentration, and platelet count. Demographic features, such as weight, age, height, and tribe, were also recorded.
Drug administration and pharmacokinetic sampling.
All participants had received 600 mg efavirenz-based ART once daily for a minimum of 2 weeks and had been prescribed a 3-day course of artemether-lumefantrine at 80/480 mg every 12 h for malaria treatment by their primary physician before study enrollment. On the first day of the study, patients received detailed information about the dosage regimen of artemether-lumefantrine. To enhance its bioavailability, patients were instructed to take artemether-lumefantrine along with 200 ml of milk provided from 20 g of an instant milk powder-filled sachet, each of which contained approximately 6 g fat, and a 200-ml plastic cup was provided for reconstitution. The last dose of artemether-lumefantrine was scheduled to be taken under supervision at the study site 60 h after the first dose. All enrollees were reminded of their artemether-lumefantrine dosage schedules by a phone call.
Patients were admitted for pharmacokinetic sampling prior to the time of taking the last dose of artemether-lumefantrine. Blood samples of about 2 ml were drawn into EDTA tubes at 0.5, 1, 2, 4, 6, 8, 12, 24, and 96 h after the last dose of artemether-lumefantrine. The samples were centrifuged at 3,000 × g for 10 min to separate the plasma, which was transferred into cryotubes and stored in a liquid nitrogen Dewar flask that was refilled fortnightly throughout the study period until it was shipped on dry ice to the HIV Pharmacology Research Laboratory, Institute of Translational Medicine, University of Liverpool, where the samples were stored at −80°C until bioanalysis.
Drug quantification and pharmacokinetic analysis.
The plasma concentrations of lumefantrine were determined using a previously validated high-pressure liquid chromatography (HPLC) assay with UV detection (35). A validated HPLC assay with UV detection method previously developed in-house to measure efavirenz in transport medium was adopted (25) and partially validated for efavirenz in plasma per the FDA recommendation for bioanalytical method validation (36). The lower limits of quantification for lumefantrine and efavirenz were 50 and 187.25 ng/ml, respectively. The desbutyl-lumefantrine plasma concentration was determined using a previously validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (37). Plasma concentration-time data were analyzed using noncompartmental analysis in Kinetica (version 4.1) pharmacokinetic software (InnaPhase Corporation, Philadelphia, PA) to obtain pharmacokinetic parameters, including the maximum plasma concentration (Cmax), the time to the peak concentration (Tmax), half-life (t1/2), the area under the concentration-time curve from 0 to 96 h (AUC0–96 h), and clearance (CL), together with the metabolic ratio, which was calculated from the ratio of the AUC0–96 for lumefantrine to the AUC0–96 for desbutyl-lumefantrine.
Statistical analysis.
The Excel program in Office software (2016; Microsoft Corporation, Redmond, WA) was used for data entry. The Kolmogorov-Smirnov normality test was used to check if the data conformed with a Gaussian distribution. The Mann-Whitney U test was conducted to test for the significance of the differences between the mean values from the two study groups using a P value of <0.05, while the 90% confidence interval for the geometric mean ratio (GMR) was used to test for clinically significant differences in pharmacokinetic parameters between the pregnant and the nonpregnant groups per U.S. Food and Drug Administration (FDA) guidelines on the investigation of bioequivalence (28). All figures were prepared using GraphPad Prism (version 5) software (GraphPad Software, La Jolla, CA).
ACKNOWLEDGMENTS
We appreciate the study participants and the members of the staff at the various clinics who assisted during patient recruitment. We are grateful for the support and training received from the Infection Pharmacology Group at the University of Liverpool.
We acknowledge TETFUND Nigeria for providing funds for this project.
REFERENCES
- 1.Takem EN, D'Alessandro U. 2013. Malaria in pregnancy. Mediter J Hematol Infect Dis 5:e2013010. doi: 10.4084/mjhid.2013.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2015. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.World Health Organization. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 4.Tarning J. 2016. Treatment of malaria in pregnancy. N Engl J Med 374:981–982. doi: 10.1056/NEJMe1601193. [DOI] [PubMed] [Google Scholar]
- 5.Pekyi D, Ampromfi AA, Tinto H, Traoré-Coulibaly M, Tahita MC, Valéa I, Mwapasa V, Kalilani-Phiri L, Kalanda G, Madanitsa M, Ravinetto R, Mutabingwa T, Gbekor P, Tagbor H, Antwi G, Menten J, De Crop M, Claeys Y, Schurmans C, Van Overmeir C, Thriemer K, Van Geertruyden JP, D'Alessandro U, Nambozi M, Mulenga M, Hachizovu S, Kabuya JB, Mulenga J. 2016. Four artemisinin-based treatments in African pregnant women with malaria. N Engl J Med 374:913–927. doi: 10.1056/NEJMoa1508606. [DOI] [PubMed] [Google Scholar]
- 6.Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. 2010. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos 38:1218–1229. doi: 10.1124/dmd.109.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Namakula R, Mayanja-Kizza H, Katabira E, Ntale M, Pakker N, Ryan M, Hanpithakpong W, Tarning J, Lindegardh N, de Vries PJ, Khoo S, Back D, Merry C. 2012. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother 67:2213–2221. doi: 10.1093/jac/dks207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoglund RM, Byakika-Kibwika P, Lamorde M, Merry C, Ashton M, Hanpithakpong W, Day NPJ, White NJ, Äbelö A, Tarning J. 2014. Artemether-lumefantrine co-administration with antiretrovirals: population pharmacokinetics and dosing implications. Br J Clin Pharmacol 79:636–649. doi: 10.1111/bcp.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kredo T, Mauff K, Workman L, Van der Walt JS, Wiesner L, Smith PJ, Maartens G, Cohen K, Barnes KI. 2016. The interaction between artemether-lumefantrine and lopinavir/ritonavir-based antiretroviral therapy in HIV-1 infected patients. BMC Infect Dis 16:30. doi: 10.1186/s12879-016-1345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh S, Kajubi R, Huang L, Ssebuliba J, Kiconco S, Gao Q, Li F, Were M, Kakuru A, Achan J, Mwebaza N, Aweeka FT. 2016. Antiretroviral choice for HIV impacts antimalarial exposure and treatment outcomes in Ugandan children. J Clin Infect Dis 63:214–222. doi: 10.1093/cid/ciw276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German PI, Aweeka FT. 2008. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet 47:91–102. doi: 10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ilett KF, Ethell BT, Maggs JL, Davis TME, Batty KT, Burchell BB, Tran Q, Thu LT, Anh H, Nguyen C, Pirmohamed M, Park BK, Edwards G. 2002. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos 30:1005–1012. doi: 10.1124/dmd.30.9.1005. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Ge S, Singh R, Basu S, Shatzer K, Zen M, Liu J, Tu Y, Zhang C, Wei J, Shi J, Zhu L, Liu Z, Wang Y, Gao S, Hu M. 2017. Glucuronidation: driving factors and their impact on glucuronide disposition. Drug Metab Rev 49:105–138. doi: 10.1080/03602532.2017.1293682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh S, Fehintola F, Huang L, Olson A, Adedeji WA, Darin KM, Morse GD, Murphy RL, Taiwo BO, Akinyinka OO, Adewole IF, Aweeka FT, Scarsi KK. 2015. Artemether-lumefantrine exposure in HIV-infected Nigerian subjects on nevirapine-containing antiretroviral therapy. Antimicrob Agents Chemother 59:7852–7856. doi: 10.1128/AAC.01153-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claessens AJ, Risler LJ, Eyal S, Shen DD, Easterling TR, Hebert MF. 2010. CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos 38:1393–1396. doi: 10.1124/dmd.110.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwinyi J, Cavaco I, Pedersen RS, Persson A, Burkhardt S, Mkrtchian S, Ingelman-Sundberg M. 2010. Regulation of CYP2C19 expression by estrogen receptor α: implications for oestrogen-dependent inhibition of drug metabolism. Mol Pharmacol 78:886–894. doi: 10.1124/mol.110.065540. [DOI] [PubMed] [Google Scholar]
- 17.Neary ML, Lamorde M, Olagunju A, Darin KM, Merry C, Byakika-Kibwika P, Back DJ, Siccardi M, Owen A, Scarsi KK. 2017. The effect of gene variants on levonorgestrel pharmacokinetics when combined with antiretroviral therapy containing efavirenz or nevirapine. J Clin Pharmacol Ther 102:529–536. doi: 10.1002/cpt.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olagunju AE, Bolaji OO, Amara A, Else L, Okafor O, Adejuyigbe E, Oyigboja J, Back D, Khoo S, Owen A. 2015. Pharmacogenetics of pregnancy-induced changes in efavirenz pharmacokinetics. J Clin Pharmacol Ther 97:298–306. doi: 10.1002/cpt.43. [DOI] [PubMed] [Google Scholar]
- 19.Dooley KE, Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, Haas DW, Hull J, Msandiwa R, Castel S. 2015. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis 211:197–205. doi: 10.1093/infdis/jiu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maganda BA, Minzi OMS, Ngaimisi E, Kamuhabwa AAR, Aklillu E. 2015. CYP2B6*6 genotype and high efavirenz plasma concentration but not nevirapine are associated with low lumefantrine plasma exposure and poor treatment response in HIV-malaria-coinfected patients. Pharmacogenomics J 16:88–95. doi: 10.1038/tpj.2015.37. [DOI] [PubMed] [Google Scholar]
- 21.Flateau C, Le Loup G, Pialoux G. 2011. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis 11:541–556. doi: 10.1016/S1473-3099(11)70031-7. [DOI] [PubMed] [Google Scholar]
- 22.Costantine MM. 2014. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 5:65. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickmann LJ, Isoherranen N. 2013. Quantitative prediction of CYP2B6 induction by estradiol during pregnancy: potential explanation for increased methadone clearance during pregnancy. Drug Metab Dispos 41:270–274. doi: 10.1124/dmd.112.047118. [DOI] [PubMed] [Google Scholar]
- 24.Cressey TR, Stek A, Capparelli E, Bowonwatanuwong C, Prommas S, Sirivatanapa P, Yuthavisuthi P, Neungton C, Huo Y, Smith E, Best BM, Mirochnick M, IMPAACT P1026s Team. 2012. Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum. J Acquir Immune Defic Syndr 59:245–252. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarsi KK, Darin KM, Nakalema S, Back DJ, Byakika-Kibwika P, Else LJ, Dilly-Penchala S, Buzibye A, Cohn SE, Merry C, Lamorde M. 2016. Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz-based antiretroviral therapy: a three-arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis 62:675–682. doi: 10.1093/cid/civ1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siccardi M, Olagunju A, Seden K, Ebrahimjee F, Rannard S, Back D, Owen A. 2013. Use of a physiologically-based pharmacokinetic model to simulate artemether dose adjustment for overcoming the drug-drug interaction with efavirenz. In Silico Pharmacol 1:4. doi: 10.1186/2193-9616-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchaparian E, Sambol NC, Arinaitwe E, McCormack SA, Bigira V, Wanzira H, Muhindo M, Creek DJ, Sukumar N, Blessborn D, Tappero JW, Kakuru A, Bergqvist Y, Aweeka FT, Parikh S. 2016. Population pharmacokinetics and pharmacodynamics of lumefantrine in young Ugandan children treated with artemether-lumefantrine for uncomplicated malaria. J Infect Dis 214:1243–1251. doi: 10.1093/infdis/jiw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Center for Drug Evaluation and Research. 2014. Guidance for industry bioavailability and bioequivalence studies submitted in NDAs or INDs—general considerations. U.S. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 29.Mutagonda RF, Kamuhawa AAR, Minzi OMS, Massawe SN, Asghar M, Homann MV, Franert A, Aklilu E. 2017. Effect of pharmacogenetics on plasma lumefantrine pharmacokinetics and malaria treatment outcome in pregnant women. Malar J 16:1–10. doi: 10.1186/s12936-016-1650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White NJ, Stepniewska K, Barnes K, Price RN, Simpson J. 2008. Simplified antimalarial therapeutic monitoring: using the day-7 drug level? Trends Parasitol 24:159–164. doi: 10.1016/j.pt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 31.McGready R, Tan SO, Ashley EA, Pimanpanarak M, Viladpai-Nguen J, Phaiphun L, Wüstefeld K, Barends M, Laochan N, Keereecharoen L, Lindegardh N, Singhasivanon P, White NJ, Nosten F. 2008. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancy. PLoS Med 5:e253. doi: 10.1371/journal.pmed.0050253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarning J, Kloprogge F, Dhorda M, Jullien V, Nosten F, White NJ, Guerin PJ, Piola P. 2013. Pharmacokinetic properties of artemether, dihydroartemisinin, lumefantrine, and quinine in pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. Antimicrob Agents Chemother 57:5096–5103. doi: 10.1128/AAC.00683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloprogge F, Piola P, Dhorda M, Muwanga S, Turyakira E, Apinan S, Lindegardh N, Nosten F, Day NP, White NJ, Guerin PJ, Tarning J. 2013. Population pharmacokinetics of lumefantrine in pregnant and nonpregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. CPT Pharmacometrics Syst Pharmacol 2:E83. doi: 10.1038/psp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piola P, Nabasumba C, Turyakira E, Dhorda M, Lindegardh N, Nyehangane D, Snounou G, Ashley EA, McGready R, Nosten F, Guerin P. 2010. Efficacy and safety of artemether-lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial. Lancet Infect Dis 10:762–769. doi: 10.1016/S1473-3099(10)70202-4. [DOI] [PubMed] [Google Scholar]
- 35.Khuda F, Iqbal Z, Shaz Y, Ahmmad L, Nasir F, Zada A, Amanullah K, Shahbaz N. 2014. Method development and validation for simultaneous determination of lumefantrine and its major metabolite, desbutyl lumefantrine in human plasma using RP-HPLC/UV detection. J Chromatogr B Analyt Technol Biomed Life Sci 944:114–122. doi: 10.1016/j.jchromb.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 36.Center for Drug Evaluation and Research. 2018. Bioanalytical method validation guidance for industry. U.S. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 37.Huang L, Li X, Marzan F, Lizak PS, Aweeka FT. 2012. Determination of lumefantrine in small-volume human plasma by LC-MS/MS: using a deuterated lumefantrine to overcome matrix effect and ionization saturation. Bioanalysis 4:157–166. doi: 10.4155/bio.11.303. [DOI] [PMC free article] [PubMed] [Google Scholar]