Although it is known that the in vitro MICs of rifampin and ethambutol are poorly correlated with the clinical response in Mycobacterium avium complex (MAC) lung disease (MAC-LD), evidence for this is limited. This study investigated the association between treatment outcome and the in vitro MICs of rifampin and ethambutol in patients with MAC-LD.
KEYWORDS: MIC, Mycobacterium avium complex, rifampin, ethambutol, treatment outcome
ABSTRACT
Although it is known that the in vitro MICs of rifampin and ethambutol are poorly correlated with the clinical response in Mycobacterium avium complex (MAC) lung disease (MAC-LD), evidence for this is limited. This study investigated the association between treatment outcome and the in vitro MICs of rifampin and ethambutol in patients with MAC-LD. Among patients diagnosed with macrolide-susceptible MAC-LD between January 2008 and December 2013, 274 patients who were treated with a standard regimen for ≥12 months until August 2017 and whose in vitro MIC results were available were enrolled at a tertiary referral center in South Korea. The MICs of antimicrobial agents were determined using the broth microdilution method. The mean age of the included patients was 60.4 years. The overall treatment success rate was 79.6% (218/274 patients) and tended to decrease with increasing MICs of rifampin and ethambutol, particularly at MICs of ≥8 μg/ml. Treatment success rate was significantly different between MAC isolates with MICs of ≥8 μg/ml for rifampin and ethambutol and those with MICs of <8 μg/ml for rifampin and/or ethambutol (64.9% versus 85.3%, P < 0.001). Multivariate analysis showed that an MIC of ≥8 μg/ml for both drugs and initial sputum acid-fast bacillus (AFB) smear positivity were independent risk factors for an unfavorable response (adjusted odds ratio [OR] = 3.154, 95% confidence interval [CI] = 1.641 to 6.063, and P = 0.001 for an MIC of ≥8 μg/ml; adjusted OR = 2.769, 95% CI = 1.420 to 5.399, and P = 0.003 for initial sputum AFB smear positivity). These findings suggest that the in vitro MICs of rifampin and ethambutol may be related to treatment outcome in MAC-LD.
INTRODUCTION
The incidence of lung disease caused by nontuberculous mycobacteria (NTM) continues to increase worldwide (1), including in South Korea (2). Mycobacterium avium complex (MAC) is the most common etiological organism of NTM lung disease in South Korea (3). Macrolides are the cornerstone of treatment for MAC lung disease (MAC-LD) (3, 4). Current guidelines for the treatment of MAC-LD recommend a three-drug macrolide-based therapy that includes macrolides, rifampin, and ethambutol (3–6). In patients with severe and advanced disease, addition of an aminoglycoside is recommended (4).
A challenge in the treatment of MAC-LD is the observation that, in contrast to the therapy of tuberculosis (7), the results of in vitro MIC testing may not be a guide for the effective in vivo response to antibiotics, with the exception of macrolides and amikacin (4, 8–10). That is, in vitro MIC data for rifampin and ethambutol have shown a poor correlation between the MIC and the clinical response (11). Therefore, the Clinical and Laboratory Standards Institute (CLSI) and the American Thoracic Society (ATS) have recommended that antimicrobial susceptibility testing (AST) of MAC isolates be performed only for macrolides and not for rifampin and ethambutol (5, 12).
However, the poor correlation of AST results for rifampin and ethambutol with treatment outcome was based on studies with a limited number of patients and the adequacy of treatment (13–16). In other words, this concept has not been confirmed in a sufficient number of patients treated with the regimen recommended by the ATS. Therefore, we aimed to investigate this correlation using a larger number of patients who received standard treatment for MAC-LD.
RESULTS
Patient characteristics and treatment outcome.
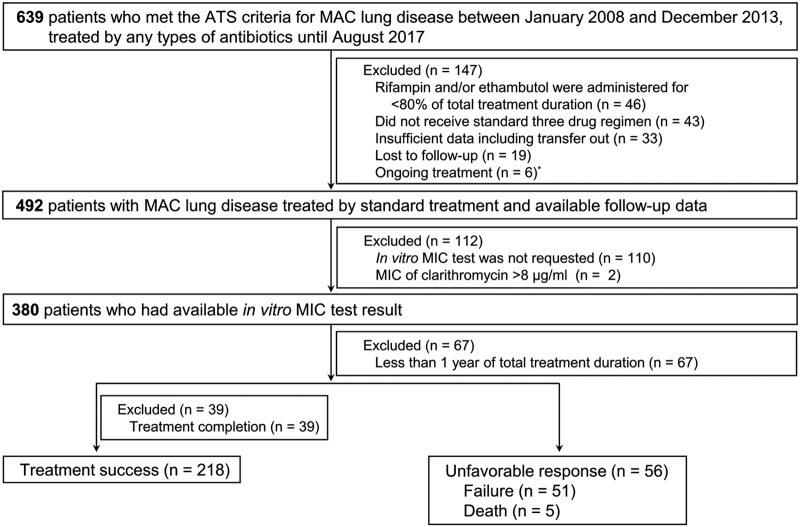
Eligibility screening identified 274 patients with MAC-LD and in vitro MIC test results for rifampin and ethambutol. The mean age was 60.4 ± 11.0 years, and the preponderance of patients were female (59.1%). Radiological features included the fibrocavitary type in 39 patients (14.2%), the nodular bronchiectatic type in 207 patients (75.5%), and an unclassifiable type in 28 patients (10.2%). The overall treatment success rate was 79.6% (218/274 patients). In the remaining 56 patients (20.4%), the response was unfavorable with treatment failure (n = 51) and death (n = 5) (Fig. 1). A total of 7 patients received surgical resection due to persistent MAC culture positivity and were classified into the treatment failure group. The treatment success rate was 69.2% (27/39 patients) for patients with the fibrocavitary type, 83.1% (172/207 patients) for patients with the nodular bronchiectatic type, and 67.9% (19/28 patients) for patients with unclassifiable types. There were significant differences between patients with treatment success and those with an unfavorable response by gender, smoking history, history of tuberculosis treatment, and the results of initial smear-positive acid-fast bacillus (AFB) testing. The baseline characteristics of the patients according to treatment outcome are shown in Table 1.
FIG 1.
Study flow chart. ATS, American Thoracic Society; MAC, Mycobacterium avium complex. *, these 6 patients were excluded because the total duration of their treatment was <12 months at the time of analysis.
TABLE 1.
Clinical characteristics of 274 patients with MAC-LD according to treatment outcomea
| Characteristic | Values for patients with: |
P value | ||
|---|---|---|---|---|
| Total (n = 274) | Treatment success (n = 218) | Unfavorable response (n = 56) | ||
| Mean ± SD age (yr) | 60.4 ± 11.0 | 60.2 ± 10.9 | 61.0 ± 11.7 | 0.647 |
| No. (%) of patients >60 yr of age | 139 (50.7) | 111 (50.9) | 28 (50.0) | 0.903 |
| No. (%) of patients by gender | 0.002 | |||
| Male | 112 (40.9) | 79 (36.2) | 33 (58.9) | |
| Female | 162 (59.1) | 139 (63.8) | 23 (41.1) | |
| Mean ± SD BMI | 20.7 ± 3.0 | 20.6 ± 2.6 | 20.8 ± 4.5 | 0.717 |
| No. (%) of patients with a BMI of ≤18.5 kg/m2 | 51 (18.6) | 40 (18.3) | 11 (19.6) | 0.730 |
| No. (%) of patients by smoking history | 0.001 | |||
| Nonsmoker | 189 (69.0) | 161 (73.9) | 28 (50.0) | |
| Current or former smoker | 85 (31.0) | 57 (26.1) | 28 (50.0) | |
| No. (%) of patients with a history of TB treatment | 118 (43.1) | 87 (39.9) | 31 (55.4) | 0.037 |
| No. (%) of patients with a history of treatment for NTM lung disease | 13 (4.7) | 12 (5.5) | 1 (1.8) | 0.478 |
| No. (%) of patients with the following comorbidities: | ||||
| Chronic obstructive lung disease | 32 (11.7) | 26 (11.9) | 6 (10.7) | 0.801 |
| Interstitial lung disease | 24 (8.8) | 17 (7.8) | 7 (12.5) | 0.267 |
| Diabetes mellitus | 24 (8.8) | 16 (7.3) | 8 (14.3) | 0.101 |
| Malignancy | 49 (17.9) | 34 (15.6) | 15 (26.8) | 0.051 |
| No. (%) of patients with the following etiology: | 0.153 | |||
| Mycobacterium avium | 126 (46.0) | 105 (48.2) | 21 (37.5) | |
| Mycobacterium intracellulare | 148 (54.0) | 113 (51.8) | 35 (62.5) | |
| No. (%) of patients with a positive AFB smear at treatment initiation | 118 (43.1) | 82 (37.6) | 36 (64.3) | <0.001 |
| No. (%) of patients with the following type of disease: | 0.820 | |||
| Fibrocavitary | 39 (14.2) | 27 (12.4) | 12 (21.4) | |
| Nodular bronchiectatic | 207 (75.5) | 172 (78.9) | 35 (62.5) | |
| Unclassifiable | 28 (10.2) | 19 (8.7) | 9 (16.1) | |
| No. (%) of patients using an injectable aminoglycoside | 137 (50.0) | 103 (47.2) | 34 (60.7) | 0.072 |
BMI, body mass index; TB, tuberculosis; NTM, nontuberculous mycobacteria; AFB, acid-fast bacilli.
Association of in vitro MICs of rifampin and ethambutol with clinical outcomes.
For the majority of patients (96.4%, 264/274 patients), AST was requested prior to (42.3%, 116 patients) or at the time of (54.0%, 148 patients) treatment initiation. The results of in vitro MIC testing for rifampin and ethambutol in MAC isolates from the 274 patients are shown in Table 2. According to the MIC of rifampin, the treatment success rate decreased with increasing MIC values (P value for trend < 0.001). Ethambutol showed a similar tendency; however, it failed to reach statistical significance (P value for trend = 0.0662).
TABLE 2.
In vitro MICs of rifampin and ethambutol for 274 Mycobacterium avium complex isolates and treatment success rate at each MIC
| MIC (μg/ml) | Rifampin |
Ethambutol |
||
|---|---|---|---|---|
| No. (%) of isolates | Treatment success rate (%) | No. (%) of isolates | Treatment success rate (%) | |
| 0.25 | 1 (0.4) | 100 | ||
| 0.5 | 10 (3.6) | 90.0 | ||
| 1 | 47 (17.2) | 89.4 | ||
| 2 | 75 (27.4) | 85.3 | 16 (5.8) | 87.5 |
| 4 | 54 (19.7) | 79.6 | 99 (36.1) | 84.8 |
| 8 | 86 (31.4) | 67.4 | 84 (30.7) | 77.4 |
| 16 | 1 (0.4) | 100 | 55 (20.1) | 74.5 |
| 32 | 20 (7.3) | 70.0 | ||
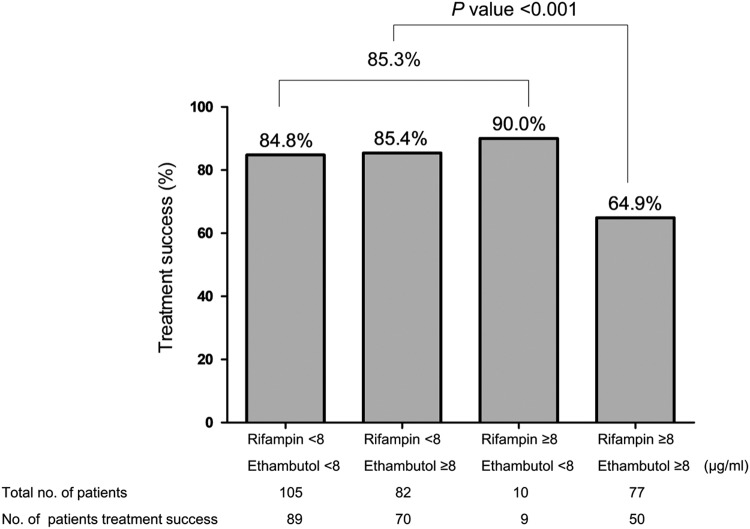
A cutoff MIC of 8 μg/ml was the most appropriate value to distinguish treatment success and an unfavorable response for both rifampin and ethambutol (P = 0.0013 and P = 0.0504, respectively). Thus, the treatment success rate for rifampin and ethambutol was assessed using this cutoff value. As shown in Fig. 2, patients with MAC isolates with MICs of ≥8 μg/ml for rifampin and ethambutol showed significantly lower rates of treatment success than those with MAC isolates with MICs of <8 μg/ml. Treatment success rates were 64.9% and 85.3% for patients with MAC isolates with MICs ≥8 μg/ml for both drugs and MICs of <8 μg/ml for rifampin and/or ethambutol, respectively (P < 0.001).
FIG 2.
Treatment success rate among 274 patients with Mycobacterium avium complex lung disease according to the MICs of rifampin and ethambutol.
Risk factors for an unfavorable response.
The univariate analysis identified male gender, smoking history, history of tuberculosis treatment, initial sputum AFB smear positivity, and in vitro MIC results for the two drugs to be significant risk factors for an unfavorable response. Multivariate analysis revealed that an MIC of ≥8 μg/ml for both drugs and initial sputum AFB smear positivity were independent risk factors for an unfavorable response (adjusted odds ratio [OR] = 3.154, 95% confidence interval [CI] = 1.641 to 6.063, and P = 0.001 for an MIC of ≥8 μg/ml; adjusted OR = 2.769, 95% CI = 1.420 to 5.399, and P = 0.003 for initial sputum AFB smear positivity; Table 3).
TABLE 3.
Risk factors for unfavorable response in 274 patients with MAC-LDa
| Characteristic | No. (%) of patients with: |
P value by univariate analysis | Multivariate analysis |
||
|---|---|---|---|---|---|
| Treatment success (n = 218) | Unfavorable response (n = 56) | Adjusted OR (95% CI) | P value | ||
| Male gender | 79 (36.2) | 33 (58.9) | 0.002 | 1.474 (0.557–3.901) | 0.434 |
| Smoking history, former or current | 57 (26.1) | 28 (50.0) | 0.001 | 1.577 (0.591–4.203) | 0.363 |
| History of TB treatment | 87 (39.9) | 31 (55.4) | 0.037 | 1.273 (0.664–2.442) | 0.467 |
| Comorbidity, malignancy | 34 (15.6) | 15 (26.8) | 0.051 | 2.172 (0.989–4.771) | 0.053 |
| Positive AFB smear at treatment initiation | 82 (37.6) | 36 (64.3) | <0.001 | 2.769 (1.420–5.399) | 0.003 |
| In vitro MICs of: | <0.001 | ||||
| <8 μg/ml for rifampin and/or ethambutol | 168 (77.1) | 29 (51.8) | 1 | ||
| ≥8 μg/ml for rifampin and ethambutol | 50 (22.9) | 27 (48.2) | 3.154 (1.641–6.063) | 0.001 | |
TB, tuberculosis; AFB, acid-fast bacilli; OR, odds ratio; CI, confidence interval.
Species analysis for M. avium and M. intracellulare.
Subgroup analysis of the Mycobacterium avium and Mycobacterium intracellulare isolates identified that an in vitro MIC of ≥8 μg/ml for both rifampin and ethambutol was an independent risk factor for an unfavorable response for both species (see Tables S1 to S6 and Fig. S1 and S2 in the supplemental material).
Follow-up AST results in patients with treatment failure.
Follow-up ASTs were performed for 22 of the 53 patients with an unfavorable treatment response. The median duration between the end of treatment and follow-up AST for these 22 patients was 10.3 months (interquartile range, 2.8 to 18.7 months). Among these patients, there were no cases of newly developed clarithromycin resistance observed, nor were any significant changes in the in vitro MICs of rifampin or ethambutol seen in isolates from these 22 patients (Fig. S3 to S5).
DISCUSSION
In the treatment of MAC-LD, clarithromycin is the only recommended agent for susceptibility testing of MAC isolates (11). Rifampin and ethambutol were not recommended for such susceptibility testing (17) due to discrepancies between the in vitro and in vivo clinical outcomes achieved with these drugs, as shown by previous studies (13–15). This lack of a correlation was thought to be related to biofilms (4), which comprise microcolonies of bacteria embedded in the extracellular matrix to provide stability and resistance to human immune mechanisms and drugs (18). However, evidence of the poor association between AST results for rifampin and ethambutol with the treatment outcome in patients with MAC-LD is limited. The present study is the first to report that the in vitro MIC test results for rifampin and ethambutol are associated with treatment outcomes in patients who received standard treatment for MAC-LD. These findings suggest that the in vitro MICs of rifampin and ethambutol may be related to the treatment outcome, highlighting the importance of AST of these drugs in the treatment of MAC-LD.
Previous studies (13–15) which showed the poor correlation between the clinical response and in vitro MIC data for rifampin and ethambutol were characterized by limitations in terms of sample size and the adequacy of the antimicrobial agent. For example, Kobashi et al. reported that there was no relationship between in vivo clinical efficacy and in vitro data for rifampin and ethambutol (13). However, the analysis of treatment outcomes in that study was performed in only 60 patients. Moreover, approximately half of the patients received a clarithromycin dosage of <800 mg, which has been shown to be inadequate for the treatment of MAC-LD (19). Another study (14) that showed similar results included only 60 patients, who also received insufficient daily doses of antimicrobial agents (clarithromycin at 600 mg, rifampin at 450 mg, and ethambutol at 400 mg). In a third study (15), the patients did not receive treatment with a clarithromycin-containing regimen. In contrast, the present study analyzed 274 patients who received the standard three-drug regimen recommended by the ATS at the proper dosage for the treatment of MAC-LD.
The outcomes of macrolide-containing regimens for the treatment of MAC-LD have thus far been unsatisfactory. One recent systematic review and meta-analysis revealed a mere 60% treatment success rate (20). Several factors, including positive AFB sputum smear, Mycobacterium intracellulare infection, old age, the fibrocavitary type of disease, and a low body mass index, have been associated with poor treatment outcomes (21–23). In the present study, the only independent risk factors for an unfavorable response were initial sputum AFB smear positivity and the in vitro MICs of rifampin and ethambutol. Thus, it is suggested that the in vitro MIC results may be more important for determining the treatment outcome in patients with MAC-LD than conventional risk factors for an unfavorable response, such as the presence of fibrocavitary lesions or low body mass index.
Because macrolides are the key drugs used for the treatment of MAC lung disease, the development of macrolide resistance indicates a poor treatment outcome and increased mortality (24). The most frustrating and inexplicable point concerning the development of macrolide resistance is that it could occur even in patients who have received the currently recommended regimen of three oral drugs (25, 26). According to our results, the development of macrolide resistance even after treatment with standard regimen medications can be speculated to be related to the in vitro MIC test results for rifampin and ethambutol. In other words, if the MICs of rifampin and ethambutol are sufficiently high for the isolates to be classified as resistant, the efficacy of a three-drug oral regimen with a macrolide, rifampin, and ethambutol would be practically the same as the efficacy of macrolide monotherapy and could eventually lead to the development of macrolide resistance. In our study, of 77 patients with clinical isolates with rifampin and ethambutol MICs of ≥8 μg/ml, 27 had unfavorable responses (Fig. 2). Follow-up ASTs were performed for 14 of those isolates, and none showed newly developed clarithromycin resistance (data not shown). We thought that further studies with more patients would address the issue.
Previously, we have shown that the spontaneous negative conversion of sputum cultures occurs in approximately half of untreated MAC lung disease patients with a stable disease course (27). One of the predictors of sputum conversion in these untreated stationary patients is transient (≥1 month) antituberculosis medication at the time of initial diagnosis. Namely, transient treatment with an antituberculosis medication for the presumptive diagnosis of pulmonary tuberculosis before the definite MAC lung disease diagnosis increases the probability of experiencing culture conversion. This suggests that transient sputum conversion may be achieved using a combination of rifampin and ethambutol without addition of macrolides. Therefore, it can be assumed that the MICs of rifampin and ethambutol are likely to be higher in ASTs performed on the MAC isolates obtained after initiation of antituberculosis treatment. However, in our study cases, we did not observe any rifampin or ethambutol MIC differences whether the patients had received the transient antituberculosis medication or not (data not shown).
A number of limitations of the present study must be acknowledged. First, the study was conducted at a single referral center, and patients were recruited over a prolonged period of time. In addition, because of its retrospective nature, this study failed to differentiate M. intracellulare from Mycobacterium chimaera, which may be less virulent and associated with different treatment outcomes (28). However, a recent study in South Korea showed that M. chimaera accounts for a minor proportion of MAC lung disease (29). Third, approximately one-quarter of patients for whom in vitro MIC testing was not requested were excluded during the eligibility screening process. Nevertheless, the clinical characteristics (except for smoking history) were similar between patients with and patients without AST. Finally, the MIC of each isolate was not retested to ascertain the reproducibility of AST.
In conclusion, the present study shows that the in vitro MICs of rifampin and ethambutol are an independent factor for predicting treatment outcome in patients treated with a standard macrolide-containing regimen for macrolide-susceptible MAC-LD. This finding suggests that AST for rifampin and ethambutol may be necessary in patients with MAC-LD.
MATERIALS AND METHODS
Study patients.
A retrospective review of the medical records at Asan Medical Center, which is a 2,700-bed referral hospital in Seoul, South Korea, revealed that 639 patients fulfilled the ATS diagnostic criteria for MAC-LD between January 2008 and December 2013. Of these 639 patients, those who received standard treatment until August 2017 were enrolled. Subsequently, patients with available in vitro MIC data and whose total treatment duration was ≥1 year were selected. Further exclusion criteria were as follows: (i) cases in which AST was not requested; (ii) patients with MAC isolates with resistance to clarithromycin; (iii) insufficient information, including transfer to another hospital during treatment; and (iv) loss to follow-up. The medical records of the remaining patients were analyzed retrospectively in August 2017.
The study protocol was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB no. 2017-0017), which waived the requirement for informed consent because of the retrospective nature of the analysis.
Definition of standard treatment.
We included only patients with MAC-LD who received the standard treatment, defined as the three-drug combination regimen of an oral macrolide (clarithromycin or azithromycin), rifampin, and ethambutol, according to the patient's weight, as recommended by the ATS guideline (5). The daily treatment regimen included the following components: (i) 1,000 mg clarithromycin or 250 mg azithromycin; (ii) 15 mg/kg of body weight ethambutol; and (iii) 450 mg/day (for patients with body weights of <50 kg) or 600 mg/day (for patients with body weights of >50 kg) rifampin. During treatment for MAC-LD, the regimen could be adjusted in some patients after treatment initiation because of newly developed adverse reactions to rifampin or ethambutol. In such cases, the treatment regimen was regarded as standard if both rifampin and ethambutol were prescribed for ≥80% of the total treatment duration. The threshold of 80% was selected a priori on the basis of the findings of a previous study (30). Because intermittent therapy was not common in our center until the year 2013, the majority of the patients with the nodular bronchiectatic type of MAC lung disease received daily therapy. Although an aminoglycoside, generally streptomycin, was administered according to the discretion of the attending physician, it was prescribed for most patients with the fibrocavitary form or advanced disease.
Definition of treatment outcomes and radiological evaluation.
In the present study, treatment outcomes were categorized as follows (3, 31–33): (i) treatment failure was no conversion to a negative sputum culture even after ≥12 months of treatment or the case received surgical resection due to persistent MAC culture positivity, (ii) treatment completion was achievement of sputum culture conversion with a treatment duration after conversion of <12 months, and (iii) treatment success was achievement of sputum culture conversion with a treatment duration after conversion of ≥12 months. Sputum culture conversion was defined as three consecutive negative sputum cultures, with the time of conversion being defined as the date of the first negative culture (34). If the patient could not expectorate sputum during the treatment duration, the sputum was considered to have converted to negative (34). Death was defined when the patient died by any cause during the treatment for MAC-LD (35). Loss to follow-up was defined for those patients with treatment interruptions of >2 consecutive months. An unfavorable response was defined as one of the following: (i) treatment failure or (ii) death. Following the exclusion of treatment completion and loss to follow-up, treatment success and an unfavorable response were selected for the analysis of the treatment results.
Radiographic abnormalities on chest computed tomography at the time of diagnosis were classified according to the distinct disease patterns as follows, as previously defined (27): (i) the fibrocavitary type, (ii) the nodular bronchiectatic type, and (iii) unclassifiable forms.
Microbiological examination and in vitro susceptibility testing.
The species of the NTM isolates were identified using the PCR and the restriction fragment length polymorphism method according to the rpoB gene or through the reverse blot hybridization assay of the rpoB gene (36–38). During the study period, the MAC isolates were classified into two species: M. avium and M. intracellulare.
The MICs of several antimicrobial agents were determined by the broth microdilution method using the Sensititre Myco susceptibility plate for slow-growing mycobacteria (Thermo Fisher Scientific Inc., Waltham, MA, USA), according to the manufacturer's instructions (39, 40). The susceptibility breakpoints, defined by CLSI, were used for the interpretation of the MIC of clarithromycin (an MIC of ≤8 μg/ml was considered susceptible, and an MIC of ≥32 μg/ml was considered resistant) (12).
Statistical analysis.
All statistical analyses were performed using SPSS software (version 20.0; SPSS, Chicago, IL, USA). The Student t test was used for continuous variables, and the χ2 or Fisher's exact test was used for categorical variables. All tests of significance were two-sided; P values of <0.05 were considered statistically significant. The OR and multivariate regression analysis were used to calculate the adjusted risk. Independent variables were selected on the basis of their statistical significance in the univariate analysis. The criterion for inclusion of a variable in the multivariate analysis was a significance level of <0.1.
Supplementary Material
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We declare that we have no conflicts of interest.
Mi-Na Kim, Heungsup Sung, Yong Pil Chong, and Kyung-Wook Jo designed the study. Byoung Soo Kwon, Younsuck Koh, Woo-Sung Kim, Jin-Woo Song, Tae Sun Shim, Yeon-Mok Oh, Sang-Do Lee, Sei Won Lee, Jae-Seung Lee, Chae-Man Lim, Chang-Min Choi, Jin-Won Huh, Sang-Bum Hong, and Sojung Park analyzed the data. Byoung Soo Kwon, Yong Pil Chong, and Kyung-Wook Jo wrote the manuscript. We all contributed to the study design, interpretation of the data, and the writing of the manuscript. We all had full access to the data and reviewed/approved the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00491-18.
REFERENCES
- 1.Brode SK, Daley CL, Marras TK. 2014. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis 18:1370–1377. doi: 10.5588/ijtld.14.0120. [DOI] [PubMed] [Google Scholar]
- 2.Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, Shim TS. 2012. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis (Seoul) 72:409–415. doi: 10.4046/trd.2012.72.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon YS, Koh WJ. 2016. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci 31:649–659. doi: 10.3346/jkms.2016.31.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu YJ, Koh WJ, Daley CL. 2016. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis (Seoul) 79:74–84. doi: 10.4046/trd.2016.79.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 6.Gale-Rowe M, Menzies D, Sutherland J, Wong T. 2014. Highlights of the new 7th edition of the Canadian Tuberculosis Standards. Can Commun Dis Rep 40:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksamit TR, Philley JV, Griffith DE. 2014. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med 108:417–425. doi: 10.1016/j.rmed.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Brown-Elliott BA, Iakhiaeva E, Griffith DE, Woods GL, Stout JE, Wolfe CR, Turenne CY, Wallace RJ Jr. 2013. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J Clin Microbiol 51:3389–3394. doi: 10.1128/JCM.01612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Ingen J, Kuijper EJ. 2014. Drug susceptibility testing of nontuberculous mycobacteria. Future Microbiol 9:1095–1110. doi: 10.2217/fmb.14.60. [DOI] [PubMed] [Google Scholar]
- 10.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. 2017. British Thoracic Society guideline for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72:ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 11.Daley CL. 2017. Mycobacterium avium complex disease. Microbiol Spectr 5:TNM17-0045-2017. doi: 10.1128/microbiolspec.TNMI7-0045-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods GL, Brown-Elliott BA, Desmond EP, Hall GS, Heifets L, Pfyffer GE, Ridderhof JC, Wallace RJ, Warren NG, Witebsky FG. 2003. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic actinomycetes. Approved standard M24-A. NCCLS, Wayne, PA. [PubMed] [Google Scholar]
- 13.Kobashi Y, Abe M, Mouri K, Obase Y, Kato S, Oka M. 2012. Relationship between clinical efficacy for pulmonary MAC and drug-sensitivity test for isolated MAC in a recent 6-year period. J Infect Chemother 18:436–443. doi: 10.1007/s10156-011-0351-x. [DOI] [PubMed] [Google Scholar]
- 14.Kobashi Y, Yoshida K, Miyashita N, Niki Y, Oka M. 2006. Relationship between clinical efficacy of treatment of pulmonary Mycobacterium avium complex disease and drug-sensitivity testing of Mycobacterium avium complex isolates. J Infect Chemother 12:195–202. doi: 10.1007/s10156-006-0457-8. [DOI] [PubMed] [Google Scholar]
- 15.The Research Committee of the British Thoracic Society. 2002. Pulmonary disease caused by Mycobacterium avium-intracellulare in HIV-negative patients: five-year follow-up of patients receiving standardised treatment. Int J Tuberc Lung Dis 6:628–634. [PubMed] [Google Scholar]
- 16.Horsburgh CR Jr, Mason UG III, Heifets LB, Southwick K, Labrecque J, Iseman MD. 1987. Response to therapy of pulmonary Mycobacterium avium-intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis 135:418–421. [DOI] [PubMed] [Google Scholar]
- 17.Somoskovi A, Salfinger M. 2014. Nontuberculous mycobacteria in respiratory infections: advances in diagnosis and identification. Clin Lab Med 34:271–295. doi: 10.1016/j.cll.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Bjarnsholt T, Hoiby N, Donelli G, Imbert C, Forsberg A. 2012. Understanding biofilms—are we there yet? FEMS Immunol Med Microbiol 65:125–126. doi: 10.1111/j.1574-695X.2012.00984.x. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa N, Nishimura T, Ohtani S, Takeshita K, Fukunaga K, Tasaka S, Urano T, Ishii K, Miyairi M, Ishizaka A. 2009. Therapeutic effects of various initial combinations of chemotherapy including clarithromycin against Mycobacterium avium complex pulmonary disease. Chest 136:1569–1575. doi: 10.1378/chest.08-2567. [DOI] [PubMed] [Google Scholar]
- 20.Kwak N, Park J, Kim E, Lee C-H, Han SK, Yim J-J. 2017. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis 65:1077–1084. doi: 10.1093/cid/cix517. [DOI] [PubMed] [Google Scholar]
- 21.Sim YS, Park HY, Jeon K, Suh GY, Kwon OJ, Koh WJ. 2010. Standardized combination antibiotic treatment of Mycobacterium avium complex lung disease. Yonsei Med J 51:888–894. doi: 10.3349/ymj.2010.51.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, Shin SJ, Kwon OJ. 2012. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest 142:1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi M, Takayanagi N, Kanauchi T, Miyahara Y, Yanagisawa T, Sugita Y. 2012. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 185:575–583. doi: 10.1164/rccm.201107-1203OC. [DOI] [PubMed] [Google Scholar]
- 24.Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, Nelson K, Caccitolo J, Alvarez J, Shepherd S, Wilson R, Graviss EA, Wallace RJ Jr. 2006. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 174:928–934. doi: 10.1164/rccm.200603-450OC. [DOI] [PubMed] [Google Scholar]
- 25.Moon SM, Park HY, Kim SY, Jhun BW, Lee H, Jeon K, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Koh WJ. 2016. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 60:6758–6765. doi: 10.1128/AAC.01240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadota T, Matsui H, Hirose T, Suzuki J, Saito M, Akaba T, Kobayashi K, Akashi S, Kawashima M, Tamura A, Nagai H, Akagawa S, Kobayashi N, Ohta K. 2016. Analysis of drug treatment outcome in clarithromycin-resistant Mycobacterium avium complex lung disease. BMC Infect Dis 16:31. doi: 10.1186/s12879-016-1877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang JA, Kim S, Jo KW, Shim TS. 2017. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 49:1600537. doi: 10.1183/13993003.00537-2016. [DOI] [PubMed] [Google Scholar]
- 28.Boyle DP, Zembower TR, Reddy S, Qi C. 2015. Comparison of clinical features, virulence, and relapse among Mycobacterium avium complex species. Am J Respir Crit Care Med 191:1310–1317. doi: 10.1164/rccm.201501-0067OC. [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Shin SH, Moon SM, Yang B, Kim H, Kwon OJ, Huh HJ, Ki CS, Lee NY, Shin SJ, Koh WJ. 2017. Distribution and clinical significance of Mycobacterium avium complex species isolated from respiratory specimens. Diagn Microbiol Infect Dis 88:125–137. doi: 10.1016/j.diagmicrobio.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Franke MF, Appleton SC, Mitnick CD, Furin JJ, Bayona J, Chalco K, Shin S, Murray M, Becerra MC. 2013. Aggressive regimens for multidrug-resistant tuberculosis reduce recurrence. Clin Infect Dis 56:770–776. doi: 10.1093/cid/cis1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, Park HY, Jeon K, Huh HJ, Ki CS, Lee NY, Chung MJ, Lee KS, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J 50:1602503. doi: 10.1183/13993003.02503-2016. [DOI] [PubMed] [Google Scholar]
- 32.Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, Ki CS, Lee NY, Shin SJ, Daley CL, Koh WJ. 2015. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 191:96–103. doi: 10.1164/rccm.201408-1545OC. [DOI] [PubMed] [Google Scholar]
- 33.Stout JE, Koh W-J, Yew WW. 2016. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Lee BY, Kim S, Hong Y, Lee SD, Kim WS, Kim DS, Shim TS, Jo KW. 2015. Risk factors for recurrence after successful treatment of Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 59:2972–2977. doi: 10.1128/AAC.04577-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Ingen J, Aksamit T, Andrejak C, Bottger EC, Cambau E, Daley CL, Griffith DE, Guglielmetti L, Holland SM, Huitt GA, Koh WJ, Lange C, Leitman P, Marras TK, Morimoto K, Olivier KN, Santin M, Stout JE, Thomson R, Tortoli E, Wallace RJ Jr, Winthrop KL, Wagner D. 2018. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J 51:1800170. doi: 10.1183/13993003.00170-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. 2000. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol 38:2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanguinetti M, Posteraro B, Ardito F, Zanetti S, Cingolani A, Sechi L, De Luca A, Ortona L, Fadda G. 1998. Routine use of PCR reverse cross-blot hybridization assay for rapid identification of Mycobacterium species growing in liquid media. J Clin Microbiol 36:1530–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adékambi T, Drancourt M, Raoult D. 2009. The rpoB gene as a tool for clinical microbiologists. Trends Microbiol 17:37–45. doi: 10.1016/j.tim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Thermo Fisher Scientific Inc. 2012. Thermo Scientific Sensititre susceptibility plates: inoculation and aerosol containment study. Thermo Fisher Scientific Inc, Waltham, MA: https://assets.thermofisher.com/TFS-Assets/MBD/Application-Notes/Sensititre%20Aerosols%20White%20Paper_EN.pdf. Accessed 19 December 2017. [Google Scholar]
- 40.Babady NE, Hall L, Abbenyi AT, Eisberner JJ, Brown-Elliott BA, Pratt CJ, McGlasson MC, Beierle KD, Wohlfiel SL, Deml SM, Wallace RJ Jr, Wengenack NL. 2010. Evaluation of Mycobacterium avium complex clarithromycin susceptibility testing using SLOMYCO Sensititre panels and JustOne strips. J Clin Microbiol 48:1749–1752. doi: 10.1128/JCM.01936-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.