LYS228 is a novel monobactam with potent activity against Enterobacteriaceae. LYS228 is stable to metallo-β-lactamases (MBLs) and serine carbapenemases, including Klebsiella pneumoniae carbapenemases (KPCs), resulting in potency against the majority of extended-spectrum β-lactamase (ESBL)-producing and carbapenem-resistant Enterobacteriaceae strains tested.
KEYWORDS: Enterobacteriaceae, LYS228, monobactams
ABSTRACT
LYS228 is a novel monobactam with potent activity against Enterobacteriaceae. LYS228 is stable to metallo-β-lactamases (MBLs) and serine carbapenemases, including Klebsiella pneumoniae carbapenemases (KPCs), resulting in potency against the majority of extended-spectrum β-lactamase (ESBL)-producing and carbapenem-resistant Enterobacteriaceae strains tested. Overall, LYS228 demonstrated potent activity against 271 Enterobacteriaceae strains, including multidrug-resistant isolates. Based on MIC90 values, LYS228 (MIC90, 1 μg/ml) was ≥32-fold more active against those strains than were aztreonam, ceftazidime, ceftazidime-avibactam, cefepime, and meropenem. The tigecycline MIC90 was 4 μg/ml against the strains tested. Against Enterobacteriaceae isolates expressing ESBLs (n = 37) or displaying carbapenem resistance (n = 77), LYS228 had MIC90 values of 1 and 4 μg/ml, respectively. LYS228 exhibited potent bactericidal activity, as indicated by low minimal bactericidal concentration (MBC) to MIC ratios (MBC/MIC ratios of ≤4) against 97.4% of the Enterobacteriaceae strains tested (264/271 strains). In time-kill studies, LYS228 consistently achieved reductions in CFU per milliliter of 3 log10 units (≥99.9% killing) at concentrations ≥4× MIC for Escherichia coli and K. pneumoniae reference strains, as well as isolates encoding TEM-1, SHV-1, CTX-M-14, CTX-M-15, KPC-2, KPC-3, and NDM-1 β-lactamases.
INTRODUCTION
Over the past 2 decades, the increasing prevalence of Gram-negative bacterial isolates expressing extended-spectrum β-lactamases (ESBLs) has eroded the effectiveness of many β-lactams, the most widely used class of antibiotics (1, 2); this has increased the usage of the carbapenem class of β-lactams as first-line therapy. Consequently, carbapenem-resistant Enterobacteriaceae (CRE) now pose yet another significant challenge. In the United States, isolates producing Klebsiella pneumoniae carbapenemase (KPC) are now endemic, and numerous large-scale outbreaks have been reported (3–6). The emergence of the New Delhi metallo-β-lactamase (NDM-1) in K. pneumoniae (7), and then in Escherichia coli, has also contributed to the reduced effectiveness of carbapenems (8–10). Such strains are frequently also resistant to other classes of antibiotics, including aminoglycosides, fluoroquinolones, and tetracyclines, and require treatment with poorly tolerated antibiotics, such as colistin, as a last resort (11, 12). Therefore, the recent identification of CRE strains that are colistin resistant, via transmissible elements such as the mobilized colistin resistance gene (e.g., MCR-1), is alarming (13). The inability to effectively treat infections caused by antibiotic-resistant Gram-negative bacteria has translated into increased morbidity and mortality rates (14–17).
Monocyclic β-lactams, such as monobactams, are a class of β-lactam antibiotics that are intrinsically stable to metallo-β-lactamases (MBLs). Unfortunately, aztreonam, the only monobactam that is clinically available in the United States and Europe, is susceptible to many serine β-lactamases (SBLs) (18); this limits the clinical use of aztreonam, since MBLs and SBLs are frequently found together in the same clinical isolates (2). Given the intrinsic stability of monobactams to MBLs and the reported liability of monobactams to inactivation by SBLs, we initiated a program to enhance the intrinsic stability of monobactams to SBLs through structural modification of the monobactam ring. Our efforts led to the identification of LYS228 (Fig. 1). LYS228 is a single-agent monobactam that is effective against Enterobacteriaceae strains, including those expressing ESBLs, SBLs, and MBLs (19, 20). This study describes the in vitro antibacterial potency and bactericidal activity of LYS228 against E. coli, K. pneumoniae, and other members of Enterobacteriaceae.
FIG 1.
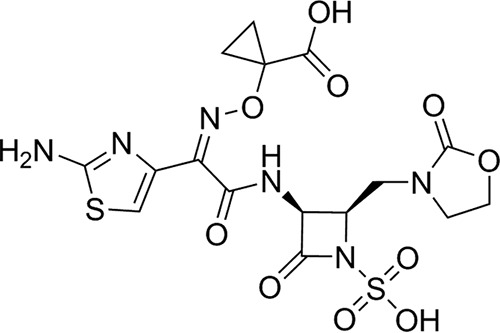
Chemical structure of LYS228.
(This work was presented, in part, at the 2017 American Society of Microbiology Microbe Meeting, New Orleans, LA.)
RESULTS AND DISCUSSION
LYS228 is a novel monobactam antibiotic. Here, we tested the in vitro antibacterial activity of LYS228 against strains with different resistance genotypes and phenotypes; some isolates produce β-lactamase enzymes with narrow substrate specificity (TEM-1), while others produce broad-spectrum enzymes (including KPCs, CTX-M, and NDM-1). LYS228 was active against a majority of the isolates tested, with MIC50 and MIC90 values of 0.25 and 1 μg/ml, respectively (Table 1). The MICs determined for LYS228 and comparators against Enterobacteriaceae are summarized in Table 2. Based on MIC90 comparisons, LYS228 was ≥32-fold more active than aztreonam, ceftazidime, ceftazidime-avibactam, cefepime, and meropenem (Table 2). LYS228 was 4-fold more potent than tigecycline, for which a MIC90 value of 4 μg/ml was observed. Overall, 95.9%, 97.9%, and 98.9% of the 271 isolates were inhibited by LYS228 concentrations of ≤2, ≤4, and ≤8 μg/ml, respectively (Table 1).
TABLE 1.
LYS228 MIC distribution among 271 Enterobacteriaceae isolates, by resistance genotype and species
| Isolate | No. (cumulative %) of isolates inhibited at MIC of: |
MIC (μg/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.06 μg/ml | 0.12 μg/ml | 0.25 μg/ml | 0.5 μg/ml | 1 μg/ml | 2 μg/ml | 4 μg/ml | 8 μg/ml | 16 μg/ml | 32 μg/ml | 64 μg/ml | MIC50 | MIC90 | |
| All (n = 271) | 70 (25.8) | 48 (43.5) | 49 (61.6) | 44 (77.9) | 35 (90.8) | 14 (95.9) | 5 (97.9) | 3 (98.9) | 2 (99.6) | 0 (99.6) | 1 (100) | 0.25 | 1 |
| ESBL producers (n = 37)a | 13 (35.1) | 8 (56.8) | 8 (78.4) | 1 (81.1) | 4 (91.9) | 1 (94.6) | 1 (97.3) | 1 (100) | 0.12 | 1 | |||
| KPC producers (n = 46)b | 4 (8.7) | 3 (15.2) | 4 (23.9) | 22 (71.7) | 8 (89.1) | 4 (97.8) | 1 (100) | 0.5 | 2 | ||||
| MBL producers (n = 33)c | 1 (3.0) | 4 (15.2) | 7 (36.4) | 8 (60.1) | 6 (78.8) | 2 (84.8) | 2 (90.9) | 1 (93.9) | 2 (100) | 0.5 | 4 | ||
| Citrobacter spp. (n = 22)d | 2 (9.1) | 2 (18.2) | 4 (36.4) | 3 (50.0) | 8 (86.4) | 3 (100) | 0.5 | 2 | |||||
| Enterobacter spp. (n = 40)e | 1 (2.5) | 2 (7.5) | 4 (17.5) | 14 (52.5) | 11 (80.0) | 6 (95.0) | 2 (100) | 0.5 | 2 | ||||
| E. coli (n = 59) | 14 (23.7) | 17 (52.5) | 13 (74.6) | 6 (84.7) | 4 (91.5) | 1 (93.2) | 2 (96.6) | 2 (100) | 0.12 | 1 | |||
| K. pneumoniae (n = 81) | 13 (16.0) | 13 (32.1) | 18 (54.3) | 20 (79.0) | 9 (90.1) | 5 (96.3) | 1 (97.5) | 1 (98.8) | 1 (100) | 0.25 | 1 | ||
| M. morganii (n = 14) | 10 (71.4) | 1 (78.6) | 2 (92.9) | 1 (100) | ≤0.06 | 0.25 | |||||||
| P. mirabilis (n = 22) | 20 (90.9) | 2 (100) | ≤0.06 | ≤0.06 | |||||||||
| S. marcescens (n = 13) | 1 (7.7) | 6 (53.8) | 4 (84.6) | 1 (92.3) | 1 (100) | 0.12 | 1 | ||||||
| Salmonella spp. (n = 16) | 7 (43.8) | 4 (68.8) | 3 (87.5) | 2 (100) | 0.12 | 1 | |||||||
Strains tested included C. freundii (n = 2), C. koseri (n = 1), E. aerogenes (n = 1), E. coli (n = 11), K. pneumoniae (n = 8), M. morganii (n = 2), P. mirabilis (n = 6), Salmonella spp. (n = 5), and S. marcescens (n = 1).
Strains tested included C. freundii (n = 1), E. cloacae (n = 4), E. hormaechei (n = 1), E. coli (n = 4), and K. pneumoniae (n = 36).
Strains tested included C. freundii (n = 1), E. aerogenes (n = 1), E. cloacae (n = 10), E. coli (n = 6), K. pneumoniae (n = 13), P. mirabilis (n = 1), and S. marcescens (n = 1).
Strains tested included C. freundii (n = 21) and C. koseri (n = 1).
Strains tested included E. aerogenes (n = 4), E. cloacae (n = 35), and E. hormaechei (n = 1).
TABLE 2.
In vitro activity of LYS228 against Enterobacteriaceae isolates
| Isolate type and test agent | MIC (μg/ml) |
% susceptiblea | ||
|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||
| Enterobacteriaceae (n = 271) | ||||
| LYS228 | ≤0.06 to 64 | 0.25 | 1 | NAb |
| Aztreonam | ≤0.06 to >64 | 16 | >64 | 45.0 |
| Ceftazidime | ≤0.06 to >64 | 16 | >64 | 42.8 |
| Ceftazidime-avibactam | ≤0.06/4 to >64/4 | 0.5/4 | >64/4 | 87.1 |
| Cefepime | ≤0.06 to >64 | 2 | >64 | 52.0 |
| Meropenem | ≤0.06 to >64 | 0.12 | 32 | 68.6 |
| Tigecycline | 0.12 to 8 | 1 | 4 | 86.0 |
| CRE isolates (n = 77)c | ||||
| LYS228 | ≤0.06 to 64 | 0.5 | 4 | NA |
| Aztreonam | ≤0.06 to >64 | >64 | >64 | 5.2 |
| Ceftazidime | 1 to >64 | >64 | >64 | 3.9 |
| Ceftazidime-avibactam | ≤0.06/4 to >64/4 | 2/4 | >64/4 | 66.2 |
| Cefepime | ≤0.06 to >64 | >64 | >64 | 3.9 |
| Meropenem | 4 to >64 | 32 | >64 | 0.0 |
| Tigecycline | 0.125 to 8 | 1 | 4 | 87.0 |
| Citrobacter spp. (n = 22)d | ||||
| LYS228 | ≤0.06 to 2 | 0.5 | 2 | NA |
| Aztreonam | 0.12 to >64 | 64 | >64 | 27.7 |
| Ceftazidime | 0.5 to >64 | 64 | >64 | 27.3 |
| Ceftazidime-avibactam | ≤0.06/4 to >64/4 | 0.5/4 | 2/4 | 95.5 |
| Cefepime | ≤0.06 to >64 | 1 | >64 | 72.7 |
| Meropenem | ≤0.06 to >64 | ≤0.06 | 4 | 86.4 |
| Tigecycline | 0.25 to 2 | 0.5 | 2 | 100 |
| Enterobacter spp. (n = 40)e | ||||
| LYS228 | ≤0.06 to 4 | 0.5 | 2 | NA |
| Aztreonam | 0.25 to >64 | >64 | >64 | 17.5 |
| Ceftazidime | 0.25 to >64 | >64 | >64 | 15.0 |
| Ceftazidime-avibactam | 0.12/4 to >64/4 | 2/4 | >64/4 | 65.0 |
| Cefepime | ≤0.06 to >64 | 16 | >64 | 35.0 |
| Meropenem | ≤0.06 to >64 | 0.5 | 64 | 57.5 |
| Tigecycline | 0.25 to 8 | 1 | 4 | 77.5 |
| E. coli (n = 59) | ||||
| LYS228 | ≤0.06 to 16 | 0.12 | 1 | NA |
| Aztreonam | ≤0.06 to >64 | 0.25 | >64 | 59.3 |
| Ceftazidime | 0.12 to >64 | 0.5 | >64 | 62.7 |
| Ceftazidime-avibactam | ≤0.06/4 to >64/4 | 0.12/4 | >64/4 | 89.8 |
| Cefepime | ≤0.06 to >64 | 0.25 | >64 | 62.7 |
| Meropenem | ≤0.06 to >64 | ≤0.06 | 32 | 83.1 |
| Tigecycline | 0.12 to 1 | 0.25 | 0.5 | 100 |
| K. pneumoniae (n = 81) | ||||
| LYS228 | ≤0.06 to 64 | 0.25 | 1 | NA |
| Aztreonam | ≤0.06 to >64 | >64 | >64 | 27.2 |
| Ceftazidime | 0.12 to >64 | >64 | >64 | 25.9 |
| Ceftazidime-avibactam | ≤0.06/4 to >64/4 | 1/4 | >64/4 | 84.0 |
| Cefepime | ≤0.06 to >64 | 32 | >64 | 32.1 |
| Meropenem | ≤0.06 to >64 | 8 | 64 | 38.3 |
| Tigecycline | 0.12 to 8 | 1 | 4 | 86.4 |
| M. morganii (n = 14) | ||||
| LYS228 | ≤0.06 to 0.5 | ≤0.06 | 0.25 | NA |
| Aztreonam | ≤0.06 to 32 | 0.12 | 16 | 78.6 |
| Ceftazidime | 0.12 to 32 | 1 | 32 | 57.1 |
| Ceftazidime-avibactam | ≤0.06/4 to 1/4 | 0.12/4 | 0.5/4 | 100 |
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.5 | 92.9 |
| Meropenem | ≤0.06 to 0.5 | 0.12 | 0.25 | 100 |
| Tigecycline | 0.5 to 8 | 2 | 8 | 78.6 |
| P. mirabilis (n = 22) | ||||
| LYS228 | ≤0.06 to 0.12 | ≤0.06 | ≤0.06 | NA |
| Aztreonam | ≤0.06 to >64 | ≤0.06 | 2 | 90.9 |
| Ceftazidime | ≤0.06 to 32 | 0.25 | 8 | 81.8 |
| Ceftazidime-avibactam | ≤0.06/4 to 4/4 | ≤0.06/4 | 0.12/4 | 100 |
| Cefepime | ≤0.06 to >64 | 0.5 | >64 | 63.6 |
| Meropenem | 0.12 to 16 | 0.12 | 0.25 | 95.5 |
| Tigecycline | 1 to 8 | 4 | 8 | 36.4 |
| S. marcescens (n = 13) | ||||
| LYS228 | ≤0.06 to 4 | 0.12 | 1 | NA |
| Aztreonam | 0.12 to >64 | 2 | >64 | 69.2 |
| Ceftazidime | 0.12 to >64 | 1 | 32 | 61.5 |
| Ceftazidime-avibactam | 0.12/4 to >64/4 | 0.25/4 | 2/4 | 92.3 |
| Cefepime | ≤0.06 to >64 | 0.25 | >64 | 61.5 |
| Meropenem | ≤0.06 to 64 | 0.25 | 32 | 69.2 |
| Tigecycline | 0.5 to 8 | 1 | 2 | 92.3 |
| Salmonella spp. (n = 16) | ||||
| LYS228 | ≤0.06 to 1 | 0.12 | 1 | NA |
| Aztreonam | ≤0.06 to >64 | 0.25 | >64 | 56.3 |
| Ceftazidime | 0.25 to >64 | 0.5 | >64 | 56.3 |
| Ceftazidime-avibactam | 0.12/4 to 2/4 | 0.25/4 | 1/4 | 100 |
| Cefepime | ≤0.06 to >64 | 0.5 | >64 | 62.5 |
| Meropenem | ≤0.06 to 0.12 | <0.06 | 0.12 | 100 |
| Tigecycline | 0.25 to 2 | 0.5 | 1 | 100 |
Susceptibility was defined by CLSI document M100 (21). In the absence of CLSI breakpoints, U.S. FDA breakpoints were applied (30, 31).
NA, not applicable. Susceptibility has not been defined for LYS228.
Strains tested included C. freundii (n = 3), E. aerogenes (n = 1), E. cloacae (n = 11), E. hormaechei (n = 1), E. coli (n = 10), K. pneumoniae (n = 47), P. mirabilis (n = 1), and S. marcescens (n = 3).
Strains tested included C. freundii (n = 21) and C. koseri (n = 1).
Strains tested included E. aerogenes (n = 4), E. cloacae (n = 35), and E. hormaechei (n = 1).
Among the 271 isolates tested, 37 strains encoded the following ESBLs: TEM-10, TEM-12, TEM-52, SHV-4, SHV-5, SHV-7, SHV-12, CTX-M-2, CTX-M-3, CTX-M-5, CTX-M-14, and CTX-M-15, as well as ESBLs that were not annotated but were identified by phenotypic screening (21). LYS228 MIC values ranged from ≤0.06 to 8 μg/ml, with MIC50 and MIC90 values of 0.12 and 1 μg/ml, respectively. A group of 46 molecularly characterized KPC-producing isolates, including 1 Citrobacter freundii isolate, 4 Enterobacter cloacae isolates, 1 Enterobacter hormaechei isolate, 4 E. coli isolates, and 36 K. pneumoniae isolates, were tested. These isolates were inhibited by LYS228 with MICs ranging from ≤0.06 to 4 μg/ml (MIC50, 0.5 μg/ml; MIC90, 2 μg/ml). Strains encoding molecularly characterized enzymes from the IMP, NDM, and VIM classes, as well as strains with uncharacterized genotypes that were positive for carbapenemase activity via both modified carbapenem inactivation method (mCIM) and eCIM carbapenemase identification tests (21), were defined as MBL producers. Against the 33 MBL-producing isolates included in this study, the LYS228 MIC50 and MIC90 values were 0.5 and 4 μg/ml, respectively.
The activity of LYS228 was evaluated against 77 strains that were defined as CRE and were resistant to meropenem (MICs of ≥4 μg/ml) under the current CLSI resistance breakpoint (21). LYS228 retained activity (MIC50, 0.5 μg/ml; MIC90, 4 μg/ml) against the CRE isolates tested (Table 2). The in vitro activity of LYS228 was superior to that of all β-lactam agents tested, as well as ceftazidime-avibactam (MIC90 values of >64 μg/ml). The tigecycline MIC90 with these strains was 4 μg/ml.
A very narrow range of LYS228 MIC values were recorded against the Citrobacter sp. isolates (n = 22), with the highest recorded value being 2 μg/ml. This group consisted of 2 species, namely, C. freundii (n = 21) and Citrobacter koseri (n = 1). LYS228 demonstrated potent activity, with MIC50 and MIC90 values of 0.5 and 2 μg/ml, respectively, against this genus (Table 2). Tested Enterobacter sp. isolates (n = 40) consisted of the following 3 species: Enterobacter aerogenes (n = 4), E. cloacae (n = 35), and E. hormaechei (n = 1). The MIC50 and MIC90 values for LYS228 against this group were 0.5 and 2 μg/ml, respectively; LYS228 MIC values ranged between ≤0.06 and 4 μg/ml (Table 1). LYS228 MIC50 and MIC90 values against 59 E. coli isolates were 0.12 and 1 μg/ml, respectively (Table 1); LYS228 was ≥128-fold more active than ceftazidime, ceftazidime-avibactam, and cefepime (MIC90 values of >64 μg/ml) and 32-fold more potent than meropenem (MIC90, 32 μg/ml), while the tigecycline MIC90 value against the E. coli strains tested was 0.5 μg/ml (Table 2). Against 81 K. pneumoniae isolates, LYS228 MIC50 and MIC90 values were 0.25 and 1 μg/ml, respectively (Table 1); LYS228 was 64-fold more potent than meropenem (MIC90, 64 μg/ml), and the tigecycline MIC90 value was 4 μg/ml (Table 2). LYS228 was very active against the 4 Klebsiella oxytoca isolates tested (MIC range, ≤0.06 to 0.12 μg/ml). The susceptibility profile for 1 of the 4 strains showed intermediate resistance to ceftazidime and cefepime and resistance to aztreonam, based on CLSI breakpoints (21). All 4 isolates were susceptible to ceftazidime-avibactam, meropenem, and tigecycline. Fourteen isolates of Morganella morganii were tested, and LYS228 was highly potent against those isolates, with MIC values ranging between ≤0.06 and 0.5 μg/ml. Five of those 14 isolates of M. morganii were resistant to ceftazidime (MIC90, 32 μg/ml) but susceptible to meropenem (MIC90, 0.25 μg/ml), ceftazidime-avibactam (MIC90, 0.5 μg/ml), and cefepime (MIC90, 0.5 μg/ml). The tigecycline MIC90 against M. morganii was 8 μg/ml (Table 2). LYS228 was highly active against the 22 isolates of Proteus mirabilis tested (MIC90, ≤0.06 μg/ml); the highest MIC value obtained for this group was 0.12 μg/ml (Table 1). Against Serratia marcescens (n = 13), LYS228 MIC values ranged from ≤0.06 to 4 μg/ml (MIC90, 1 μg/ml) (Table 1). While the MIC90 values for ceftazidime-avibactam and tigecycline were 2 μg/ml, the MIC90 values for the other tested comparators were high (MIC90 values of 32 to ≥64 μg/ml) (Table 2). LYS228 demonstrated potent activity, with MIC values ranging from ≤0.06 to 1 μg/ml and MIC50 and MIC90 values of 0.12 and 1 μg/ml, respectively, against 16 isolates of Salmonella spp.
The preclinical pharmacokinetic (PK) profile of LYS228 predicts a human PK profile similar to that of aztreonam (19). Based on the current CLSI resistance breakpoint for aztreonam (21), LYS228 showed elevated MIC values (MICs ranging from 16 to 64 μg/ml) against 3 of the 271 Enterobacteriaceae isolates tested (Table 1), i.e., 2 E. coli strains and 1 K. pneumoniae strain. The highest LYS228 MIC observed was 64 μg/ml for 1 strain of K. pneumoniae producing CTX-M-15, OXA-48, and VEB-1 β-lactamases. This finding is consistent with the instability of LYS228 to VEB-1, which we observed previously when LYS228 was profiled against a wide panel of isogenic strains producing individual β-lactamases (19). LYS228 MIC values of 16 μg/ml were obtained for 2 strains of E. coli producing NDM-1, and the MICs did not appear to be associated with the β-lactamases expressed in those isolates. Both isolates harbored a mutation in ftsI, encoding the YRIN amino acid insertion in penicillin-binding protein 3 (PBP3), which is known to reduce susceptibility to some monobactams in clinical isolates of E. coli expressing NDM-1 (22). This mechanism likely accounts, at least in part, for the elevated LYS228 MIC values observed for those 2 isolates.
The minimal bactericidal concentration (MBC) was defined as the lowest concentration of compound that produced a reduction in the CFU per milliliter of ≥99.9% (i.e., ≥3 log10 units) in 24 h, compared to the starting inoculum. Bactericidal activity was defined as a MBC/MIC ratio of ≤4. MBC values for LYS228 against the 271 isolates tested ranged from ≤0.06 to 64 μg/ml, with MBC50 and MBC90 values of 0.25 and 2 μg/ml, respectively. LYS228 MBC/MIC ratios for all isolates tested were equal to 1 (72.3%), 2 (19.2%), 4 (5.9%), or 8 (2.6%) (Table 3). For the 7 strains with MBC/MIC ratios of 8 (1 C. freundii, 1 E. cloacae, 1 E. coli, 2 K. pneumoniae, and 2 Salmonella sp. isolates), LYS228 MICs ranged from ≤0.06 to 1 μg/ml. These strains produced either a class A or a class C β-lactamase enzyme; none of them was a KPC or MBL producer. Overall, LYS228 was bactericidal against 264 (97.4%) of the 271 strains tested, as indicated by MBC/MIC ratios of ≤4 (Table 3). The other β-lactam agents tested had similarly low MBC/MIC ratios. In comparison, tigecycline, a bacteriostatic agent, had MBC/MIC ratios of >4 for 193 (71.2%) of the 271 strains tested. Time-kill studies were performed to characterize further the bactericidal activity of LYS228. Tested isolates included reference strains and clinical isolates encoding the following β-lactamases: TEM-1, SHV-1, CTX-M-14, CTX-M-15, KPC-2, KPC-3, and NDM-1. At concentrations ≥4× MIC, LYS228 produced reductions in CFU per milliliter of ≥3 log10 units for all 10 E. coli and K. pneumoniae strains tested (Table 4). Against an E. coli reference strain (ATCC 25922) and E. coli NB27236, expressing NDM-1, LYS228 was bactericidal at 2× MIC at 4 and 8 h; however, bacterial regrowth was observed at 24 h (Table 4). Reductions in CFU per milliliter of ≥3 log10 units were achieved by LYS228 at all concentrations tested (≥2× MIC) for strains of E. coli expressing TEM-1, CTX-M-15, and KPC-3. For the K. pneumoniae isolates tested, LYS228 conferred reductions in CFU per milliliter of ≥3 log10 units at concentrations of ≥0.5 μg/ml (≥4× MIC) for strain ATCC 43816 and strain NB29254, expressing SHV-1 (Table 4). Reductions in CFU per milliliter of ≥3 log10 units were achieved by LYS228 against K. pneumoniae strains NB29084 (CTX-M-14), NB29082 (KPC-2), and ATCC 1100975 (NDM-1) at all concentrations tested (Table 4).
TABLE 3.
Bactericidal activity of LYS228 against 271 Enterobacteriaceae isolates
| Test agent | No. (%) of isolates with MBC/MIC ratioa of: |
||||
|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | >8 | |
| LYS228 | 196 (72.3) | 52 (19.2) | 16 (5.9) | 7 (2.6) | |
| Aztreonam | 216 (79.7) | 37 (13.7) | 10 (3.7) | 8 (3.0) | |
| Ceftazidime | 223 (82.3) | 29 (10.7) | 12 (4.4) | 7 (2.6) | |
| Ceftazidime-avibactamb | 196 (72.3) | 51 (18.8) | 16 (5.9) | 8 (3.0) | |
| Cefepime | 194 (71.6) | 45 (16.6) | 16 (5.9) | 16 (5.9) | |
| Meropenem | 197 (72.7) | 40 (14.7) | 21 (7.7) | 13 (4.8) | |
| Tigecycline | 46 (17.0) | 20 (7.4) | 12 (4.4) | 14 (5.2) | 179 (66.1) |
For MBC/MIC ratio calculations, MIC and MBC values of ≤0.06 μg/ml were set to 0.06 μg/ml and MIC and MBC values of >64 μg/ml were set to 128 μg/ml.
Avibactam was tested at a fixed concentration of 4 μg/ml.
TABLE 4.
Kill kinetics of LYS228 and comparators against E. coli and K. pneumoniae strains expressing various β-lactamases
| Organism (β-lactamase) and test agent | MIC (μg/ml) | Concentration tested (μg/ml) | Δlog10 CFU/ml at: |
|||
|---|---|---|---|---|---|---|
| 2 h | 4 h | 8 h | 24 h | |||
| E. coli ATCC 25922 | ||||||
| LYS228 | 0.25 | 0.5 | −2.0 | −4.6 | −4.1 | 2.0 |
| 1 | −2.2 | −4.6 | −4.6 | −4.6 | ||
| 2 | −2.1 | −4.6 | −4.6 | −4.6 | ||
| Aztreonam | 0.12 | 0.25 | −2.1 | −3.8 | −3.8 | −4.4 |
| 1 | −1.7 | −4.1 | −4.4 | −4.4 | ||
| Meropenem | 0.03 | 0.06 | −1.6 | −3.8 | −4.4 | −4.4 |
| 0.25 | −3.6 | −4.1 | −4.4 | −4.4 | ||
| E. coli ATCC 35218 (TEM-1) | ||||||
| LYS228 | 0.06 | 0.12 | −2.8 | −3.8 | −4.6 | −4.9 |
| 0.25 | −2.8 | −4.1 | −4.9 | −4.9 | ||
| 0.5 | −2.9 | −3.9 | −4.1 | −4.9 | ||
| Meropenem | 0.03 | 0.06 | −2.2 | −2.9 | −4.1 | −4.1 |
| 0.25 | −2.5 | −3.0 | −4.1 | −4.1 | ||
| E. coli NB27235 (CTX-M-15) | ||||||
| LYS228 | 0.25 | 0.5 | −3.0 | −3.1 | −4.1 | −4.8 |
| 1 | −3.1 | −3.2 | −4.2 | −4.5 | ||
| 2 | −3.5 | −3.4 | −4.2 | −4.8 | ||
| Meropenem | 0.03 | 0.06 | −3.0 | −3.6 | −1.7 | 3.7 |
| 0.25 | −3.3 | −4.1 | −4.8 | −4.8 | ||
| E. coli NB27169 (KPC-3) | ||||||
| LYS228 | 0.5 | 1 | −0.2 | −2.1 | −2.6 | −4.2 |
| 2 | −1.0 | −2.2 | −2.9 | −4.9 | ||
| 4 | −1.2 | −2.0 | −2.9 | −4.3 | ||
| E. coli NB27236 (NDM-1) | ||||||
| LYS228 | 4 | 8 | −4.4 | −4.4 | −4.4 | −2.1 |
| 16 | −2.9 | −4.1 | −4.4 | −4.4 | ||
| 32 | −4.4 | −3.9 | −4.4 | −4.4 | ||
| K. pneumoniae ATCC 43816 | ||||||
| LYS228 | 0.06 | 0.12 | −0.7 | −1.9 | −0.9 | 2.7 |
| 0.25 | −1.0 | −1.7 | −3.2 | −4.6 | ||
| 0.5 | −1.1 | −2.1 | −3.3 | −4.6 | ||
| Aztreonam | 0.06 | 0.12 | −0.6 | −2.0 | −0.5 | 0.7 |
| 0.5 | −0.3 | −2.1 | −3.3 | −4.6 | ||
| Meropenem | 0.06 | 0.12 | −2.3 | −4.9 | −4.9 | −4.9 |
| 0.5 | −3.0 | −4.9 | −4.9 | −4.9 | ||
| K. pneumoniae NB29254 (SHV-1) | ||||||
| LYS228 | 0.12 | 0.25 | −1.7 | −2.6 | −4.9 | −1.4 |
| 0.5 | −1.9 | −2.9 | −4.9 | −4.9 | ||
| 1 | −1.7 | −2.7 | −4.9 | −4.9 | ||
| Meropenem | 0.03 | 0.06 | −2.3 | −3.4 | −4.9 | −1.8 |
| 0.25 | −2.8 | −3.7 | −4.9 | −4.9 | ||
| K. pneumoniae NB29084 (CTX-M-14) | ||||||
| LYS228 | 0.5 | 1 | −0.3 | −1.5 | −2.7 | −4.9 |
| 2 | −0.9 | −2.0 | −3.2 | −4.9 | ||
| 4 | −0.8 | −2.4 | −3.7 | −4.9 | ||
| Meropenem | 0.03 | 0.06 | −1.4 | −2.8 | −0.7 | 4.0 |
| 0.25 | −3.2 | −3.5 | −1.6 | 3.9 | ||
| K. pneumoniae NB29082 (KPC-2) | ||||||
| LYS228 | 0.5 | 1 | −1.4 | −2.4 | −4.5 | −5.0 |
| 2 | −1.6 | −2.5 | −4.2 | −5.0 | ||
| 4 | −1.6 | −2.8 | −4.1 | −5.0 | ||
| K. pneumoniae ATCC 1100975 (NDM-1) | ||||||
| LYS228 | 1 | 2 | −2.0 | −1.9 | −3.8 | −5.0 |
| 4 | −1.9 | −2.9 | −4.1 | −5.0 | ||
| 8 | −2.0 | −1.6 | −4.5 | −5.0 | ||
In conclusion, LYS228 is a novel monobactam with potent in vitro activity against a broad panel of clinically relevant Enterobacteriaceae strains, including CRE strains. LYS228 is stable to MBLs, such as NDMs, and to most characterized SBLs, such as KPCs. These findings are consistent with data for a panel of isogenic strains expressing various β-lactamases (19). Here, we show that the stability of LYS228 translates to potent activity against a panel of multidrug-resistant Enterobacteriaceae clinical isolates, including CREs, which clearly differentiates LYS228 from other reported monobactams (23–25). In recent studies, LYS228 demonstrated efficacy in vivo, in a neutropenic murine thigh infection model, against E. coli and K. pneumoniae, including strains expressing KPC or NDM carbapenemases (26); the PK/pharmacodynamic driver for LYS228 efficacy in this model of infection was the time that the unbound drug concentration exceeded the MIC (27). Together, these in vitro and in vivo findings support the continued development of this new agent for the treatment of serious infections due to ESBL-producing and carbapenem-resistant Enterobacteriaceae strains. LYS228 is currently under evaluation in phase 2 studies among patients with complicated urinary tract infections (ClinicalTrials registration no. NCT03377426) and patients with complicated intra-abdominal infections (ClinicalTrials registration no. NCT03354754).
MATERIALS AND METHODS
Antimicrobial agents.
LYS228 and avibactam were synthesized at Novartis. Aztreonam, ceftazidime, cefepime, meropenem, and tigecycline were obtained from commercial sources.
Bacterial strains.
A total of 271 Enterobacteriaceae clinical isolates from the Novartis collection, which had been obtained from various geographic locations, were used in these studies. The isolates were acquired between 2000 and 2016 and included the following bacterial species: Citrobacter freundii, C. koseri, Enterobacter aerogenes, E. cloacae, E. hormaechei, Escherichia coli, Klebsiella oxytoca, K. pneumoniae, Morganella morganii, Proteus mirabilis, Serratia marcescens, and Salmonella spp. The E. coli ATCC 25922, K. pneumoniae ATCC 700603, and ATCC 43816 reference strains used in this study were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). E. coli BW25113 was obtained from the Keio collection (Institute for Advanced Biosciences, Keio University, Tsuruoka City, Yamagata, Japan).
Antibiotic susceptibility testing.
Susceptibility testing was performed using a broth microdilution assay, following the recommended CLSI methodology (28). The MIC50 and MIC90 were defined as the MIC values at which 50% and 90% of the isolates, respectively, were inhibited.
PCR and sequencing analysis of the ftsI gene encoding E. coli PBP3.
Oligonucleotide primers (Table 5) were synthesized by IDT (Coralville, IA). The ftsI gene, which encodes E. coli PBP3, was PCR amplified and sequenced from 2 clinical isolates (NB27307 and NB27326; LYS228 MICs, 16 μg/ml), E. coli ATCC 25922 (LYS228 MIC, 0.25 μg/ml), and E. coli BW25113. PCR amplification was conducted in several sections using Phusion high-fidelity polymerase, according to the supplied protocol. DNA sequencing was performed by ELIM Biopharmaceuticals, Inc. (Hayward, CA). The sequencing results were analyzed for mutations using Vector NTI 11.5.1 (Thermo-Fisher, Waltham, MA) and Sequencher 5.0 (GeneCodes, Ann Arbor, MI), according to the manufacturer's instructions, by comparison to E. coli MG1655 ftsI reference sequences obtained from the EcoGene database (ecogene.org), as well as gene sequences obtained for E. coli ATCC 25922.
TABLE 5.
Primers and primer pairs used in this study
| Primer or primer pair | Sequence (5′ to 3′) | PCR product size (bp) |
|---|---|---|
| ftsI (PBP3) primer | ||
| SR170 | GCATGTTGATCCGTCACAAG | |
| SR171 | ATGGCTAACAGCCCGTCATA | |
| SR172 | ACGGCTGTCGAGTGTCATCT | |
| SR173 | TAACGCCAGCTTGGAAACAC | |
| SR174 | CGAGACTCTTCACGCAGATG | |
| SR175 | TTATCGCCCACTGTCGATTAC | |
| Primer pair | ||
| SR170-SR174 | 555 | |
| SR170-SR173 | 1,165 | |
| SR175-SR172 | 524 | |
| SR171-SR172 | 1,021 | |
| SR170-SR172 | 1,901 |
Bactericidal activity.
For MBC determinations, 2 μl was removed from each well and aseptically plated on a tryptic soy agar plate. The plates were incubated at 35°C in ambient air for 24 h. After incubation, the number of colonies that grew from each well was counted and recorded. The MBC was defined as the lowest concentration of drug that resulted in ≥99.9% reductions (≥3 log10 units) in titers from the original inoculum. The MBC50 and MBC90 were defined as the MBCs at which 50% and 90% of the isolates, respectively, were killed.
Time-kill experiments were performed against 10 strains of E. coli and K. pneumoniae with various β-lactamases in cation-adjusted Mueller-Hinton broth, following CLSI methodology (29). Antibiotics were added to the culture medium at concentrations equivalent to multiples of the MIC for each organism tested. Tubes were inoculated with early log-phase cultures of bacteria, which were diluted to yield a final cell density of 1 × 106 CFU/ml. The samples taken at that time constituted the 0-h time point. Cultures were then incubated at 35°C in ambient air for 24 h, with constant agitation using an orbital shaker (Innova 43; New Brunswick Scientific, Enfield, CT), and were sampled at various times. Prior to each sampling, tubes were mixed carefully. Viable cell counts were determined by performing 10-fold serial dilutions in sterile saline; 0.1 ml of undiluted sample and diluted samples was applied directly to the Mueller-Hinton agar using sterile glass beads. Colonies were counted after 24 h of incubation at 35°C in ambient air.
ACKNOWLEDGMENTS
We thank Katherine R. Prosen for technical assistance. We thank JMI Laboratories for strains NB27235, NB27236, and NB29084 and William Weiss for strain NB27169. We thank Bret Benton, Ellena Growcott, and Catherine Jones for editorial support.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Bush K. 2010. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol 13:558–564. doi: 10.1016/j.mib.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Kanamori H, Parobek CM, Juliano JJ, van Duin D, Cairns BA, Weber DJ, Rutala WA. 2017. A prolonged outbreak of KPC-3-producing Enterobacter cloacae and Klebsiella pneumoniae driven by multiple mechanisms of resistance transmission at a large academic burn center. Antimicrob Agents Chemother 61:e01516-16. doi: 10.1128/AAC.00912-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hargreaves ML, Shaw KM, Dobbins G, Snippes Vagnone PM, Harper JE, Boxrud D, Lynfield R, Aziz M, Price LB, Silverstein KA, Danzeisen JL, Youmans B, Case K, Sreevatsan S, Johnson TJ. 2015. Clonal dissemination of Enterobacter cloacae harboring blaKPC-3 in the upper midwestern United States. Antimicrob Agents Chemother 59:7723–7734. doi: 10.1128/AAC.01291-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn C, Syed A, Hu F, O'Hara JA, Rivera JI, Doi Y. 2014. Microbiological features of KPC-producing Enterobacter isolates identified in a U.S. hospital system. Diagn Microbiol Infect Dis 80:154–158. doi: 10.1016/j.diagmicrobio.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiedrowski LM, Guerrero DM, Perez F, Viau RA, Rojas LJ, Mojica MF, Rudin SD, Hujer AM, Marshall SH, Bonomo RA. 2014. Carbapenem-resistant Enterobacter cloacae isolates producing KPC-3, North Dakota, USA. Emerg Infect Dis 20:1583–1585. doi: 10.3201/eid2009.140344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 8.Biedenbach D, Bouchillon S, Hackel M, Hoban D, Kazmierczak K, Hawser S, Badal R. 2015. Dissemination of NDM metallo-β-lactamase genes among clinical isolates of Enterobacteriaceae collected during the SMART global surveillance study from 2008 to 2012. Antimicrob Agents Chemother 59:826–830. doi: 10.1128/AAC.03938-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan LK, Bonomo RA. 2016. Metallo-β-lactamase (MBL)-producing Enterobacteriaceae in United States children. Open Forum Infect Dis 3:ofw090. doi: 10.1093/ofid/ofw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacconelli E, Magrini N. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 11.Pogue JM, Lee J, Marchaim D, Yee D, Zhoa JJ, Chopra T, Lephart P, Kaye KS. 2011. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 53:879–884. doi: 10.1093/cid/cir611. [DOI] [PubMed] [Google Scholar]
- 12.Navarro-San Francisco C, Mora-Rillo M, Romero-Gómez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gómez-Gil R, Arribas-López JR, Mingorance J, Paño-Pardo JR. 2013. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect 19:E72–E79. doi: 10.1111/1469-0691.12091. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 14.Hauck C, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Scalera NM, Doi Y, Kaye KS, Evans S, Fowler VG Jr, Bonomo RA, van Duin D. 2016. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect 22:513–519. doi: 10.1016/j.cmi.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. 2014. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 20:1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 17.Slama TG. 2008. Gram-negative antibiotic resistance: there is a price to pay. Crit Care 12(Suppl 4):S4. doi: 10.1186/cc6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore DM. 2009. Has the era of untreatable infections arrived? J Antimicrob Chemother 64(Suppl 1):i29–i36. doi: 10.1093/jac/dkp255. [DOI] [PubMed] [Google Scholar]
- 19.Reck F, Bermingham A, Blais J, Capka V, Cariaga T, Casarez A, Colvin R, Dean CR, Fekete A, Growcott E, Guo H, Gong W, Jones AK, Li C, Li F, Lin X, Lindvall M, Lopez S, McKenney D, Metzger L, Moser HE, Prathapam R, Rasper D, Rudewicz P, Sethuraman V, Shen X, Shaul J, Simmons RL, Tashiro K, Tang D, Tjandra M, Turner N, Uehara T, Vitt C, Whitebread S, Yifru A, Zang X, Zhu Q. 2018. Optimization of novel monobactams with activity against carbapenem-resistant Enterobacteriaceae: identification of LYS228. Bioorg Med Chem Lett 28:748–755. doi: 10.1016/j.bmcl.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Mendes R, Rhomberg PR, Schaefer B, Huband MD, Flamm RK. 2017. In vitro activity of LYS228 against Enterobacteriaceae, including molecularly characterized multidrug-resistant isolates, abstr Saturday-293. Abstr 2017 Am Soc Microbiol Microbe Meet, New Orleans, LA. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing—28th ed CLSI document M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Alm RA, Johnstone MR, Lahiri SD. 2015. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother 70:1420–1428. doi: 10.1093/jac/dku568. [DOI] [PubMed] [Google Scholar]
- 23.Page MGP, Dantier C, Desarbre E. 2010. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant Gram-negative bacilli. Antimicrob Agents Chemother 54:2291–2302. doi: 10.1128/AAC.01525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka SK, Summerill RA, Minassian BF, Bush K, Visnic DA, Bonner DP, Sykes RB. 1987. In vitro evaluation of tigemonam, a novel oral monobactam. Antimicrob Agents Chemother 31:219–225. doi: 10.1128/AAC.31.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imada A, Kondo M, Okonogi K, Yukishige K, Kuno M. 1985. In vitro and in vivo antibacterial activities of carumonam (AMA-1080), a new N-sulfonated monocyclic beta-lactam antibiotic. Antimicrob Agents Chemother 27:821–827. doi: 10.1128/AAC.27.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Growcott EJ, Cariagat T, Gold J, Camboa L, Lopez S, Simmons RL, Osborne CS. 2017. In vivo efficacy of the novel monobactam LYS228 in a neutropenic murine thigh model of infection with carbapenem-resistant Enterobacteriaceae, abstr Saturday-291. Abstr 2017 Am Soc Microbiol Microbe Meet, New Orleans, LA. [Google Scholar]
- 27.Growcott EJ, Zang X, Cariaga TA, Lopez S, Simmons RL, Osborne CS. 2017. Pharmacokinetics and pharmacodynamics of the novel monobactam LYS228 in the neutropenic murine thigh infection model, abstr Saturday-289. Abstr 2017 Am Soc Microbiol Microbe Meet, New Orleans, LA. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2015. Methods for dilution susceptibility tests for bacteria that grow aerobically; approved standard—10th ed CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2009. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. CLSI document M26-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Actavis, Inc. 2015. Avycaz: highlights of prescribing information. Actavis, Inc, Parsippany, NJ: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206494s000lbl.pdf. [Google Scholar]
- 31.Wyeth Pharmaceuticals, Inc. 2016. Tygacil: highlights of prescribing information. Wyeth Pharmaceuticals, Inc, Philadelphia, PA: http://labeling.pfizer.com/ShowLabeling.aspx?id=491. [Google Scholar]


