Mycobacterium abscessus causes a difficult-to-treat pulmonary disease (MAb-PD). After initial intravenous treatment, minocycline is recommended in the oral continuation phase of treatment.
KEYWORDS: Mycobacterium abscessus, minocycline, pharmacokinetics
ABSTRACT
Mycobacterium abscessus causes a difficult-to-treat pulmonary disease (MAb-PD). After initial intravenous treatment, minocycline is recommended in the oral continuation phase of treatment. We determined the MICs, synergy, and time-kill kinetics of minocycline against M. abscessus. With MICs of 8 to 512 mg/liter, rapid emergence of tolerance in time-kill assays, and no synergy with other drugs used to treat MAb-PD, minocycline appears ineffective against M. abscessus. These in vitro data question its role as a MAb-PD treatment modality.
TEXT
Mycobacterium abscessus is an opportunistic pathogen that can cause severe and very difficult-to-treat infections. Its most frequent disease manifestation is chronic pulmonary infection in patients with preexisting pulmonary disease, particularly, but not exclusively, those with cystic fibrosis. Because of its intrinsic resistance to most classes of antibiotics, it has been rightfully dubbed an “antibiotic nightmare” (1). Available treatment guidelines for M. abscessus pulmonary disease (MAb-PD) recommend an intensive phase of 2 to 3 intravenous drugs followed by a continuation phase of oral and inhaled antibiotics (2, 3). Minocycline, a tetracycline antibiotic, is among the recommended oral antibiotics for the continuation phase (2, 3), despite the absence of clinical and microbiological data supporting its use.
We investigated the activity of minocycline against M. abscessus and other rapidly growing nontuberculous mycobacteria (RGM). Minocycline hydrochloride was obtained from Sigma-Aldrich (Zwijndrecht, the Netherlands; lot no. 027M4012V). First, we determined the MICs of 14 clinical isolates as well as the reference strains M. abscessus CIP 104536 and Mycobacterium fortuitum ATCC 6841 using broth microdilution in cation-adjusted Mueller-Hinton (CAMH) broth as recommended by CLSI guidelines (4), as well as in Middlebrook 7H9 (M7H9) broth. M. fortuitum and Mycobacterium chelonae isolates served as further controls for consistency with the literature. For M. abscessus CIP 104536, we also determined the minimum bactericidal concentration (MBC) by plating conditions with no visible bacterial growth from the MIC determination on Columbia (III) agar with 5% sheep blood and subsequently incubating for 3 days at 30°C. The corresponding concentration of the first plate without any growth was used to determine the MBC. Synergy between minocycline and key antimycobacterial drugs against M. abscessus CIP 104536 was assessed using checkerboard microdilution assays and the fractional inhibitory concentration index (FICI) calculation (5). We defined synergy as a FICI of <0.5, no interaction as a FICI between 0.5 and 2, and antagonism as a FICI of >2. A dose-response time-kill kinetics assay of minocycline was performed with M. abscessus CIP 104536 in CAMH broth as previously described (6) using drug concentrations ranging from 0.25 to 32× the MIC.
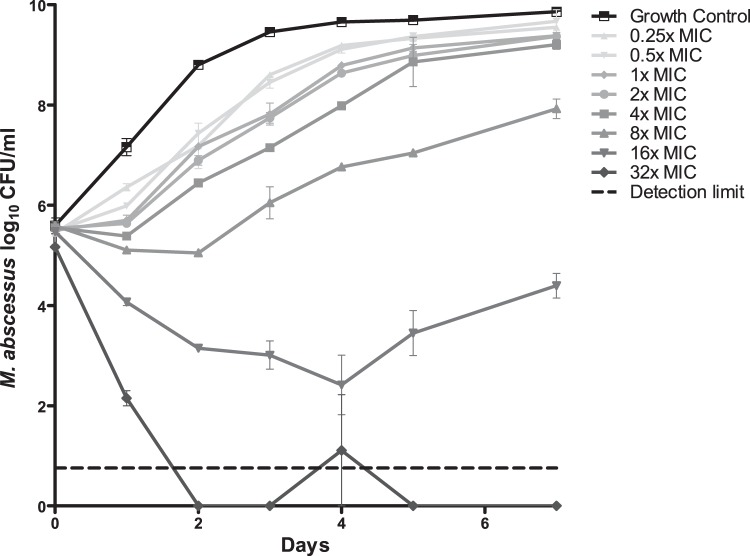
The MICs of minocycline are given in Table 1. The MIC50 for M. abscessus was 128 mg/liter in both CAMH and M7H9, and the MIC90 was >512 mg/liter, but we found MICs as low as 8 mg/liter for one isolate. The MBC was >512 mg/liter in both broths, with a MBC/MIC ratio of >8, suggesting a bacteriostatic effect only, according to definitions published previously (7). The results of synergy testing are shown in Table 2. No synergistic or antagonistic interactions were found with any tested drug, although FICI values for linezolid, bedaquiline, and cefoxitin were close to the cutoff value for synergy. Kill curves of the time-kill kinetics assay are shown in Fig. 1. At concentrations of 4× MIC and lower, there is rapid outgrowth reaching bacterial loads similar to those of the growth control; concentrations of 8× and 16× MIC show some killing followed by a sustained regrowth by day 7. Only a concentration of 32× MIC shows sustained killing.
TABLE 1.
MICs of minocycline against M. abscessus, M. fortuitum, and M. chelonae isolates
| Species | Isolatea | MIC (mg/liter) in CAMH | MIC (mg/liter) in M7H9 |
|---|---|---|---|
| M. abscessus | CIP 104536 | 64 | 64 |
| B16022328 | 512 | 256 | |
| B16037315 | 128 | 256 | |
| B16074282 | 8 | 8 | |
| B16119949 | 16 | 16 | |
| B16084679 | >512 | 256 | |
| B16045866 | 128 | 64 | |
| B15134898 | >512 | 128 | |
| B16124985 | 128 | 256 | |
| B16126240 | 256 | 128 | |
| M. fortuitum | ATCC 6841 | <0.5 | 0.5 |
| B16092122 | 16 | 16 | |
| B16099804 | 4 | 16 | |
| M. chelonae | B15092030 | 64 | >512 |
| B15142145 | 256 | 512 | |
| B15120359 | 64 | 128 |
aIsolates with a “B” identification are clinical isolates.
TABLE 2.
FICIs of minocycline in combination with different established antimycobacterial drugs against M. abscessus CIP 104536
| Compound | FICI |
|---|---|
| Clofazimine | 1 |
| Clarithromycin | 1.125 |
| Amikacin | 2 |
| Linezolid | 0.625 |
| Cefoxitin | 0.75 |
| Bedaquiline | 0.75 |
| Thioridazine | 1 |
FIG 1.
Time-kill curves of minocycline against M. abscessus CIP 104536 (MIC = 64 μg/ml). Error bars indicate standard error of the mean.
This is the first report assessing the time-kill kinetics of minocycline, which indicates that even at drug concentrations higher than the MIC, M. abscessus can readily tolerate minocycline. Our MIC data confirm those of an earlier study, which also reported minocycline MICs of >64 mg/liter for M. abscessus and a MIC of <0.125 mg/liter for M. fortuitum ATCC 6841 (8).
Little is known about synergistic interactions between minocycline and other drugs used in MAb-PD treatment. Our synergy tests support data generated by Miyasaka et al., who found that the combination of minocycline and imipenem is not synergistic against M. abscessus (9). Combined with our similar observation regarding cefoxitin, acknowledging great differences in activity between individual β-lactams against M. abscessus, as shown by Lefebvre et al. (10), this suggests a lack of synergy between minocycline and β-lactams.
There are some limitations in our work to consider. MIC and synergy data may underestimate the effect of tetracyclines against intracellular pathogens, because these drugs accumulate in macrophages where the mycobacteria also reside (11), reaching concentrations 2 to 5 times higher than those in plasma; this concept has been established for tetracycline but not specifically for minocycline (12). A recent study conducted by Gotfried et al. found that omadacycline, a derivative of minocycline, reaches sustained high concentrations in epithelial lining fluid and alveolar macrophages even after a single dose, indicating that tetracyclines inherently penetrate and accumulate in tissue (13). However, the time-kill kinetics show that the effect of minocycline, even at very high concentrations, is limited and rapidly abrogated by the emergence of tolerance and subsequent outgrowth, also indicating a limited therapeutic value.
In conclusion, minocycline alone is inactive against M. abscessus, and it is not synergistic with clarithromycin, cefoxitin, amikacin, bedaquiline, linezolid, and clofazimine, which are also used in M. abscessus therapy. These in vitro data raise doubt about its role as a treatment modality for MAb-PD, even in the continuation phase, although a clinical evaluation is needed. To optimize MAb-PD treatment, new evidence-based treatment modalities are urgently needed.
REFERENCES
- 1.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 2.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71:88–90. doi: 10.1136/thoraxjnl-2015-207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. 2017. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72:ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes: approved standard, second edition. CLSI document M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 5.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 6.Ferro BE, van Ingen J, Wattenberg M, van Soolingen D, Mouton JW. 2015. Time-kill kinetics of antibiotics active against rapidly growing mycobacteria. J Antimicrob Chemother 70:811–817. doi: 10.1093/jac/dku431. [DOI] [PubMed] [Google Scholar]
- 7.French GL. 2006. Bactericidal agents in the treatment of MRSA infections—the potential role of daptomycin. J Antimicrob Chemother 58:1107–1117. doi: 10.1093/jac/dkl393. [DOI] [PubMed] [Google Scholar]
- 8.Wallace RJ, Brown-Elliott BA, Crist CJ, Mann L, Wilson RW. 2002. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob Agents Chemother 46:3164–3167. doi: 10.1128/AAC.46.10.3164-3167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyasaka T, Kunishima H, Komatsu M, Tamai K, Mitsutake K, Kanemitsu K, Ohisa Y, Yanagisawa H, Kaku M. 2007. In vitro efficacy of imipenem in combination with six antimicrobial agents against Mycobacterium abscessus. Int J Antimicrob Agents 30:255–258. doi: 10.1016/j.ijantimicag.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre A-L, Le Moigne V, Bernut A, Veckerlé C, Compain F, Herrmann J-L, Kremer L, Arthur M, Mainardi J-L. 2017. Inhibition of the β-lactamase BlaMab by avibactam improves the in vitro and in vivo efficacy of imipenem against Mycobacterium abscessus. Antimicrob Agents Chemother 61:e02440-16. doi: 10.1128/AAC.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler IC, Chapman SJ, Clayton A, Cullen M, Daniels T, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Humphrey H, Isalska B, Jensen-Fangel S, Jönsson B, Jones AM, Katzenstein TL, Lillebaek T, MacGregor G, Mayell S, Millar M, Modha D, Nash EF, O'Brien C, O'Brien D, Ohri C, Pao CS, Peckham D, Perrin F, Perry A, Pressler T, Prtak L, Qvist T, Robb A, Rodgers H, Schaffer K, Shafi N, van Ingen J, Walshaw M, Watson D, West N, Whitehouse J, Haworth CS, Harris SR, Ordway D, Parkhill J, Floto RA. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hand WL, Corwin RW, Steinberg TH, Grossman GD. 1984. Uptake of antibiotics by human alveolar macrophages. Am Rev Respir Dis 129:933–937. [DOI] [PubMed] [Google Scholar]
- 13.Gotfried MH, Horn K, Garrity-Ryan L, Villano S, Tzanis E, Chitra S, Manley A, Tanaka SK, Rodvold KA. 2017. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother 61:e01135-17. doi: 10.1128/AAC.01135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]