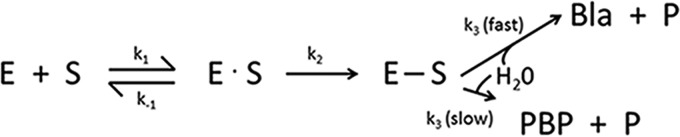β-Lactamases, the major resistance determinant for β-lactam antibiotics in Gram-negative bacteria, are ancient enzymes whose origins can be traced back millions of years ago. These well-studied enzymes, currently numbering almost 2,800 unique proteins, initially emerged from environmental sources, most likely to protect a producing bacterium from attack by naturally occurring β-lactams.
KEYWORDS: ESBL, MBL, β-lactam, β-lactamase, carbapenemase, cephalosporinase, penicillinase
ABSTRACT
β-Lactamases, the major resistance determinant for β-lactam antibiotics in Gram-negative bacteria, are ancient enzymes whose origins can be traced back millions of years ago. These well-studied enzymes, currently numbering almost 2,800 unique proteins, initially emerged from environmental sources, most likely to protect a producing bacterium from attack by naturally occurring β-lactams. Their ancestors were presumably penicillin-binding proteins that share sequence homology with β-lactamases possessing an active-site serine. Metallo-β-lactamases also exist, with one or two catalytically functional zinc ions. Although penicillinases in Gram-positive bacteria were reported shortly after penicillin was introduced clinically, transmissible β-lactamases that could hydrolyze recently approved cephalosporins, monobactams, and carbapenems later became important in Gram-negative pathogens. Nomenclature is based on one of two major systems. Originally, functional classifications were used, based on substrate and inhibitor profiles. A later scheme classifies β-lactamases according to amino acid sequences, resulting in class A, B, C, and D enzymes. A more recent nomenclature combines the molecular and biochemical classifications into 17 functional groups that describe most β-lactamases. Some of the most problematic enzymes in the clinical community include extended-spectrum β-lactamases (ESBLs) and the serine and metallo-carbapenemases, all of which are at least partially addressed with new β-lactamase inhibitor combinations. New enzyme variants continue to be described, partly because of the ease of obtaining sequence data from whole-genome sequencing studies. Often, these new enzymes are devoid of any phenotypic descriptions, making it more difficult for clinicians and antibiotic researchers to address new challenges that may be posed by unusual β-lactamases.
INTRODUCTION
One of the most studied enzyme families is the group of enzymes known as β-lactamases, with more than 28,900 citations in Medline (https://www.ncbi.nlm.nih.gov/pubmed/). These enzymes, whose most obvious role is to inactivate β-lactam antibiotics, have driven research in academic laboratories since the early 1940s (1, 2). Perhaps more importantly, they have been responsible for scores of pharmaceutical research programs that have attempted to find ways to protect effective antibiotics from destruction. β-Lactamases provide intellectual challenges for academic investigators with their deceptively simple mechanisms of action, as enzymes whose hydrolysis activity approaches that of a “fully efficient enzyme” with diffusion-limited reaction rates (3). Attempts to provide economically viable anti-infective agents that can circumvent the action of these enzymes have yielded substantial economic rewards to successful pharmaceutical companies. As a result, the safe and effective β-lactam antibiotics have become one of the most widely prescribed classes of antibacterial agents (4). Many review articles have been published about various aspects of these intriguing enzymes (5–11), but they have tended to focus on narrowly targeted sets of β-lactamases. In this minireview, a short history of the β-lactamases is presented, emphasizing key points that have driven both academic and pharmaceutical science over the past 75 years. High points in their history will be discussed, based on the “Table of Firsts” shown as Table 1. This history is not meant to be a comprehensive review of all the current literature on β-lactamases but is meant to tell a story about where these enzymes came from, how they have driven antibiotic discovery programs, and what challenges they pose for today.
TABLE 1.
Table of Firsts: the dates, organisms, and locations of the first of a series of β-lactamase-producing isolates with long-term clinical significance
| Original β-lactamase name (currently recognized name) | Yr of first verified isolation | Organism | Location | First description in literature | Reference(s) |
|---|---|---|---|---|---|
| Penicillinase (chromosomal AmpC) | 1940 | Bacillus coli (Escherichia coli) | England | 1940 | 1 |
| Penicillinase | 1942 | Staphylococcus aureus | England | 1942 | 65 |
| OXA | 1962 | Salmonella enterica serovar Typhimurium, Escherichia colia | England | 1965 | 87, 215 |
| 1967 | |||||
| TEM-1 | 1963 | Escherichia coli | Greece | 1965 | 85 |
| SHV-1 | 1972 | Klebsiella pneumoniae | Unknown | 1972 | 216 |
| Transferable ESBL (SHV-2) | Pre-1983 | K. pneumoniae | Germany | 1983 | 217 |
| Serine (class A, group 2f) carbapenemase (SME-1) | 1982 | Serratia marcescens | England (London) USA (Minnesota) | 1990 | 148, 150 |
| 1985 | 1986 | ||||
| Plasmid-encoded AmpC (MIR-1) | 1988 | K. pneumoniae | USA (Massachusetts) | 1990 | 141 |
| Plasmid-encoded MBL (IMP-1) | 1988 | Pseudomonas aeruginosa | Japan | 1991 | 151 |
| Inhibitor-resistant TEM (TEM-30) | 1991 | E. coli | France (Paris) | 1994 | 118 |
| KPC-type (KPC-2) | 1996 | K. pneumoniae | USA (North Carolina) | 2000 | 158 |
| NDM-1 | 2006 | K. pneumoniae | India (New Delhi) | 2009 | 175, 176 |
TOPLINE VIEW OF β-LACTAMASES
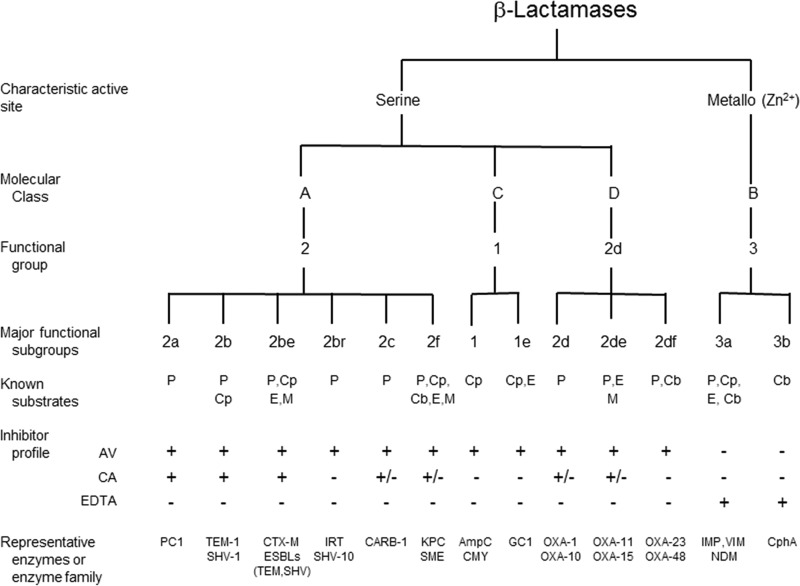
β-Lactamases are versatile enzymes with a limited range of molecular structures found in a diversity of bacterial sources. Their commonality is the ability to hydrolyze chemical compounds containing a β-lactam ring. As shown in Fig. 1, they have been classified biochemically into two broad divisions according to the mechanism by which they perform hydrolysis, either through the formation of an acyl enzyme with an active-site serine (Fig. 2) (12) or via a hydrolytic reaction facilitated by one or two essential zinc ions in the active sites of metallo-β-lactamases (MBLs) (13). After sequence analyses became available for key β-lactamases, four molecular classes, A, B, C, and D, were eventually assigned based on molecular size and homology between active-site amino acid motifs (14–16). However, biochemical differences between penicillinases and cephalosporinases had been recognized well before sequences were available (17), and known β-lactamases were distinguished based on functional capabilities related to substrate and inhibitor profiles (Table 2). Early classification schemes relied upon relative hydrolysis rates of penicillins and early cephalosporins, together with the enzymatic response to protein-modifying agents (18, 19). As additional substrates and inhibitors were introduced into clinical practice and gene sequencing became inexpensive and routine, both molecular and functional characteristics were combined into a more comprehensive classification scheme (20, 21). Today, at least 17 functional groups associated with the four molecular classes have been distinguished (20), with the major groups shown in Fig. 1. Enzymes are still differentiated with respect to the relative hydrolysis of the β-lactam substrates, penicillins, cephalosporins, carbapenems, and monobactams. Further differentiation is possible based on reactions with the class A β-lactamase inhibitor clavulanic acid (22), the broad-spectrum serine β-lactamase inhibitor avibactam (23), and the metal ion chelator EDTA to identify MBLs (24).
FIG 1.
Molecular and functional relationships among β-lactamases (adapted from references 20 and 201 with permission). AV, avibactam; CA, clavulanic acid; Cb, carbapenem; Cp, cephalosporin; E, expanded-spectrum cephalosporin; M, monobactam; P, penicillin.
FIG 2.
General reaction mechanism for binding of a β-lactam substrate (S) to a PBP (E) or a serine β-lactamase (E). Reversible formation of a Michaelis complex (E · S) which proceeds to a stable acyl enzyme (E—S) caused by reaction with the active-site serine. Hydrolysis occurs to form the microbiologically inactive ring-opened β-lactam (P) and either enzymatically active PBP (slow hydrolysis of acyl enzyme) or β-lactamase (Bla, high hydrolysis rate).
TABLE 2.
Characterization of β-lactamases based on information existing at the time of publication
| Yr | Basis for characterizationa | Conclusion(s) | Reference or source |
|---|---|---|---|
| 1968 | Substrate profile, reaction with antiserum | Three different groups of enzymes, typical cephalosporinases, cephalosporinase/penicillinase, and penicillinases (including those produced by R factors) | Sawai et al. (18) |
| 1970 | Substrate profile, inhibition by pCMB and cloxacillin, reaction with antiserum, electrical charge | Eight distinctive types of β-lactamases | Jack and Richmond (92) |
| 1973 | Substrate profiles, inhibition by cloxacillin and pCMB, electrophoretic mobility, molecular wt | Five classes of β-lactamases (I, II, III, IV, and V) | Richmond and Sykes (19) |
| 1976 | Substrate profiles, inhibition by cloxacillin and pCMB, isoelectric focusing, immunological relatedness, molecular wt; chromosomal or plasmid location considered | Five classes of β-lactamases (distinct from Richmond and Sykes) | Sykes and Matthew (218) |
| 1980 | Amino acid sequences from purified proteins | Molecular classes A and B defined based on four class A enzymes and one class B enzyme | Ambler (14) |
| 1981 | Amino acid sequence translated from ampC nucleotide sequence | Molecular class C defined | Jaurin and Grundström (15) |
| 1988 | Amino acid sequence translated from blaPSE-2 nucleotide sequence | Molecular class D defined | Huovinen et al. (16) |
| 1988 | Substrate profile, inhibition by clavulanic acid, aztreonam, and EDTA, molecular class designations for eight enzymes based on active site or full amino acid sequence data | Functional classification scheme with three groupings proposed for 28 enzymes | Bush (187) |
| 1989 | Substrate profile, inhibition by clavulanic acid, aztreonam, and EDTA, isoelectric point, molecular size, molecular class designations based on nucleotide sequences | Functional classification assigned to groups 1, 2 (six subgroups), 3, and 4 for 84 enzymes, aligned with molecular classes for 21 enzymes | Bush (219-221) |
| 1995 | Substrate profile, inhibition by clavulanic acid, sulbactam, tazobactam, aztreonam, cloxacillin, pCMB, and EDTA, molecular size, isoelectric point, molecular class designations based on nucleotide sequences | Functional classification for groups 1, 2 (eight subgroups), 3, and 4, for 217 enzymes, aligned with molecular classes for 118 enzymes | Bush et al. (21) |
| 1996–2015 | Amino acid sequences, initially for naturally occurring acquired TEM, SHV, and OXA ESBLs and IRTs; later expanded to all β-lactamase families | Curated numbering assignments for sequential β-lactamases in all major families | Lahey database (http://www.lahey.org/Studies/) |
| 2010 | Substrate profile, inhibition by clavulanic acid, tazobactam, or EDTA; molecular classification | Functional classification for groups 1 (2 subgroups), 2 (12 subgroups), 3 (2 subgroups), and 4 | Bush and Jacoby (20) |
| 2012 | Genomic (DNA) data | Genotypic data for Klebsiella spp.¸ including curation of nomenclature for OKP, LEN, and OXY β-lactamases | Institut Pasteur (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html) |
| 2013 | Nucleotide or amino acid sequences for multiple resistance determinants for all antibiotic classes | Curated collection of characterized molecular sequences and phenotypes to predict functionality associated with antibiotic resistance genes | Comprehensive Antibiotic Resistance Database (CARD; https://card.mcmaster.ca) (222, 223) |
| 2015 | DNA sequences in NCBI database that encode proteins conferring or contributing to resistance to β-lactam and other antibiotics | Annotated sequence records for representative DNA sequences in the NCBI database, including both naturally occurring and laboratory-derived β-lactamases | NCBI (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047) |
| 2016 | Commonalities of amino acid sequences of 285 class A β-lactamases | Separation of class A β-lactamases into two distinct subclasses, A1 and A2 | Philippon et al. (73) |
| 2017 | Amino acid sequences, biochemical data (kinetics of hydrolysis), X-ray crystallographic structures | Well-annotated compilation of structure-function relationships of β-lactamases from all classes, including laboratory mutants | BLDB (http://bldb.eu) (197) |
pCMB, p-chloromercuribenzoate.
β-LACTAMASE ORIGINS
The antibacterial effect of a β-lactam results in inhibition of the growth of replicating bacteria by acylating an active-site serine in essential penicillin-binding proteins (PBPs) (25, 26). During the terminal stages of cell wall biosynthesis, these enzymes are thus prevented from effecting the cross-linking of peptide chains to form peptidoglycan, resulting in cell death. Tipper and Strominger proposed similarities between the structures of penicillin and d-Ala-d-Ala, the terminal amino acids of the nascent acetylmuramyl-pentapeptide fragment, to explain the antimicrobial activity of penicillin (27). Because the majority of β-lactamases contain an active-site serine that also can be acylated by β-lactam molecules, it is not unexpected to find mechanistic and structural similarities between the two sets of enzymes (28, 29). Fisher and Mobashery have cogently outlined a history of studies demonstrating the biochemical commonality between the two acyl enzymes (30). Molecular modeling of various serine β-lactamases and PBP structures has demonstrated three-dimensional similarities with conserved folding patterns and preservation of topology at the active site, in spite of low amino acid identities (28, 31).
The general reaction mechanism for both enzymes is shown in Fig. 2. It is commonly assumed that PBPs were the precursors of the β-lactamases, with the k3 rate for deacylation increased dramatically for β-lactamases, compared to PBPs that exhibit a fast acylation step (k2) compared to a slow deacylation step. Formation of the PBP-acyl enzyme complex is considered by many to be an irreversible reaction compared to the lifetime of a bacterial cell, with half-lives of acyl enzymes ranging from a relatively short 9 to 15 min for Pseudomonas aeruginosa PBP2 and PBP3 with amdinocillin or aztreonam (32, 33) to a half-life of more than 24 h for nitrocefin with PBP2a from Staphylococcus aureus (34). Some PBPs may have evolved to function as weak β-lactamases with a slow turnover of the β-lactam substrate (28); for example, cefotaxime deacylation rates are 70- to 80-fold higher for PBP2x variants than that for the wild-type enzyme in Streptococcus pneumoniae (35).
β-Lactamases are ancient enzymes that existed even in the absence of the pressure of therapeutic antibiotics. In 1979, Hamilton-Miller claimed that “penicillinase was born on December 28, 1940” (36), the date Abraham and Chain reported that a bacterial enzyme was capable of destroying penicillin (1). Little did he know that modern phylogenetic analyses have estimated the age of serine β-lactamases to be more than 2 billion years old (37), with plasmid-encoded β-lactamases appearing millions of years ago (38). Recent explorations of remote environmental niches have identified functional β-lactamase-like activity in locations, such as a region of the Lechuguilla Cave in New Mexico (USA) estimated to have been isolated for over 4 million years (39). Sampling of the cave resulted in the growth of 93 bacterial strains, with as many as 62% capable of hydrolyzing β-lactams. However, no sequences of known β-lactamase sequences were identified. The same group also examined metagenomic DNA from 30,000-year-old permafrost sediments east of Dawson City, Yukon (Canada) (40). In these samples, deduced amino acid sequences with 53% to 84% identities to the ubiquitous TEM β-lactamases clustered into the following two enzyme groups: (i) β-lactamases from various streptomycetes, and (ii) uncharacterized β-lactamase-like hydrolytic proteins. In a slightly younger sample of cold-seep sediments of Edison seamount (Papua, New Guinea) estimated to be 10,000 years old, 30 clones produced genes encoding β-lactamases primarily belonging to enzymes in the TEM family (41). Additional metagenomic analyses of ancient and naive environmental samples identified a novel metallo-β-lactamase (MBL), isolated from a 14th century bone sample, with an unusual substrate profile unlike that of other known MBLs; its sensitivity to clavulanic acid and to carbapenems suggested that it may have emerged to provide resistance to currently unknown β-lactam-containing molecules (42). In addition to these β-lactamase-type enzymes in historical samples, β-lactamase production has been identified in multiple remote pristine Antarctic soil samples (43), in ice cores in glaciers outside Antarctica (44), and in remote South American populations that have rarely, or never, been treated with commercial β-lactam antibiotics (45, 46). Curiously, an IMP MBL, a somewhat infrequent contributor to β-lactam resistance in contemporary clinical isolates (47), was found in approximately half of the glacial samples but not from Greenland or Antarctica (44). β-Lactamases have also been identified in fungi, where they are suspected of serving to inactivate plant or microbial naturally occurring β-lactam compounds (48). However, there are still organisms with no confirmed β-lactamases, notably S. pneumoniae, Streptococcus pyogenes, Helicobacter pylori, Mycoplasma spp. and the Chlamydiae, possibly due to the production of homologous enzymes with the ability to inactivate β-lactams (49).
The history of β-lactamases can hardly be written without referring to the history of β-lactam-containing antibacterial agents, including the penicillins, cephalosporins, carbapenems, and monobactams, all of which contain a 4-membered 2-azetidinone ring (50). Although most of the β-lactam-containing agents in use today are synthetic or semisynthetic molecules, β-lactams as therapeutic agents originate from naturally occurring sources, identified initially because of their antibacterial activities in agar-based assays. Penicillin (penicillin G or benzylpenicillin) was first recognized because it prevented the growth of staphylococci on a petri dish contaminated with the common mold Penicillium notatum (51). Cephalosporin C, the starting point for the cephalosporins, was isolated from sewage sludge in Sardinia (52). Carbapenems were found in various β-lactam-producing actinomycetes from soil samples (53), and monobactams were isolated from bacteria in New Jersey soil and water samples (54). All these iterations of unmodified β-lactams existed in natural environments and placed selective pressure on the neighboring bacteria to evade a milieu of deleterious agents. When β-lactam-producing organisms compete with nonproducing bacteria in the same environmental niche, survival strategies are quickly developed. Because bacteria, possibly the first living organisms, have existed for 2.5 to 4 billion years (55, 56), there has been a long incubation period for naturally occurring β-lactam biosynthesis to be perfected and for neighboring bacteria to learn how to survive in the presence of these molecules. Interestingly, mammalian enzymes, including serum proteins (57) and renal dehydropeptidase (dipeptidase) (58), are also capable of hydrolyzing β-lactams.
β-LACTAMASES IN GRAM-POSITIVE BACTERIA
Resistance to β-lactams can be mediated by multiple mechanisms. Because it is in the best interest of the bacteria to maintain active PBPs, the first line of defense would appear to be for PBPs to alter their affinities for β-lactams while maintaining their physiological function (28). This approach has been utilized most effectively by Gram-positive bacteria, where a primary resistance mechanism has been acquisition of PBPs with decreased affinity for common β-lactams (59). However, it took over 20 years of penicillin usage before clinical isolates of penicillin-resistant S. pneumoniae (60, 61) and penicillin/methicillin-resistant S. aureus (MRSA) were reported as a result of production of low-affinity PBP variants (62–64). Instead, penicillinases, or β-lactamases that hydrolyze penicillin, emerged as the initial resistance mechanism in S. aureus to challenge the therapeutic use of this agent (65). The drug was used more extensively and promiscuously to treat nosocomial streptococcal infections previously associated with high mortality (66), but, as collateral damage, staphylococci initially fully susceptible to penicillin rapidly developed resistance as a result of penicillinase production. In a single English hospital, the percentage of penicillinase-producing staphylococci increased from 14% in 1946 to 59% in 1948 (67) and up to 80% in 1953 (66). More recently, a 2013 study of U.S. clinical isolates showed that 86.5% of all S. aureus isolates (75.6% of methicillin-susceptible isolates) contained the penicillinase gene blaZ (68), indicating that a subpopulation of methicillin-susceptible staphylococci has survived in the absence of either a low-affinity PBP or the production of penicillinase. A transferable penicillinase was also reported in a few clinical isolates of Enterococcus faecalis in the 1980s (69), with identical or closely homologous amino acid sequences as staphylococcal penicillinases (70). A genomic analysis in 2005 demonstrated at least two divergent origins (71). However, penicillinase-producing enterococci have not been observed for the past decade and appear to have disappeared from these pathogens (72). Another analysis of recent genomic data noted similarities among a set of 88 class A β-lactamases from Gram-positive bacteria and two enzymes from Gram-negative bacteria, the ROB-1 penicillinase from Haemophilus influenzae, and the ACl-1 enzyme from Acidaminococcus fermentans (73), possibly due to “trans-Gram transfer” of β-lactamase genes between Gram-positive and Gram-negative bacteria.
In addition to the observed penicillinase production in Gram-positive clinical isolates, enzymes with β-lactam-hydrolyzing properties from numerous environmental bacilli were used by theoretical enzymologists as prototypical biochemical examples of well-behaved enzymes (74, 75). Early studies with β-lactamases from Bacillus cereus (76), Bacillus anthracis (77), and Bacillus licheniformis (78) provided tools on which future biochemical studies of β-lactamases were based. Assay development, enzyme purification, induction properties, and identification of a metal-dependent β-lactamase from these bacilli were activities critical to the establishment of β-lactamases as important enzymological entities (75, 79, 80). It is notable that the classical structural classification of β-lactamases initially proposed by Ambler in 1980 was based on five known amino acid sequences, four of which were from enzymes originating in Gram-positive bacteria. These included three class A penicillinases, PC1 from S. aureus, penicillinase from B. licheniformis 749C, and the B. cereus 569/H β-lactamase I. The only class B metallo-β-lactamases (MBL) that had been sequenced and studied in any detail at the time was the zinc-containing B. cereus 569/H β-lactamase II (14). The first report of β-lactamases other than class A and B enzymes in Gram-positive bacilli described a set of oxacillin-hydrolyzing (class D) penicillinases from a set of environmental strains in 2016 (81).
β-LACTAMASES IN GRAM-NEGATIVE BACTERIA
1940 to 1985 and the emergence of modern β-lactamases.
In Gram-negative bacteria, β-lactamases have played a critical clinical role and have served as the primary resistance mechanism for the β-lactam antibiotics. The first enzyme with β-lactamase activity reported in the literature in 1940 was from Bacillus coli (1), now assumed to be the class C, AmpC chromosomal cephalosporinase from Escherichia coli (Table 1). As β-lactam resistance began to be more frequently recognized in Gram-negative pathogens, it was shown that many enteric bacteria and P. aeruginosa produced species-specific inducible chromosomal β-lactamases (82, 83). However, it is the mobile β-lactamases in Gram-negative bacteria that have created a more insidious threat to the β-lactams. Although plasmid-encoded penicillinases were first reported in staphylococci (84), the genes encoding these enzymes were not readily transferred to other species, other than for the few enterococcal strains that appeared in the 1980s carrying a staphylococcal penicillinase gene (69). In contrast, transferable genetic elements encoding a wide variety of β-lactamases became the most prevalent mechanism leading to the emergence of β-lactam resistance among Gram-negative bacteria, with few species barriers existing for their transmission. When β-lactamases on “R-factors” were first described in 1965 (85), only a limited number of these enzymes were identified, related either to the transferable penicillinase on RTEM (86), now known to be TEM-1, or to a transferable enzyme that hydrolyzed cloxacillin (87), an enzyme in the OXA family of β-lactamases. These mobile bla genes were soon disseminated among most enteric bacteria (88). We now understand that β-lactamase-encoding genes can be acquired horizontally by different means but mainly by plasmid acquisition. Gene mobilization mechanisms may include genetic elements, such as transposons, gene cassettes, integrons, and insertion sequences (89–91).
Sawai et al. in 1968 (18), Jack and Richmond in 1970 (92), and Richmond and Sykes in 1973 (19) attempted to group the known β-lactamases from Gram-negative rods in a meaningful way based on biochemical properties and functionality. Over time, these approaches have seen considerable refinement. As shown in Table 2, a limited number of different β-lactamases served as the starting point for a logical classification scheme based on biochemical characteristics.
The consensus was that the β-lactamases known by the mid-1970s constituted the range of β-lactam-hydrolyzing enzymes in relevant Gram-negative bacteria. The introduction of isoelectric focusing (IEF) (82, 93) aided by the ease of β-lactamase detection using nitrocefin as a colorimetric activity indicator (94) allowed investigators to analyze bacterial extracts for the presence of β-lactamase activity with minimal effort. Until the mid-1980s, it was possible to make a reasonable estimate of the number of β-lactamases produced per strain and the putative identity of an enzyme, based on isoelectric points from IEF gels. Biochemical properties were determined using purified enzymes; published molecular sizes, many of which were incorrect, were based on data obtained from gel exclusion chromatography, and amino acid composition was deduced from peptide analyses of purified proteins in a process that took months to obtain a single enzyme sequence. This was the environment in which the first definitions of molecular classes A, B, and C emerged (14, 15). Eventually, class D was added, based on nucleotide sequencing of the blaPSE-2 gene (16), and it represents the most diverse of the molecular classes.
During the late 1970s and early 1980s, a number of surveillance studies were conducted to assess β-lactamase production in Gram-negative bacteria. In a set of five studies published between 1979 and 1985, the distribution of β-lactamases from almost 1,800 ampicillin-resistant enteric bacteria were evaluated, based primarily on IEF profiles (95–99). The TEM-1 and TEM-2 penicillinases were the most common enzymes, with an average of 63% (range, 42 to 85%) of the isolates producing one of these enzymes. In these isolates, an enzyme designated SHV-1 was produced by an average of 9.9% of the isolates (range, 1.7 to 24.0%), primarily as a chromosomal enzyme in Klebsiella pneumoniae, with OXA enzymes produced on average at 7.8% (range, 0.4 to 15.4%). As was common in the early years of β-lactam resistance due to transferable β-lactamases, few isolates produced more than one plasmid-encoded enzyme, with multiple transferable β-lactamases generally reported in no more than 3% of the isolates. An exception was a set of Klebsiella isolates from a 1983 Spanish study in which 30% of the strains produced at least two plasmidic enzymes, primarily combinations of SHV-1 with TEM-1 or TEM-2 (97). This compilation set the stage for the future evolution of β-lactamases.
The facile transmission of the R-factor encoded “RTEM” (TEM-1) into a multiplicity of Gram-negative pathogens is associated with triggering one of the most productive periods of antibiotic development, especially for the β-lactams. In 1974, reports of ampicillin resistance in meningitis patients infected with Haemophilus influenzae led to the discovery that these strains produced an acquired TEM-type β-lactamase (100). At that time, ampicillin was the preferred treatment of sepsis or pediatric meningitis caused by H. influenzae type b, and the new TEM-producing strains were no longer responsive to therapeutic levels of ampicillin (101). This observation was followed quite soon by the alarming finding that the TEM β-lactamase was freely being transferred into Neisseria gonorrhoeae by conjugal mating when a 24.5-kDa gonococcal plasmid was present (102). Two geographically distinct plasmids of different sizes emerged almost simultaneously, each of which encoded the blaTEM gene in gonococci (103). Epidemiologically, this was attributed in part to the promiscuity of naval populations and prostitutes who were exchanging organisms with R-factor-associated TEM enzymes during trips between the Philippines and Europe or between western Africa and England (104). Prior to this, a single dose of penicillin had been demonstrated to cure >95% of the cases of gonorrhea (105), so the loss of an effective, safe, and inexpensive drug was devastating (106). To add insult to injury, the penicillin-resistant organisms originating from the Asia-Pacific region also were tetracycline resistant due to the tet(M) gene that appeared to travel on a different plasmid from blaTEM. This loss of useful therapeutic approaches to a widespread venereal disease resulted in panic, not only from military organizations operating in the western Pacific, but also from operators of clinics designed to treat outpatients with a single injection of either penicillin or tetracycline. As a result of concerns from the medical community (107), pharmaceutical companies quickly took notice and increased research efforts to identify either inhibitors of the TEM β-lactamase or molecules that were stable to β-lactamases from Gram-negative pathogens (4).
Because of the relatively small number of known β-lactamases in the mid-1970s, TEM-stable molecules, or TEM inhibitors, were evaluated for antimicrobial activities against a few standard β-lactamases and β-lactamase-producing strains that were common in multiple pharmaceutical companies. The enzymes studied were those that were easily purified in large amounts and that could be assayed with relatively rapid spectrophotometric methods (79). Although TEM-1 was the primary target for many research groups, other enzymes in the testing panels often included a class A penicillinase from S. aureus, a class C cephalosporinase from Enterobacter cloacae (frequently the P99-hyperproducing strain), and the class A K1 enzyme from Klebsiella pneumoniae (now Klebsiella oxytoca), the earliest extended-spectrum β-lactamase (ESBL) that served as the most stringent enzyme to test the stability of oxyimino-cephalosporins and monobactams (22, 108). As a result of this research, 24 new β-lactam-containing agents, including 15 cephalosporins, were approved by the U.S. Food and Drug Administration (FDA) in the 1980s (109). In addition to the TEM-stable oxyimino-substituted cephalosporins (e.g., cefotaxime, cefuroxime, ceftriaxone, and ceftazidime), monobactam (aztreonam) and carbapenem (imipenem), two β-lactamase inhibitors targeting the TEM β-lactamase and staphylococcal penicillinases, were also approved between 1981 and 1986 in three different combinations, as follows: clavulanic acid with amoxicillin (orally administered) or ticarcillin (parenterally administered), and sulbactam with ampicillin as an intravenous drug (4).
1985 to 2000, new β-lactams as drivers of resistance.
When microbiologists were asked to predict how resistance would develop to these new agents, the most common response involved the selection of derepressed AmpC mutants from the Enterobacteriaceae and P. aeruginosa, bacteria known to have inducible cephalosporinases whose hyperproduction could be selected clinically by the oxyimino-cephalosporins, or expanded-spectrum cephalosporins (110). E. cloacae in particular, as well as Citrobacter spp. and P. aeruginosa (111), were reported to be prone to this kind of selection, with clinical reports of 25% of patients treated with ceftazidime exhibiting resistance to the drug as a result of a derepressed AmpC enzyme in E. cloacae (112). However, outbreaks of cefotaxime- or ceftazidime-resistant Enterobacteriaceae reported in Clermont-Ferrand, France, in the mid-1908s heralded a different broad-based resistance to these new agents (113). These resistant isolates were the result of transferable β-lactamases, now known as ESBLs, which were capable of hydrolyzing the new β-lactams substituted with an oxyimino side chain, e.g., aztreonam and the third-generation or expanded-spectrum cephalosporins. Initially, ESBLs were derived from the common SHV-1, TEM-1, or TEM-2 β-lactamases and differed from the parental enzymes by no more than two or three amino acids in the coding region (http://www.lahey.org/Studies/temtable.asp) (5). In both Europe and the United States where these enzymes were identified almost simultaneously in the late 1980s, the prominent ESBLs were almost all SHV or TEM variants (114–117). Early ESBL-producing isolates were resistant to penicillins and most cephalosporins but susceptible to β-lactamase inhibitor combinations, a differentiating feature used to define ESBL producers phenotypically. As a result of the selective use of agents, such as amoxicillin-clavulanic acid and piperacillin-tazobactam, “inhibitor-resistant” TEM enzymes (IRTs) (118), followed by inhibitor-resistant SHV enzymes (119) emerged. Curiously, organisms that produced many of these enzymes were resistant to penicillin combinations with clavulanic acid or tazobactam but were susceptible to cephalothin (120). Although these enzymes have not played a major role in resistance to the inhibitor combinations in most parts of the world, a recent report from Spain indicates that IRTs are still prevalent in Spanish E. coli isolates, from both community and hospital sources (121).
Over the past decade, the common TEM, IRT, and SHV variants have diminished in numbers, only to be replaced by the CTX-M family of ESBLs, a dominating contributor to the multidrug-resistant profile in many Gram-negative bacteria (122, 123). In contrast to the TEM and SHV variants that arose from prevalent European and North American plasmid-encoded penicillinases, the CTX-M enzymes are closely related to chromosomal β-lactamases from the genus Kluyvera (124–126), a genus rarely associated with clinical disease (127). SHV ESBL variants are still identified, but few new TEM-related enzymes are now seen. Only recently have tazobactam-resistant CTX-M β-lactamases been identified, unusual enzymes that are still inhibited by clavulanic acid (128, 129). The family of GES enzymes was initially believed to represent another set of ESBLs, but some GES variants with single point mutations have acquired the ability to hydrolyze carbapenems (130, 131). The numbers of enzymes in major β-lactamase families are reflected in Table 3, where the high rate of increase in TEM and SHV novel naturally occurring variants from the 1990s/early 2000s has diminished over time compared to other families. In current isolates, multiple ESBLs may be produced in the same organism, together with enzymes from any of the other molecular classes (132–134).
TABLE 3.
Increasing numbers of β-lactamases in well-characterized familiesa
| Enzyme type | Class | Functional group | No. in class by yr |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1961 | 1995 | 2000 | 2005 | 2010 | 2015 | 2018 | |||
| CMY | C | 1 | 0 | 1 | 6 | 22 | 64 | 136 | 139 |
| PDCb | C | 1 | 0 | (1) | (1) | (1) | 10 | 30 | 226 |
| ADCc | C | 1 | 0 | 0 | (1) | 7 | 7 | 7 | 81 |
| All TEMs | A | 2b, 2be, 2br | 0 | 36 | 86 | 153 | 178 | 219 | 224 |
| All SHVs | A | 2b, 2be, 2br | 0 | 6 | 26 | 89 | 134 | 182 | 193 |
| CTX-M | A | 2be | 0 | 2 | 9 | 51 | 103 | 172 | 182 |
| KPC | A | 2f | 0 | 0 | 0 | 3 | 11 | 22 | 24 |
| All OXAs | D | 2d | 0 | 18 | 28 | 88 | 202 | 498 | 520 |
| IMP | B | 3 | 0 | 1 | 3 | 23 | 29 | 48 | 53 |
| VIM | B | 3 | 0 | 0 | 2 | 12 | 27 | 41 | 46 |
| NDM | B | 3 | 0 | 0 | 0 | 0 | 1 | 12 | 14 |
| Estimated total of all unique β-lactamases | (<13)d | 217 | 309 | 584 | 1,003 | 1,855 | 2,771 | ||
Some data are from reference 224, as well as from http://www.lahey.org/Studies/ and https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047.
PDC, Pseudomonas-derived cephalosporinase family, first named in 2009 (136). AmpC pseudomonal cephalosporinases were described as early as 1965 (137). This family was not included in the Lahey database http://www.lahey.org/Studies/.
ADC, Acinetobacter-derived cephalosporinase, family first named in 2005 (190). The name ADC-1 was assigned to an enzyme first described in 2000 (135). This family was not included in the Lahey database http://www.lahey.org/Studies/.
Class C, AmpC-related, cephalosporinases also played a role in the resistance to cephalosporins, cephamycins, and, to a lesser extent, to carbapenems in both enteric bacteria and nonfermentative pathogens (135–137). Until the late 1980s, they were most worrisome when derepressed AmpC production resulted in high levels of chromosomal/species-specific enzymes. Even enzymes with poor hydrolysis rates for these β-lactams could inactivate enough of the drug to cause clinical resistance, particularly in strains with decreased permeability or increased efflux (138–140). However, when plasmid-encoded AmpC-type enzymes on high-copy-number plasmids began to emerge by 1990 (141), a new threat was introduced, as these enzymes were freely transferable among species, resulting in increased resistance to multiple β-lactam classes, including carbapenems (142).
As ESBL-producing isolates became a more prominent segment of the Gram-negative population in hospitalized patients, carbapenems were used more frequently in those health care centers with large outbreaks of cephalosporin-resistant infections (143). This often occurred when isolates produced multiple β-lactamases and a β-lactamase inhibitor combination was not potent enough to overcome all the enzymes in the resistant isolate (144). Selection of carbapenem-resistant pathogens was the fully predictable consequence. Resistance to carbapenems or alternative β-lactam-containing agents emerged due to a multiplicity of the following factors: altered PBPs in Acinetobacter spp. (145), overproduction of plasmid-encoded class C cephalosporinases together with porin deficiencies in the Enterobacteriaceae (144), and the production of carbapenemases, both chromosomal and plasmid encoded (132).
Carbapenemases remain the major resistance mechanism for carbapenems in the Gram-negative bacteria. Initially they were regarded as nontraditional clinically irrelevant zinc-containing β-lactamases occurring only in the occasional Stenotrophomonas (Pseudomonas) maltophilia clinical isolate (146) and in isolates of Bacillus spp. (other than B. anthracis) that are infrequent causes of nongastrointestinal human infections (147). However, class A carbapenemases, such as the species-specific chromosomal SME enzymes in Serratia marcescens, began to be identified intermittently in Europe (148, 149) and the United States (150) in the 1980s. Plasmid-encoded MBLs emerged in Japan in 1990 with the IMP family of enzymes (151) and in Italy in 1997 with the VIM β-lactamases (152), causing some to predict a global epidemic of multidrug-resistant MBL-producing Gram-negative bacteria. Although the IMP and VIM MBL families began to expand after 2000 (Table 3), outbreaks associated with these enzymes have tended to be small, limited in time, and localized to specific geographical regions (153–157).
2001 to 2018, major epidemic β-lactamases.
Identification of the plasmid-encoded KPC serine carbapenemases in the early 2000s (158, 159) soon led to major epidemics caused by carbapenemase-producing Enterobacteriaceae in many areas of the world (160–162). These enzymes can appear in almost any Gram-negative pathogen (9, 163, 164), although they are predominantly identified in K. pneumoniae. The most prominent of the KPCs are KPC-2 and KPC-3, frequently found in K. pneumoniae clonal complex 258 (CC258) (165, 166). Within CC258, the common sequence type 258 (ST258) found in the United States and Europe, are two major clades, clade I associated with KPC-2 dissemination and clade II associated with KPC-3 (167). KPC-producing organisms have been associated with high rates of mortality, as high as 51% in patients with infections caused by colistin-resistant K. pneumoniae strains (168). Other serine carbapenemases have not become quite as prolific or deleterious as the KPCs. Only the Serratia-specific chromosomal SME enzymes have occasionally caused small outbreaks (169). A recent unusual multidrug-resistant S. marcescens isolate was selected during therapy due to the selection of hyperproduction of both the AmpC and SME chromosomal β-lactamases (170).
Until recently, plasmid-encoded MBL dissemination had been a minor threat in most geographical regions, even in countries that have recorded sporadic outbreaks with enzymes, such as VIM-1 in Greece (171), IMP-8 in Taiwan (172), and IMP-1 in Japan (173, 174). However, MBLs became more menacing after the NDM-1 zinc-containing carbapenemase was identified in 2009 from an isolate originating from New Delhi, India (175). Retrospective studies have traced the origins of this MBL to at least 2006 (176). In contrast to the other MBLs, NDM-1 quickly spread worldwide, being the predominant carbapenemase in the Indian subcontinent, but with major outbreaks also reported in the Balkans and the Middle East (9, 166). Of great concern is the widespread occurrence of the blaNDM gene that has been identified in environmental water samples in India (177, 178).
A third transferable carbapenemase associated with outbreaks is the OXA-48 enzyme, originally identified as a class D oxacillinase from Turkey in 2001 (179). This enzyme, found in multidrug-resistant Enterobacteriaceae, slowly hydrolyzes carbapenems and expanded-spectrum cephalosporins and is poorly inhibited by most β-lactamase inhibitors, with the exception of avibactam (180). It is most prevalent in the Mediterranean region and southern Europe. Outbreaks have been reported in France (181) and Spain (182), where 74% and 32% of the carbapenemases in E. coli and Citrobacter spp., respectively, were recently identified as OXA-48 (182, 183). Class D oxacillinases are also frequently found in Acinetobacter spp. and are the primary cause for carbapenem resistance in those organisms. As Acinetobacter-related infections increased during the early 2000s, the contributions of the chromosomally encoded OXA-51 in Acinetobacter baumannii (184) and plasmid-encoded OXA-23, OXA-24/33/40, and OXA-58 enzymes were more fully appreciated (185). Although multidrug-resistant Acinetobacter spp. have been associated with a number of outbreaks, the contribution of β-lactamases to these outbreaks has been contributory, but not necessarily the driving factor, due to the intrinsic resistance of these pathogens to most antibiotics (185, 186).
β-LACTAMASE CLASSIFICATIONS
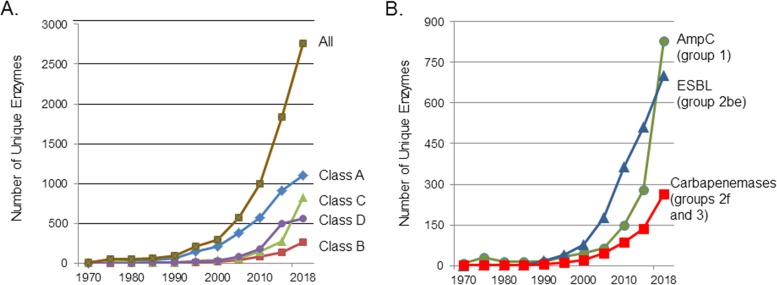
With the variety of unique β-lactamases that have been identified in natural isolates now exceeding 2,770 (Table 3 and Fig. 3), it is important to have reliable and easily understandable nomenclature to refer to these enzymes. Beginning with Sawai and colleagues, who classified β-lactamases as penicillinases or cephalosporinases according to substrate profiles (18), other classification schemes arose (Table 2) based traditionally on the functional characteristics of these enzymes (19). As nucleotide and amino acid sequences became available, molecular relatedness was added as a defining characteristic (14–16). One of the most cited classification schemes using functional group designations was proposed by Bush, initially in 1988 (187), and then in collaboration with Jacoby and Medeiros (21), as discussed above. Their updated scheme described in 2010 (20) has been further expanded, as shown in Fig. 1, with the addition of avibactam as a differentiating inhibitor to separate serine carbapenemases from MBLs, potentially a useful diagnostic characteristic in whole-cell phenotypic assays (188). Fortuitously, assignments to functional groups generally aligned with molecular classes, although exceptions were noted, particularly with the intrinsically diverse OXA enzymes (189).
FIG 3.
Increase in numbers of unique, naturally occurring β-lactamases (some data from reference 224 as well as from http://www.lahey.org/Studies/ and https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047). (A) β-Lactamases enumerated according to molecular classes A, B, C, and D, with the total number of enzymes (all) equal to 2,771. (B) β-Lactamases enumerated according to major functional groups with their trivial names, AmpC, group 1; ESBLs, group 2be; and carbapenemases, groups 2f and 3.
Numbering of variants within β-lactamase families has been a challenge, beginning with the emergence of numerous ESBLs resulting from point mutations in both nucleotide and amino acid sequences (5). As a result, in 1996, Jacoby offered to serve as the curator for assignments of new allele numbers for naturally occurring plasmid-encoded ESBLs, with the establishment of the website at http://www.lahey.org/Studies/. The site was later expanded to include assignments for natural alleles in all major β-lactamase families containing more than three known variants. Functional assignments were made for the TEM, SHV, and OXA enzymes if substrate and inhibitor profiles were available. In 2015, the task of assigning new numbers for novel β-lactamases was transferred to NCBI (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047), with a request for phenotypic information and genotype data. Tracking of the LEN, OXY, and OKP enzyme families in Klebsiella spp. is being curated by investigators at the Institut Pasteur (http://bigsdb.web.pasteur.fr/klebsiella/klebsiella.html). Bonomo and colleagues have established numbering systems for the Acinetobacter-derived cephalosporinases (ADCs) (190), followed by similar numbering of Pseudomonas-derived cephalosporinases (PDCs) (136, 191), families whose numbers have increased greatly in the past 5 years as a result of whole-genome sequencing projects (185) (Table 3 and Fig. 3).
The agreement within the β-lactamase community to provide a unique designation for each unique (natural) allele has met with criticism from others in the antibiotic resistance field who advocate that there should be at least a 2% change in nucleotide or predicted amino acid sequence before a new enzyme or gene name is assigned (192). However, a single amino acid substitution in a TEM or SHV β-lactamase can alter its biochemical properties, such that the substrate spectrum is dramatically altered to provide for an ESBL phenotype (5), or resistance to β-lactamase inhibitors can occur (118). These enzymes clearly have different functional characteristics and as such warrant a unique designation (193). A recent attempt to lump ESBLs and carbapenemases into a single ESBL category (194) was challenged by many in the β-lactamase community (195). Although the original intention was to simplify β-lactamase nomenclature for the practicing physician, many thought that this would be confusing, especially in situations in which carbapenems, which are routinely used to treat traditional ESBL-producing pathogens (196), would not be effective against infections caused by carbapenemase-producing organisms if carbapenemases were also named ESBLs.
More recent classification schemes are based on the association between 3-dimensional structures and functional information, particularly for the class A/group 2 β-lactamases (197–200). As crystallographic analyses become available for additional β-lactamases, determination of structure-function relationships will become more common. However, for this approach to be successful, high-quality phenotypic data must be provided for novel β-lactamases. The numbers of β-lactamases continue to increase almost exponentially (see Fig. 3 and reference 201), partly because of easy access to inexpensive and rapid gene sequencing. Laboratories now have the ability to identify dozens of “new” β-lactamase sequences (185), with no functional information provided. Unfortunately, whole-genome sequencing provides more data than we perhaps need to know. Some of the genes that are identified as β-lactamase-encoding genes are incomplete, some are misannotated, and some are not expressed, so that assignments for new alleles are not aligned with functionality (202). In addition, “β-lactamase” may be included in the annotation for a new protein sequence that has no β-lactamase hydrolytic capability. An example is the NCBI annotation for sequences in the metallo-β-lactamase superfamily, a family of more than 6,000 enzymes that includes oxidoreductases, as well as enzymes which hydrolyze thiol-ester, phosphodiester, and sulfuric ester bonds (203) but <300 verified MBLs (Fig. 3). Naive investigators may incorrectly assume that this commonly identified set of sequences signals the presence of an MBL, potentially triggering aggressive medical treatment in the hospital laboratory. Even for legitimate enumerations of new β-lactamases based only on sequence data, a lack of functional data minimizes the usefulness of the information. Attempts by structural biologists to align structures with function will suffer due to the lack of phenotypic information to correlate with novel sequences. Although minimally, it is recommended that any novel β-lactamase gene be transferred to a non-β-lactamase-producing strain and its resistance phenotype confirmed, it is preferable that a novel enzyme be purified and its biochemical properties determined and then correlated with microbiological properties in the producing organism (20, 193). Ideally, both genomic and biochemical information should be available before classifying enzymes into families (20).
DISCUSSION
Selective pressure from both naturally occurring β-lactams and clinically overused β-lactam-containing drugs has created an environment in which new β-lactamases readily emerge, together with maintenance of some of the older, more fit, enzymes. Recent laboratory studies have demonstrated that conjugal plasmids with antibiotic resistance genes are stable in multispecies communities over long periods of time, even in the absence of antibiotic pressure (204). This partially explains the continued presence of antibiotic resistance genes in apparently naive environments (45, 46) and the perpetuation of β-lactamase genes encoding common TEM, SHV, and OXA enzymes. It is notable that TEM-1 and, to a lesser extent, OXA-1, two of the first known plasmid-encoded β-lactamases, have not disappeared and are frequently identified in contemporary, multidrug-resistant, clinical, and environmental isolates (132, 205, 206), perhaps because of their exquisite ability to hydrolyze inexpensive penicillins that are still used therapeutically in community settings. However, there is great concern about environmental contamination in public water supplies by β-lactamase-producing antibiotic-resistant pathogens. A recent study in Hyderabad, India, in both urban and rural areas, found that 100% of all water samples collected from bulk drug manufacturing facilities and multiple water sources, including those contaminated by sewage treatment plants, were positive for ESBL genes, and 95% of them contained carbapenemase genes, often with multiple genes per sample (e.g., blaOXA-48, blaKPC, and blaNDM) (178). Several studies have identified environmental sources of carbapenem-resistant organisms carrying primarily blaKPC and blaVIM genes in hospital drains and sinks, thereby providing sources for these pathogens to enter water supplies outside health care centers (153, 207).
One of the most worrisome threats described by the Centers for Disease Control and Prevention (CDC) is carbapenemase production in recent Gram-negative clinical isolates, especially those enzymes with genes carried on mobile genetic elements (208). Fortunately, serine carbapenemases are readily inhibited by new β-lactamase inhibitors, including the diazabicyclooctanone (DBO) inhibitors, such as avibactam (209), and the boronic acid derivative vaborbactam (210), both of which have been recently approved for marketing in combination with ceftazidime or meropenem, respectively. These inhibitor combinations allow relatively effective clinical treatment of infections caused by organisms producing serine carbapenemases (211), thus lowering the risk of clinical failures. A combination of avibactam with the MBL-stable monobactam aztreonam is currently in phase 3 clinical trials to treat Gram-negative bacterial infections, including those with MBL-producing pathogens (https://clinicaltrials.gov/ct2/show/NCT03329092?term=avibactam&draw=2&rank=19). Although resistance has been reported in patients treated with avibactam-ceftazidime, the numbers are currently small (212). As can be expected, however, resistance will increase as the new agents are used. At this time, a β-lactam-containing agent inhibiting a broad spectrum of pathogens, including nonfermentative and anaerobic pathogens, is still to be identified. This is due in part to the increase in multidrug-resistant organisms producing MBLs and/or OXA-48 carbapenemases in combination with β-lactamases of different classes and the lack of an effective inhibitor of all the enzymes (213). Thus, it is possible that MBLs rather than serine β-lactamases may become the predominant carbapenemases in multidrug-resistant bacteria. It is also possible that β-lactamase-stable β-lactams, such as cefiderocol (S-649266), or non-β-lactam-containing agents, such as plazomicin, omadacycline, or eravacycline, will serve as reliable and safe therapeutic alternatives against the most resistant carbapenem-resistant pathogens (214).
In conclusion, β-lactamases, some of our oldest enzymes, have emerged as perhaps the most studied, and most troublesome, of the antibiotic resistance determinants. The fact that many β-lactamase-encoding genes travel together on mobile elements with transmissible resistance factors for other antibiotic classes means that resistance can arise due to a multiplicity of selection pressures. One can anticipate that additional unique β-lactamases with unusual properties will be identified due to the widespread existence of these genes in both environmental and clinical sources and to continued pressure from the use of β-lactam antibiotics. It will be up to the scientific community to monitor their presence with detailed structural and functional studies to provide a scientific basis for the design of new antimicrobial agents that may evade these emerging enzymes, at least for the immediate future.
ACKNOWLEDGMENTS
This article is an updated version of the 2014 ICAAC Lecture “β-Lactamases: Ubiquitous and Formidable.” It is dedicated to the many investigators who set the stage for those of us who have continued to study β-lactam resistance, especially as it drives the discovery and development of new antibacterial agents.
I particularly thank my many collaborators and β-lactamase friends with whom I have worked in this area, including those named below (in alphabetical order): Robert Bonomo, Patricia Bradford, Jed Fisher, Jean-Marie Frère, Janet Hindler, Romney Humphries, George Jacoby, Steve Jenkins, David Livermore, Antone Medeiros, Shahriar Mobashery, Malcolm Page, Timothy Palzkill, Anne Marie Queenan, the late John Quinn, Gian Maria Rossolini, Christine Sanders, Richard Sykes, the late Frank Tally, Fred Tenover, Carl Urban, and Youjun Yang.
There was no financial support associated with the writing of this work.
REFERENCES
- 1.Abraham EP, Chain E. 1940. An enzyme from bacteria able to destroy penicillin. Nature 146:837. doi: 10.1038/146837a0. [DOI] [PubMed] [Google Scholar]
- 2.Kirby WMN. 1944. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science 99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 3.Fisher JF, Knowles JR. 1978. Bacterial resistance to β-lactams: the β-lactamases. Ann Rep Med Chem 13:239–248. doi: 10.1016/S0065-7743(08)60628-4. [DOI] [Google Scholar]
- 4.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview, p 23–44. In Silver LL, Bush K (ed), Antibiotics and antibiotic resistance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford PA. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacoby GA. 2009. AmpC beta-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 9.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palzkill T. 2018. Structural and mechanistic basis for extended-spectrum drug-resistance mutations in altering the specificity of TEM, CTX-M, and KPC beta-lactamases. Front Mol Biosci 5:16. doi: 10.3389/fmolb.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. 2018. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 66:1290–1297. doi: 10.1093/cid/cix893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knott-Hunziker V, Waley SG, Orlek BS, Sammes PG. 1979. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett 99:59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HM, Hao Q. 2011. Crystal structure of NDM-1 reveals a common β-lactam hydrolysis mechanism. FASEB J 25:2574–2582. doi: 10.1096/fj.11-184036. [DOI] [PubMed] [Google Scholar]
- 14.Ambler RP. 1980. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci 289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 15.Jaurin B, Grundstrom T. 1981. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of β-lactamases of the penicillinase type. Proc Natl Acad Sci U S A 78:4897–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huovinen P, Huovinen S, Jacoby GA. 1988. Sequence of PSE-2 beta-lactamase. Antimicrob Agents Chemother 32:134–136. doi: 10.1128/AAC.32.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwabara S, Abraham EP. 1967. Some properties of two extracellular β-lactamases from Bacillus cereus 569/H. Biochem J 103:27c–30c. doi: 10.1042/bj1030027C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawai T, Mitsuhashi S, Yamagishi S. 1968. Drug resistance of enteric bacteria. XIV. Comparison of β-lactamases in Gram-negative rod bacteria resistant to α-aminobenzylpenicillin. Jpn J Microbiol 12:423–434. [DOI] [PubMed] [Google Scholar]
- 19.Richmond MH, Sykes RB. 1973. The β-lactamases of Gram-negative bacteria and their possible physiological role, p 31–88. In Rose AH, Tempest DW (ed), Advances in microbial physiology, vol 9 Academic Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 20.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush K, Jacoby GA, Medeiros AA. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother 39:1211–1233. doi: 10.1128/AAC.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reading C, Cole M. 1977. Clavulanic acid: a beta-lactamase inhibitor from Streptomyces clavuligerus. Antimicrob Agents Chemother 11:852–857. doi: 10.1128/AAC.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stachyra T, Pechereau MC, Bruneau JM, Claudon M, Frere JM, Miossec C, Coleman K, Black MT. 2010. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob Agents Chemother 54:5132–5138. doi: 10.1128/AAC.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saino Y, Kobayashi F, Inoue M, Mitsuhashi S. 1982. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother 22:564–570. doi: 10.1128/AAC.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frère J-M, Dues C, Ghuysen J-M, Vandekerkhove J. 1976. Occurrence of a serine in the penicillin-binding site of the exocellular dd-carboxypeptidase-transpeptidase from Streptomyces R6 I. FEBS Lett 70:257–260. doi: 10.1016/0014-5793(76)80770-3. [DOI] [PubMed] [Google Scholar]
- 26.Spratt BG. 1983. Penicillin-binding proteins and the future of ß-lactam antibiotics. J Gen Microbiol 129:1247–1260. [DOI] [PubMed] [Google Scholar]
- 27.Tipper DJ, Strominger JL. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-d-alanyl-d-alanine. Proc Natl Acad Sci U S A 54:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massova I, Mobashery S. 1998. Kinship and diversification of bacterial penicillin-binding proteins and β-lactamases. Antimicrob Agents Chemother 42:1–17. doi: 10.1093/jac/42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meroueh SO, Minasov G, Lee W, Shoichet BK, Mobashery S. 2003. Structural aspects for evolution of beta-lactamases from penicillin-binding proteins. J Am Chem Soc 125:9612–9618. doi: 10.1021/ja034861u. [DOI] [PubMed] [Google Scholar]
- 30.Fisher JF, Mobashery S. 2009. Three decades of the class A beta-lactamase acyl-enzyme. Curr Protein Pept Sci 10:401–407. doi: 10.2174/138920309789351967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JD, Kumarasiri M, Zhang W, Hesek D, Lee M, Toth M, Vakulenko S, Fisher JF, Mobashery S, Chen Y. 2013. Structural analysis of the role of Pseudomonas aeruginosa penicillin-binding protein 5 in beta-lactam resistance. Antimicrob Agents Chemother 57:3137–3146. doi: 10.1128/AAC.00505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro AB, Gu RF, Gao N, Livchak S, Thresher J. 2013. Continuous fluorescence anisotropy-based assay of BOCILLIN FL penicillin reaction with penicillin binding protein 3. Analyt Biochem 439:37–43. doi: 10.1016/j.ab.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro AB, Gao N, Gu RF, Thresher J. 2014. Fluorescence anisotropy-based measurement of Pseudomonas aeruginosa penicillin-binding protein 2 transpeptidase inhibitor acylation rate constants. Analyt Biochem 463:15–22. doi: 10.1016/j.ab.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Lemaire S, Fuda C, Van Bambeke F, Tulkens PM, Mobashery S. 2008. Restoration of susceptibility of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics by acidic pH: role of penicillin-binding protein PBP 2a. J Biol Chem 283:12769–12776. doi: 10.1074/jbc.M800079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu WP, Kincaid E, Sun Y, Bauer MD. 2001. Kinetics of beta-lactam interactions with penicillin-susceptible and -resistant penicillin-binding protein 2x proteins from Streptococcus pneumoniae. Involvement of acylation and deacylation in beta-lactam resistance. J Biol Chem 276:31494–31501. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton-Miller JMT. 1979. An historical introduction to beta-lactamases, p 1–16. In Hamilton-Miller JMT, Smith JT (ed), Beta-lactamases. Academic Press, London, United Kingdom. [Google Scholar]
- 37.Hall BG, Barlow M. 2004. Evolution of the serine beta-lactamases: past, present and future. Drug Resist Updat 7:111–123. doi: 10.1016/j.drup.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Barlow M, Hall BG. 2002. Phylogenetic analysis shows that the OXA beta-lactamase genes have been on plasmids for millions of years. J Mol Evol 55:314–321. doi: 10.1007/s00239-002-2328-y. [DOI] [PubMed] [Google Scholar]
- 39.Bhullar K, Waglechner N, Pawlowski A, Koteva K, Banks ED, Johnston MD, Barton HA, Wright GD. 2012. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One 7:e34953. doi: 10.1371/journal.pone.0034953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, Golding GB, Poinar HN, Wright GD. 2011. Antibiotic resistance is ancient. Nature 477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 41.Song JS, Jeon JH, Lee JH, Jeong SH, Jeong BC, Kim SJ, Lee JH, Lee SH. 2005. Molecular characterization of TEM-type beta-lactamases identified in cold-seep sediments of Edison Seamount (south of Lihir Island, Papua New Guinea). J Microbiol 43:172–178. [PubMed] [Google Scholar]
- 42.Rascovan N, Telke A, Raoult D, Rolain JM, Desnues C. 2016. Exploring divergent antibiotic resistance genes in ancient metagenomes and discovery of a novel beta-lactamase family. Environ Microbiol Rep 8:886–895. doi: 10.1111/1758-2229.12453. [DOI] [PubMed] [Google Scholar]
- 43.Van Goethem MW, Pierneef R, Bezuidt OKI, Van De Peer Y, Cowan DA, Makhalanyane TP. 2018. A reservoir of ‘historical’ antibiotic resistance genes in remote pristine Antarctic soils. Microbiome 6:40. doi: 10.1186/s40168-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segawa T, Takeuchi N, Rivera A, Yamada A, Yoshimura Y, Barcaza G, Shinbori K, Motoyama H, Kohshima S, Ushida K. 2013. Distribution of antibiotic resistance genes in glacier environments. Environ Microbiol Rep 5:127–134. doi: 10.1111/1758-2229.12011. [DOI] [PubMed] [Google Scholar]
- 45.Bartoloni A, Bartalesi F, Mantella A, Dell'Amico E, Roselli M, Strohmeyer M, Barahona HG, Barron VP, Paradisi F, Rossolini GM. 2004. High prevalence of acquired antimicrobial resistance unrelated to heavy antimicrobial consumption. J Infect Dis 189:1291–1294. doi: 10.1086/382191. [DOI] [PubMed] [Google Scholar]
- 46.Bartoloni A, Pallecchi L, Rodriguez H, Fernandez C, Mantella A, Bartalesi F, Strohmeyer M, Kristiansson C, Gotuzzo E, Paradisi F, Rossolini GM. 2009. Antibiotic resistance in a very remote Amazonas community. Int J Antimicrob Agents 33:125–129. doi: 10.1016/j.ijantimicag.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Karlowsky JA, Lob SH, Kazmierczak KM, Badal RE, Young K, Motyl MR, Sahm DF. 2017. In vitro activity of imipenem against carbapenemase-positive Enterobacteriaceae isolates collected by the SMART global surveillance program from 2008 to 2014. J Clin Microbiol 55:1638–1649. doi: 10.1128/JCM.02316-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao M, Glenn AE, Blacutt AA, Gold SE. 2017. Fungal lactamases: their occurrence and function. Front Microbiol 8:1775. doi: 10.3389/fmicb.2017.01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittl PR, Luthy L, Hunziker P, Grutter MG. 2000. The cysteine-rich protein A from Helicobacter pylori is a beta-lactamase. J Biol Chem 275:17693–17699. doi: 10.1074/jbc.M001869200. [DOI] [PubMed] [Google Scholar]
- 50.Abeylath SC, Turos E. 2008. Drug delivery approaches to overcome bacterial resistance to beta-lactam antibiotics. Expert Opin Drug Deliv 5:931–949. doi: 10.1517/17425247.5.9.931. [DOI] [PubMed] [Google Scholar]
- 51.Fleming A. 1929. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol 10:226–236. [Google Scholar]
- 52.Newton GGF, Abraham EP. 1956. Isolation of cephalosporin C, a penicillin-like antibiotic containing d-alpha aminoadipic acid. Biochem J 62:651–658. doi: 10.1042/bj0620651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahan JS, Kahan FM, Goegelman R, Currie SA, Jackson M, Stapley EO, Miller TW, Miller AK, Hendlin D, Mochales S, Hernandez S, Woodruff HB, Birnbaum J. 1979. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J Antibiot (Tokyo) 32:1–12. [DOI] [PubMed] [Google Scholar]
- 54.Sykes RB, Wells JS, Parker WL, Koster WH, Cimarusti CM. 1986. Aztreonam: discovery and development of the monobactams. N J Med Spec No:8–15. [PubMed] [Google Scholar]
- 55.Moxon ER. 2011. Darwin, microbes and evolution by natural selection. Adv Exp Med Biol 697:77–86. doi: 10.1007/978-1-4419-7185-2_6. [DOI] [PubMed] [Google Scholar]
- 56.Knoll AH, Bergmann KD, Strauss JV. 2016. Life: the first two billion years. Philos Trans R Soc Lond B Biol Sci 371:05. doi: 10.1098/rstb.2015.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Callaghan CH. 1978. Irreversible effects of serum proteins on beta-lactam antibiotics. Antimicrob Agents Chemother 13:628–633. doi: 10.1128/AAC.13.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kropp H, Sundelof JG, Hadju R, Kahan FM. 1982. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase-I. Antimicrob Agents Chemother 22:62–70. doi: 10.1128/AAC.22.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher JF, Mobashery S. 2016. β-Lactam resistance mechanisms: Gram-positive bacteria and Mycobacterium tuberculosis, p 45–63. In Silver LL, Bush K (ed), Antibiotics and antibiotic resistance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansman D, Bullen M. 1967. A resistant pneumococcus. Lancet ii:264–265. doi: 10.1016/S0140-6736(67)92346-X. [DOI] [PubMed] [Google Scholar]
- 61.Dowson CG, Hutchison A, Brannigan JA, George RC, Hansman D, Linares J, Tomasz A, Smith JM, Spratt BG. 1989. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci U S A 86:8842–8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hemmer RJ, Vaudaux P, Waldvogel FA. 1979. Methicillin potentiates the effect of gentamicin on methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 15:34–41. doi: 10.1128/AAC.15.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saroglou G, Cromer M, Bisno AL. 1980. Methicillin-resistant Staphylococcus aureus: interstate spread of nosocomial infections with emergence of gentamicin-methicillin resistant strains. Infect Control 1:81–89. doi: 10.1017/S0195941700052590. [DOI] [PubMed] [Google Scholar]
- 64.Hartman BJ, Tomasz A. 1984. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol 158:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rammelkamp CH, Maxon T. 1942. Resistance of Staphylococcus aureus to the action of penicillin. Proc Soc Exp Biol Med 51:386–389. doi: 10.3181/00379727-51-13986. [DOI] [Google Scholar]
- 66.Medeiros AA. 1997. Evolution and dissemination of β-lactamases accelerated by generations of beta-lactam antibiotics. Clin Infect Dis 24:S19–S45. doi: 10.1093/clinids/24.Supplement_1.S19. [DOI] [PubMed] [Google Scholar]
- 67.Barber M, Whitehead JE. 1949. Bacteriophage types in penicillin-resistant staphylococcal infection. Br Med J 2:565–569. doi: 10.1136/bmj.2.4627.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richter SS, Doern GV, Heilmann KP, Miner S, Tendolkar S, Riahi F, Diekema DJ. 2016. Detection and prevalence of penicillin-susceptible Staphylococcus aureus in the United States in 2013. J Clin Microbiol 54:812–814. doi: 10.1128/JCM.03109-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murray BE, Mederski-Samaroj B. 1983. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest 72:1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zscheck KK, Murray BE. 1991. Nucleotide sequence of the beta-lactamase gene from Enterococcus faecalis HH22 and its similarity to staphylococcal beta-lactamase genes. Antimicrob Agents Chemother 35:1736–1740. doi: 10.1128/AAC.35.9.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nallapareddy SR, Wenxiang H, Weinstock GM, Murray BE. 2005. Molecular characterization of a widespread, pathogenic, and antibiotic resistance-receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity island. J Bacteriol 187:5709–5718. doi: 10.1128/JB.187.16.5709-5718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Belhaj M, Boutiba-Ben Boubaker I, Slim A. 2016. Penicillin-binding protein 5 sequence alteration and levels of plp5 mRNA expression in clinical isolates of Enterococcus faecium with different levels of ampicillin resistance. Microb Drug Resist 22:202–210. doi: 10.1089/mdr.2015.0211. [DOI] [PubMed] [Google Scholar]
- 73.Philippon A, Slama P, Deny P, Labia R. 2016. A structure-based classification of class A beta-lactamases, a broadly diverse family of enzymes. Clin Microbiol Rev 29:29–57. doi: 10.1128/CMR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pollock MR. 1965. Purification and properties of penicillinases from two strains of Bacillus licheniformis: a chemical physicochemical and physiological comparison. Biochem J 94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabath LD, Abraham EP. 1966. Zinc as a cofactor for cephalosporinase from Bacillus cereus 569. Biochem J 98:11c–13c. doi: 10.1042/bj0980011C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benedict RG, Schmidt WH, Coghill RD. 1945. Penicillin VII. Penicillinase. Arch Biochem 8:377–384. [PubMed] [Google Scholar]
- 77.Barnes JM. 1947. Penicillin and B. anthracis. J Pathol Bacteriol 59:113–125. [DOI] [PubMed] [Google Scholar]
- 78.Duthie ES. 1947. The production of stable potent preparations of penicillinase. J Gen Microbiol 1:370–377. doi: 10.1099/00221287-1-3-370. [DOI] [PubMed] [Google Scholar]
- 79.Jansson JA. 1965. A direct spectrophotometric assay for penicillin β-lactamase (penicillinase). Biochim Biophys Acta 99:171–172. doi: 10.1016/S0926-6593(65)80018-2. [DOI] [PubMed] [Google Scholar]
- 80.Citri N, Pollock MR. 1966. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol 28:237–323. [DOI] [PubMed] [Google Scholar]
- 81.Toth M, Antunes NT, Stewart NK, Frase H, Bhattacharya M, Smith CA, Vakulenko SB. 2016. Class D beta-lactamases do exist in Gram-positive bacteria. Nat Chem Biol 12:9–14. doi: 10.1038/nchembio.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthew M, Harris AM. 1976. Identification of β-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol 94:55–67. doi: 10.1099/00221287-94-1-55. [DOI] [PubMed] [Google Scholar]
- 83.Labia R, Guionie M, Masson JM, Philippon A, Barthelemy M. 1977. Beta-lactamases produced by a Pseudomonas aeruginosa strain highly resistant to carbenicillin. Antimicrob Agents Chemother 11:785–790. doi: 10.1128/AAC.11.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitsuhashi S, Hashimoto H, Kono M, Morimura M. 1965. Drug resistance of staphylococci. II. Joint elimination and joint transduction of the determinants of penicillinase production and resistance to macrolide antibiotics. J Bacteriol 89:988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Datta N, Kontomichalou P. 1965. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 208:239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- 86.Datta N, Richmond MH. 1966. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J 98:204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Egawa R, Sawai T, Mitsuhashi S. 1967. Drug resistance of enteric bacteria. XII. Unique substrate specificity of penicillinase produced by R-factor. Jpn J Microbiol 11:179–186. [Google Scholar]
- 88.Matthew M, Hedges RW. 1976. Analytical isoelectric focusing of R factor-determined β-lactamases: correlation with plasmid compatibility. J Bacteriol 125:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pfeifer Y, Cullik A, Witte W. 2010. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol 300:371–379. doi: 10.1016/j.ijmm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 90.Mathers A. 2016. Mobilization of carbapenemase-mediated resistance in Enterobacteriaceae. Microbiol Spectr EI10-0010-2015. doi: 10.1128/microbiolspec.EI10-0010-2015. [DOI] [PubMed] [Google Scholar]
- 91.Brolund A, Sandegren L. 2016. Characterization of ESBL disseminating plasmids. Infect Dis 48:18–25. doi: 10.3109/23744235.2015.1062536. [DOI] [PubMed] [Google Scholar]
- 92.Jack GW, Richmond MH. 1970. Comparative amino acid contents of purified β-lactamases from enteric bacteria. FEBS Lett 12:30–32. doi: 10.1016/0014-5793(70)80587-7. [DOI] [PubMed] [Google Scholar]
- 93.Matthew M, Harris AM, Marshall MJ, Ross GW. 1975. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol 88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 94.O'Callaghan C, Morris A, Kirby SM, Shingler AH. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother 1:283–288. doi: 10.1128/AAC.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matthew M. 1979. Plasmid mediated β-lactamases of Gram-negative bacteria: properties and distribution. J Antimicrob Chemother 5:349–358. doi: 10.1093/jac/5.4.349. [DOI] [PubMed] [Google Scholar]
- 96.Simpson IN, Harper PB, O'Callaghan CH. 1980. Principal β-lactamases responsible for resistance to β-lactam antibiotics in urinary tract infections. Antimicrob Agents Chemother 17:929–936. doi: 10.1128/AAC.17.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roy C, Foz A, Segura C, Tirado M, Fuster C, Reig R. 1983. Plasmid-determined β-lactamases identified in a group of 204 ampicillin-resistant Enterobacteriaceae. J Antimicrob Chemother 12:507–510. doi: 10.1093/jac/12.5.507. [DOI] [PubMed] [Google Scholar]
- 98.Medeiros AA. 1984. β-Lactamases. Br Med Bull 40:18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- 99.Roy C, Segura C, Tirado M, Reig R, Hermida M, Teruel D, Foz A. 1985. Frequency of plasmid-determined beta-lactamases in 680 consecutively isolated strains of Enterobacteriaceae. Eur J Clin Microbiol 4:146–147. doi: 10.1007/BF02013586. [DOI] [PubMed] [Google Scholar]
- 100.Medeiros AA, O'Brien TF. 1975. Ampicillin-resistant Haemophilus influenzae type B possessing a TEM-type beta-lactamase but little permeability barrier to ampicillin. Lancet i:716–719. doi: 10.1016/S0140-6736(75)91630-X. [DOI] [PubMed] [Google Scholar]
- 101.Khan W, Ross S, Rodriguez W, Controni G, Saz AK. 1974. Haemophilus influenzae type B resistant to ampicillin: a report of two cases. JAMA 229:298–301. doi: 10.1001/jama.1974.03230410022016. [DOI] [PubMed] [Google Scholar]
- 102.Eisenstein BI, Sox T, Biswas G, Blackman E, Sparling PF. 1977. Conjugal transfer of the gonococcal penicillinase plasmid. Science 195:998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- 103.Roberts M, Elwell LP, Falkow S. 1977. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J Bacteriol 131:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arya OP, Rees E, Turner GC, Percival A, Bartzokas CA, Annels EH, Carey PB, Ghosh AK, Jephcott AE, Johnston NA. 1984. Epidemiology of penicillinase-producing Neisseria gonorrhoeae in Liverpool from 1977 to 1982. J Infect 8:70–83. doi: 10.1016/S0163-4453(84)93462-5. [DOI] [PubMed] [Google Scholar]
- 105.McCormack WM. 1977. Treatment of gonorrhea–is penicillin passé? N Engl J Med 296:934–936. doi: 10.1056/NEJM197704212961610. [DOI] [PubMed] [Google Scholar]
- 106.McCormack WM. 1982. Penicillinase-producing Neisseria gonorrhoeae–a retrospective. N Engl J Med 307:438–439. doi: 10.1056/NEJM198208123070712. [DOI] [PubMed] [Google Scholar]
- 107.Sparling PF, Holmes KK, Wiesner PJ, Puziss M. 1977. Summary of the conference on the problem of penicillin-resistant gonococci. J Infect Dis 135:865–867. doi: 10.1093/infdis/135.5.865. [DOI] [Google Scholar]
- 108.Bush K, Freudenberger JS, Sykes RB. 1982. Interaction of azthreonam and related monobactams with β-lactamases from gram-negative bacteria. Antimicrob Agents Chemother 22:414–420. doi: 10.1128/AAC.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Powers JH. 2004. Antimicrobial drug development–the past, the present, and the future. Clin Microbiol Infect 10(Suppl 4):S23–S31. [DOI] [PubMed] [Google Scholar]
- 110.Sanders WE Jr, Sanders CC. 1988. Inducible β-lactamases: clinical and epidemiologic implications for use of newer cephalosporins. Rev Infect Dis 10:830–838. doi: 10.1093/clinids/10.4.830. [DOI] [PubMed] [Google Scholar]
- 111.Livermore DM. 1987. Clinical significance of beta-lactamase induction and stable derepression in Gram-negative rods. Eur J Clin Microbiol 6:439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- 112.Quinn JP, DiVincenzo CA, Foster J. 1987. Emergence of resistance to ceftazidime during therapy for Enterobacter cloacae infections. J Infect Dis 155:942–947. doi: 10.1093/infdis/155.5.942. [DOI] [PubMed] [Google Scholar]
- 113.Sirot J, Chanal C, Petit A, Sirot D, Labia R, Gerbaud G. 1988. Klebsiella pneumoniae and other Enterobacteriaceae producing novel plasmid-mediated β-lactamases markedly active against third-generation cephalosporins: epidemiological studies. Rev Infect Dis 10:850–859. doi: 10.1093/clinids/10.4.850. [DOI] [PubMed] [Google Scholar]
- 114.Kliebe C, Nies BA, Meyer JF, Tolxdorff-Neutzling RM, Wiedemann B. 1985. Evolution of plasmid-coded resistance to broad spectrum cephalosporins. Antimicrob Agents Chemother 28:302–307. doi: 10.1128/AAC.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sirot D, Sirot J, Labia R, Morand A, Courvalin P, Darfeuille-Michaud A, Perroux R, Cluzel R. 1987. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel β-lactamase. J Antimicrob Chemother 20:323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- 116.Jacoby GA, Medeiros AA, O'Brien TF, Pinto ME, Jiang H. 1988. Broad-spectrum, transmissible β-lactamases. N Engl J Med 319:723–723. doi: 10.1056/NEJM198809153191114. [DOI] [PubMed] [Google Scholar]
- 117.Meyer KS, Urban C, Eagan JA, Berger BJ, Rahal JJ. 1993. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann Intern Med 119:353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- 118.Zhou XY, Bordon F, Sirot D, Kitzis M-D, Gutmann L. 1994. Emergence of clinical isolates of Escherichia coli producing TEM-1 derivatives or an OXA-1β-lactamase conferring resistance to β-lactamase inhibitors. Antimicrob Agents Chemother 38:1085–1089. doi: 10.1128/AAC.38.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prinarakis EE, Miriagou V, Tzelepi E, Gazouli M, Tzouvelekis L. 1997. Emergence of an inhibitor-resistant β-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob Agents Chemother 41:838–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bret L, Chaibi EB, Chanal-Claris C, Sirot D, Labia R, Sirot J. 1997. Inhibitor-resistant TEM (IRT) β-lactamases with different substitutions at position 244. Antimicrob Agents Chemother 41:2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ríos E, Lopez MC, Rodriguez-Avial I, Pena I, Picazo JJ. 2015. Characterization of inhibitor-resistant TEM β-lactamases and mechanisms of fluoroquinolone resistance in Escherichia coli isolates. Microb Drug Resist 21:512–515. doi: 10.1089/mdr.2015.0039. [DOI] [PubMed] [Google Scholar]
- 122.Peirano G, van der Bij AK, Gregson DB, Pitout JD. 2012. Molecular epidemiology over an 11-year period (2000 to 2010) of extended-spectrum beta-lactamase-producing Escherichia coli causing bacteremia in a centralized Canadian region. J Clin Microbiol 50:294–299. doi: 10.1128/JCM.06025-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barrios H, Garza-Ramos U, Mejia-Miranda I, Reyna-Flores F, Sanchez-Perez A, Mosqueda-Garcia D, Silva-Sanchez J, Bacterial Resistance Consortium. 2017. ESBL-producing Escherichia coli and Klebsiella pneumoniae: the most prevalent clinical isolates obtained between 2005 and 2012 in Mexico. J Glob Antimicrob Resist 10:243–246. doi: 10.1016/j.jgar.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 124.Decousser JW, Poirel L, Nordmann P. 2001. Characterization of a chromosomally encoded extended-spectrum class A beta-lactamase from Kluyvera cryocrescens. Antimicrob Agents Chemother 45:3595–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Humeniuk C, Arlet G, Gautier V, Grimont P, Labia R, Philippon A. 2002. Beta-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob Agents Chemother 46:3045–3049. doi: 10.1128/AAC.46.9.3045-3049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poirel L, Kampfer P, Nordmann P. 2002. Chromosome-encoded Ambler class A beta-lactamase of Kluyvera georgiana, a probable progenitor of a subgroup of CTX-M extended-spectrum beta-lactamases. Antimicrob Agents Chemother 46:4038–4040. doi: 10.1128/AAC.46.12.4038-4040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Narchi H. 2005. Kluyvera urinary tract infection: case report and review of the literature. Pediatr Infect Dis J 24:570–572. doi: 10.1097/01.inf.0000164702.21798.22. [DOI] [PubMed] [Google Scholar]
- 128.Shen Z, Ding B, Bi Y, Wu S, Xu S, Xu X, Guo Q, Wang M. 2017. CTX-M-190, a novel beta-lactamase resistant to tazobactam and sulbactam, identified in an Escherichia coli clinical isolate. Antimicrob Agents Chemother 61:01. doi: 10.1128/AAC.01848-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai J, Cheng Q, Shen Y, Gu D, Fang Y, Chan EW, Chen S. 2017. Genetic and functional characterization of blaCTX-M-199, a novel tazobactam and sulbactam resistance-encoding gene located in a conjugative mcr-1-bearing IncI2 plasmid. Antimicrob Agents Chemother 61:e00562-17. doi: 10.1128/AAC.00562-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bontron S, Poirel L, Nordmann P. 2015. In vitro prediction of the evolution of GES-1 beta-lactamase hydrolytic activity. Antimicrob Agents Chemother 59:1664–1670. doi: 10.1128/AAC.04450-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Poirel L, Le Thomas I, Naas T, Karim A, Nordmann P. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum beta-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob Agents Chemother 44:622–632. doi: 10.1128/AAC.44.3.622-632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bush K. 2013. Carbapenemases: partners in crime. J Glob Antimicrob Resist 1:7–16. doi: 10.1016/j.jgar.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 133.Wang JT, Chang SC, Chang FY, Fung CP, Chuang YC, Chen YS, Shiau YR, Tan MC, Wang HY, Lai JF, Huang IW, Yang Lauderdale TL. 2015. Antimicrobial non-susceptibility of Escherichia coli from outpatients and patients visiting emergency rooms in Taiwan. PLoS One 10:e0144103. doi: 10.1371/journal.pone.0144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shaw E, Rombauts A, Tubau F, Padulles A, Camara J, Lozano T, Cobo-Sacristan S, Sabe N, Grau I, Rigo-Bonnin R, Dominguez MA, Carratala J. 2018. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother 73:1104–1106. doi: 10.1093/jac/dkx496. [DOI] [PubMed] [Google Scholar]
- 135.Bou G, Martinez-Beltran J. 2000. Cloning, nucleotide sequencing, and analysis of the gene encoding an AmpC β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother 44:428–432. doi: 10.1128/AAC.44.2.428-432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodríguez-Martínez JM, Poirel L, Nordmann P. 2009. Extended-spectrum cephalosporinases in Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:1766–1771. doi: 10.1128/AAC.01410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sabath LD, Jago M, Abraham EP. 1965. Cephalosporinase and penicillinase activity of beta-lactamase from Pseudomonas pyocyanea. Biochem J 96:739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vu H, Nikaido H. 1985. Role of β-lactam hydrolysis in the mechanism of resistance of a β-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum β-lactams. Antimicrob Agents Chemother 27:393–398. doi: 10.1128/AAC.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bush K, Tanaka SK, Bonner DP, Sykes RB. 1985. Resistance caused by decreased penetration of β-lactam antibiotics into Enterobacter cloacae. Antimicrob Agents Chemother 27:555–560. doi: 10.1128/AAC.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee EH, Nicolas MH, Kitzis MD, Pialoux G, Collatz E, Gutmann L. 1991. Association of two resistance mechanisms in a high-level imipenem-resistant clinical isolate of Enterobacter cloacae. Antimicrob Agents Chemother 35:1093–1098. doi: 10.1128/AAC.35.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Papanicolaou G, Medeiros AA, Jacoby GA. 1990. Novel plasmid-mediated β-lactamase (MIR-1) conferring resistance to oxyimino- and α-methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother 34:2200–2209. doi: 10.1128/AAC.34.11.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cao VT, Arlet G, Ericsson BM, Tammelin A, Courvalin P, Lambert T. 2000. Emergence of imipenem resistance in Klebsiella pneumoniae owing to combination of plasmid-mediated CMY-4 and permeability alteration. J Antimicrob Chemother 46:895–900. doi: 10.1093/jac/46.6.895. [DOI] [PubMed] [Google Scholar]
- 143.Rahal JJ, Urban C, Horn D, Freeman K, Segal-Maurer S, Maurer J, Mariano N, Marks S, Burns JM, Dominick D, Lim M. 1998. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 280:1233–1237. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 144.Bradford PA, Urban C, Mariano N, Projan SJ, Rahal JJ, Bush K. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC beta-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother 41:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Urban C, Go E, Mariano N, Rahal JJ. 1995. Interaction of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and -susceptible Acinetobacter baumannii. FEMS Microbiol Lett 125:193–198. doi: 10.1111/j.1574-6968.1995.tb07357.x. [DOI] [Google Scholar]
- 146.Cullmann W, Dick W. 1990. Heterogeneity of beta-lactamase production in Pseudomonas maltophilia, a nosocomial pathogen. Chemotherapy 36:117–126. doi: 10.1159/000238757. [DOI] [PubMed] [Google Scholar]
- 147.Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang Y, Wu P, Livermore DM. 1990. Biochemical characterization of a β-lactamase that hydrolyzes penems and carbapenems for two Serratia marcescens isolates. Antimicrob Agents Chemother 34:755–758. doi: 10.1128/AAC.34.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nordmann P, Mariotte S, Naas T, Labia R, Nicolas M-H. 1993. Biochemical properties of a carbapenem-hydrolyzing β-lactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob Agents Chemother 37:939–946. doi: 10.1128/AAC.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Medeiros AA, Hare RS. 1986. Beta-lactamase mediated resistance to penems and carbapenems amongst Enterobacteriaceae, abstr 116. 26th Intersci Conf Antimicrob Agents Chemother, 28 September to 1 October 1986, New Orleans, LA. [Google Scholar]
- 151.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 35:147–151. doi: 10.1128/AAC.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini GM. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother 43:1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, Peto TEA, Crook DW, Stoesser N. 2017. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections-a systematic review of the literature. Clin Infect Dis 64:1435–1444. doi: 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- 154.Breathnach AS, Cubbon MD, Karunaharan RN, Pope CF, Planche TD. 2012. Multidrug-resistant Pseudomonas aeruginosa outbreaks in two hospitals: association with contaminated hospital waste-water systems. J Hosp Infect 82:19–24. doi: 10.1016/j.jhin.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 155.Turton JF, Wright L, Underwood A, Witney AA, Chan YT, Al-Shahib A, Arnold C, Doumith M, Patel B, Planche TD, Green J, Holliman R, Woodford N. 2015. High-resolution analysis by whole-genome sequencing of an international lineage (sequence type 111) of Pseudomonas aeruginosa associated with metallo-carbapenemases in the United Kingdom. J Clin Microbiol 53:2622–2631. doi: 10.1128/JCM.00505-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chouchani C, Marrakchi R, Ferchichi L, El Salabi A, Walsh TR. 2011. VIM and IMP metallo-beta-lactamases and other extended-spectrum beta-lactamases in Escherichia coli and Klebsiella pneumoniae from environmental samples in a Tunisian hospital. APMIS 119:725–732. doi: 10.1111/j.1600-0463.2011.02793.x. [DOI] [PubMed] [Google Scholar]
- 157.Herbert S, Halvorsen DS, Leong T, Franklin C, Harrington G, Spelman D. 2007. Large outbreak of infection and colonization with gram-negative pathogens carrying the metallo-β-lactamase gene blaIMP-4 at a 320-bed tertiary hospital in Australia. Infect Control Hosp Epidemiol 28:98–101. doi: 10.1086/508841. [DOI] [PubMed] [Google Scholar]
- 158.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yigit H, Queenan AM, Rasheed JK, Biddle JW, Domenech-Sanchez A, Alberti S, Bush K, Tenover FC. 2003. Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing beta-lactamase KPC-2. Antimicrob Agents Chemother 47:3881–3889. doi: 10.1128/AAC.47.12.3881-3889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, Rahal JJ, Brooks S, Cebular S, Quale J. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin Infect Dis 39:55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]
- 161.Woodford N, Tierno PM Jr, Young K, Tysall L, Palepou MF, Ward E, Painter RE, Suber DF, Shungu D, Silver LL, Inglima K, Kornblum J, Livermore DM. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 48:4793–4799. doi: 10.1128/AAC.48.12.4793-4799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leavitt A, Navon-Venezia S, Chmelnitsky I, Schwaber MJ, Carmeli Y. 2007. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother 51:3026–3029. doi: 10.1128/AAC.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kazmierczak KM, Biedenbach DJ, Hackel M, Rabine S, de Jonge BL, Bouchillon SK, Sahm DF, Bradford PA. 2016. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother 60:4490–4500. doi: 10.1128/AAC.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y, Brolund A, Giske CG. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 53:3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, Bonomo RA, Musser JM, Kreiswirth BN. 2014. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U S A 111:4988–4993. doi: 10.1073/pnas.1321364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, Mantengoli E, Saffioti C, Pagani N, Tedeschi S, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viale P, Viscoli C, Tumbarello M, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). 2015. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 21:1106.e1–8. doi: 10.1016/j.cmi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 169.Queenan AM, Torres-Viera C, Gold HS, Carmeli Y, Eliopoulos GM, Moellering RC, Quinn JP, Hindler J, Medeiros AA, Bush K. 2000. SME-type carbapenem-hydrolyzing class A beta-lactamases from geographically diverse Serratia marcescens strains. Antimicrob Agents Chemother 44:3035–3039. doi: 10.1128/AAC.44.11.3035-3039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hemarajata P, Amick T, Yang S, Gregson E, Holzmeyer C, Bush K, Humphries RM. 2018. Selection of hyperproduction of AmpC and SME-1 in a carbapenem-resistant Serratia marcescens isolate during antibiotic therapy. J Antimicrob Chemother 73:1256–1262. doi: 10.1093/jac/dky028. [DOI] [PubMed] [Google Scholar]
- 171.Zagorianou A, Sianou E, Iosifidis E, Dimou V, Protonotariou E, Miyakis S, Roilides E, Sofianou D. 2012. Microbiological and molecular characteristics of carbapenemase-producing Klebsiella pneumoniae endemic in a tertiary Greek hospital during 2004–2010. Euro Surveill 17:pii=20088. doi: 10.2807/ese.17.07.20088-en. [DOI] [PubMed] [Google Scholar]
- 172.Liao IC, Chen HM, Wu JJ, Tsai PF, Wang LR, Yan JJ. 2011. Metallo-beta-lactamase-producing Enterobacteriaceae isolates at a Taiwanese hospital: lack of distinctive phenotypes for screening. APMIS 119:543–550. doi: 10.1111/j.1600-0463.2011.02772.x. [DOI] [PubMed] [Google Scholar]
- 173.Fukigai S, Alba J, Kimura S, Iida T, Nishikura N, Ishii Y, Yamaguchi K. 2007. Nosocomial outbreak of genetically related IMP-1 beta-lactamase-producing Klebsiella pneumoniae in a general hospital in Japan. Int J Antimicrob Agents 29:306–310. doi: 10.1016/j.ijantimicag.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 174.Mano Y, Saga T, Ishii Y, Yoshizumi A, Bonomo RA, Yamaguchi K, Tateda K. 2015. Molecular analysis of the integrons of metallo-beta-lactamase-producing Pseudomonas aeruginosa isolates collected by nationwide surveillance programs across Japan. BMC Microbiol 15:41. doi: 10.1186/s12866-015-0378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. 2011. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program (2006—2007). Antimicrob Agents Chemother 55:1274–1278. doi: 10.1128/AAC.01497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 178.Lübbert C, Baars C, Dayakar A, Lippmann N, Rodloff AC, Kinzig M, Sorgel F. 2017. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection 45:479–491. doi: 10.1007/s15010-017-1007-2. [DOI] [PubMed] [Google Scholar]
- 179.Poirel L, Heritier C, Tolun V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sader HS, Mendes RE, Pfaller MA, Shortridge D, Flamm RK, Castanheira M. 2018. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) clinical Enterobacteriaceae isolates. Antimicrob Agents Chemother 62:e01856-17. doi: 10.1128/AAC.00311-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gauthier L, Dortet L, Cotellon G, Creton E, Cuzon G, Ponties V, Bonnin RA, Naas T. 2018. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012–2013. Antimicrob Agents Chemother 62:e00266-18. doi: 10.1128/AAC.00266-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ortega A, Saez D, Bautista V, Fernandez-Romero S, Lara N, Aracil B, Perez-Vazquez M, Campos J, Oteo J, Spanish Collaborating Group for the Antibiotic Resistance Surveillance Programme. 2016. Carbapenemase-producing Escherichia coli is becoming more prevalent in Spain mainly because of the polyclonal dissemination of OXA-48. J Antimicrob Chemother 71:2131–2138. doi: 10.1093/jac/dkw148. [DOI] [PubMed] [Google Scholar]
- 183.Arana DM, Ortega A, Gonzalez-Barbera E, Lara N, Bautista V, Gomez-Ruiz D, Saez D, Fernandez-Romero S, Aracil B, Perez-Vazquez M, Campos J, Oteo J, Spanish Antibiotic Resistance Surveillance Programme Collaborating Group. 2017. Carbapenem-resistant Citrobacter spp. isolated in Spain from 2013 to 2015 produced a variety of carbapenemases including VIM-1, OXA-48, KPC-2, NDM-1 and VIM-2. J Antimicrob Chemother 72:3283–3287. doi: 10.1093/jac/dkx325. [DOI] [PubMed] [Google Scholar]
- 184.Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol 44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Périchon B, Goussard S, Walewski V, Krizova L, Cerqueira G, Murphy C, Feldgarden M, Wortman J, Clermont D, Nemec A, Courvalin P. 2014. Identification of 50 class D β-lactamases and 65 Acinetobacter-derived cephalosporinases in Acinetobacter spp. Antimicrob Agents Chemother 58:936–949. doi: 10.1128/AAC.01261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bonomo RA, Szabo D. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis 43(Suppl 2):S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 187.Bush K. 1988. Recent developments in β-lactamase research and their implications for the future. Rev Infect Dis 10:681–690. doi: 10.1093/clinids/10.4.681. [DOI] [PubMed] [Google Scholar]
- 188.Thomson G, Turner D, Brasso W, Kircher S, Guillet T, Thomson K. 2017. High-stringency evaluation of the automated BD Phoenix CPO detect and Rapidec Carba NP tests for detection and classification of carbapenemases. J Clin Microbiol 55:3437–3443. doi: 10.1128/JCM.01215-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Antunes NT, Lamoureaux TL, Toth M, Stewart NK, Frase H, Vakulenko SB. 2014. Class D β-lactamases: are they all carbapenemases? Antimicrob Agents Chemother 58:2119–2125. doi: 10.1128/AAC.02522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hujer KM, Hamza NS, Hujer AM, Perez F, Helfand MS, Bethel CR, Thomson JM, Anderson VE, Barlow M, Rice LB, Tenover FC, Bonomo RA. 2005. Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 beta-lactamase: defining a unique family of class C enzymes. Antimicrob Agents Chemother 49:2941–2948. doi: 10.1128/AAC.49.7.2941-2948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Winkler ML, Papp-Wallace KM, Hujer AM, Domitrovic TN, Hujer KM, Hurless KN, Tuohy M, Halld G, Bonomo RA. 2015. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 59:1020–1029. doi: 10.1128/AAC.04238-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hall RM, Schwarz S. 2016. Resistance gene naming and numbering: is it a new gene or not? J Antimicrob Chemother 71:569–571. doi: 10.1093/jac/dkv351. [DOI] [PubMed] [Google Scholar]
- 193.Jacoby GA, Bonomo RA, Bradford PA, Bush K, Doi Y, Feldgarden M, Haft D, Klimke W, Nordmann P, Palzkill T, Poirel L, Prasad A, Rossolini GM, Walsh T. 2016. Comment on: resistance gene naming and numbering: is it a new gene or not? J Antimicrob Chemother 71:2677–2678. doi: 10.1093/jac/dkw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Giske CG, Sundsfjord AS, Kahlmeter G, Woodford N, Nordmann P, Paterson DL, Canton R, Walsh TR. 2009. Redefining extended-spectrum β-lactamases: balancing science and clinical need. J Antimicrob Chemother 63:1–4. doi: 10.1093/jac/dkn444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bush K, Jacoby GA, Amicosante G, Bonomo RA, Bradford P, Cornaglia G, Garau J, Giamarellou H, Jarlier V, Martinez-Martinez L, Miriagou V, Palzkill T, Pascual A, Rodriguez-Bano J, Rossolini GM, Sougakoff W, Vatopoulos A. 2009. Comment on: redefining extended-spectrum beta-lactamases: balancing science and clinical need. J Antimicrob Chemother 64:212–213. doi: 10.1093/jac/dkp104. [DOI] [PubMed] [Google Scholar]
- 196.Bhavnani SM, Ambrose PG, Craig WA, Dudley MN, Jones RN, SENTRY Antimicrobial Surveillance Program. 2006. Outcomes evaluation of patients with ESBL- and non-ESBL-producing Escherichia coli and Klebsiella species as defined by CLSI reference methods: report from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis 54:231–236. doi: 10.1016/j.diagmicrobio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 197.Naas T, Oueslati S, Bonnin RA, Dabos ML, Zavala A, Dortet L, Retailleau P, Iorga BI. 2017. Beta-lactamase database (BLDB)–structure and function. J Enzyme Inhib Med Chem 32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee D, Das S, Dawson NL, Dobrijevic D, Ward J, Orengo C. 2016. Novel computational protocols for functionally classifying and characterising serine β-lactamases. PLoS Comput Biol 12:e1004926. doi: 10.1371/journal.pcbi.1004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zeil C, Widmann M, Fademrecht S, Vogel C, Pleiss J. 2016. Network analysis of sequence-function relationships and exploration of sequence space of TEM β-lactamases. Antimicrob Agents Chemother 60:2709–2717. doi: 10.1128/AAC.02930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Öztürk H, Ozkirimli E, Özgür A. 2015. Classification of beta-lactamases and penicillin binding proteins using ligand-centric network models. PLoS One 10:e0117874. doi: 10.1371/journal.pone.0117874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bush K. 2013. Proliferation and significance of clinically relevant β-lactamases. Ann N Y Acad Sci 1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 202.Moran RA, Anantham S, Holt KE, Hall RM. 2017. Prediction of antibiotic resistance from antibiotic resistance genes detected in antibiotic-resistant commensal Escherichia coli using PCR or WGS. J Antimicrob Chemother 72:700–704. [DOI] [PubMed] [Google Scholar]
- 203.Bebrone C. 2007. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem Pharmacol 74:1686–1701. doi: 10.1016/j.bcp.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 204.Lopatkin AJ, Meredith HR, Srimani JK, Pfeiffer C, Durrett R, You L. 2017. Persistence and reversal of plasmid-mediated antibiotic resistance. Nat Commun 8:1689. doi: 10.1038/s41467-017-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Poirel L, Kieffer N, Nordmann P. 2016. In vitro evaluation of dual carbapenem combinations against carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 71:156–161. doi: 10.1093/jac/dkv294. [DOI] [PubMed] [Google Scholar]
- 206.de Oliveira DV, Nunes LS, Barth AL, Van Der Sand ST. 2017. Genetic background of β-lactamases in Enterobacteriaceae isolates from environmental samples. Microb Ecol 74:599–607. doi: 10.1007/s00248-017-0970-6. [DOI] [PubMed] [Google Scholar]
- 207.Vergara-López S, Dominguez MC, Conejo MC, Pascual A, Rodriguez-Bano J. 2013. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-beta-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect 19:E490–E498. doi: 10.1111/1469-0691.12288. [DOI] [PubMed] [Google Scholar]
- 208.Centers for Disease Control. 2013. Antibiotic resistance threats in the United States, 2013. Atlanta, GA. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 209.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, Dudley MN. 2017. Vaborbactam: spectrum of β-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 61:e01443-17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Castanheira M, Huband MD, Mendes RE, Flamm RK. 2017. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 61:e00567-17. doi: 10.1128/AAC.00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bush K, Page MG. 2017. What we may expect from novel antibacterial agents in the pipeline with respect to resistance and pharmacodynamic principles. J Pharmacokinet Pharmacodyn 44:113–132. doi: 10.1007/s10928-017-9506-4. [DOI] [PubMed] [Google Scholar]
- 215.Anderson ES, Datta N. 1965. Resistance to penicillins and its transfer in Enterobacteriaceae. Lancet i:407–409. [DOI] [PubMed] [Google Scholar]
- 216.Pitton JS. 1972. Mechanisms of bacterial resistance to antibiotics, p 15–93. In Adrian RH. (ed), Reviews of physiology, vol 65 Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 217.Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- 218.Sykes RB, Matthew M. 1976. The β-lactamases of Gram-negative bacteria and their role in resistance to β-lactam antibiotics. J Antimicrob Chemother 2:115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- 219.Bush K. 1989. Characterization of β-lactamases. Antimicrob Agents Chemother 33:259–263. doi: 10.1128/AAC.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bush K. 1989. Classification of β-lactamases: groups 1, 2a, 2b, and 2b′. Antimicrob Agents Chemother 33:264–270. doi: 10.1128/AAC.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bush K. 1989. Classification of β-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother 33:271–276. doi: 10.1128/AAC.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acid Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.McArthur AG, Waglechner N, Nizam F, Yan A, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, Kalan L, King AM, Koteva K, Morar M, Mulvey MR, O'Brien JS, Pawlowski AC, Piddock LJ, Spanogiannopoulos P, Sutherland AD, Tang I, Taylor PL, Thaker M, Wang W, Yan M, Yu T, Wright GD. 2013. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother 57:3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bush K, Fisher JF. 2011. Epidemiological expansion, structural studies, and clinical challenges of new beta-lactamases from Gram-negative bacteria. Annu Rev Microbiol 65:455–478. doi: 10.1146/annurev-micro-090110-102911. [DOI] [PubMed] [Google Scholar]