APX001A is the active moiety of the first-in-class drug candidate APX001. So far, most susceptibility testing studies have examined ≤30 isolates/species, and only one used the EUCAST method.
KEYWORDS: APX001A, EUCAST, Aspergillus, Candida, Candida auris, amphotericin B, antifungal susceptibility testing, azoles, candidemia, echinocandins
ABSTRACT
APX001A is the active moiety of the first-in-class drug candidate APX001. So far, most susceptibility testing studies have examined ≤30 isolates/species, and only one used the EUCAST method. Here, we investigated the in vitro activity of APX001A and five comparators against 540 candidemia and 122 C. auris isolates. Isolates (17 Candida and 3 yeast species) were identified using CHROMagar, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) and, when needed, internal transcribed space (ITS) sequencing. EUCAST E.Def 7.3.1 susceptibility testing included APX001A, amphotericin B, anidulafungin, micafungin, fluconazole, and voriconazole. Wild-type upper limits (WT-UL) were established following the EUCAST principles for epidemiological cutoff value setting for APX001A, allowing classification as wild type (WT) or non-WT. APX001A MIC50 values (mg/liter) were as follows: Candida albicans, Candida dubliniensis, and Candida tropicalis, 0.004 to 0.008; Candida parapsilosis and Candida auris, 0.016; Candida glabrata, 0.06; and Candida krusei, >0.5. APX001A MICs against the rare species varied from ≤0.0005 (C. pelliculosa) to >0.5 (Candida norvegensis). APX001A was equally or more active in vitro than the comparators against all species except C. krusei and C. norvegensis. Four isolates were APX001A non-WT; all were fluconazole resistant. A correlation was observed between APX001A and fluconazole MICs across all species except Candida guilliermondii and C. auris, and when comparing high and low fluconazole MIC isolates of C. albicans, C. dubliniensis, C. glabrata, C. tropicalis, and C. auris. APX001A showed promising in vitro activity against most Candida and other yeast species, including C. auris, compared to five comparators. WT-UL were suggested for the common species, and a new and unexplained correlation to fluconazole susceptibility was observed.
INTRODUCTION
APX001A (formerly E1210) is the active moiety of the first-in-class small-molecule drug candidate APX001, currently in phase 1 clinical trials. It has broad-spectrum activity that includes Candida, Aspergillus, and rare molds. APX001A inhibits the conserved fungal inositol acyltransferase enzyme GWT1, thereby preventing glycosylphosphatidylinositol (GPI)-anchored protein maturation and compromising fungal growth.
In vitro activity against Candida spp. has been investigated using the Clinical and Laboratory Standards Institute (CLSI) M27A-3 methodology.1 CLSI MIC90 values of <0.008 to 0.06 mg/liter were observed across the different species, except for Candida krusei (CLSI MICs of 2 to >32 mg/liter) (2, 3). APX001A retained activity against isolates with acquired resistance to fluconazole and echinocandins (2). Moreover, in vitro activity was recently demonstrated against 16 Candida auris isolates (4). APX001A has been investigated in murine models of oropharyngeal and invasive candidiasis and demonstrates in vivo activity against Candida albicans, Candida tropicalis, and C. auris (4–6). The area under the concentration-time curve over 24 h in the steady state for the free, unbound fraction divided by the MIC (fAUC0–24/MIC ratio) has been proposed to be the pharmacokinetic/pharmacodynamic (PK/PD) index that best correlates with efficacy (7). A lower stasis fAUC/MIC target was found for Candida glabrata (1.31 ± 0.27) compared to those for C. albicans (20.60 ± 6.50) and C. auris (14.67 ± 8.30), suggesting that clinical breakpoints should be species specific.
APX001A in vitro susceptibility evaluations utilizing the European Committee on Antimicrobial Susceptibility Testing (EUCAST) E.Def 7.3.1 method are very scarce and are limited to a study investigating 12 to 25 Candida isolates from each of the 5 most common species (C. albicans, C. glabrata, C. krusei, Candida parapsilosis, and C. tropicalis) (3). We therefore included APX001A in our prospective EUCAST antifungal susceptibility testing of bloodstream isolates referred as part of the nationwide fungemia surveillance program in Denmark to generate population-based contemporary EUCAST MIC data for this compound and for future epidemiologic cutoff (ECOFF) and clinical breakpoint setting. In parallel, we investigated the susceptibility pattern of 122 Indian clinical C. auris isolates to APX001A and comparator drugs by the EUCAST method to also generate MIC data for this multidrug-resistant yeast that is rapidly emerging as a significant cause of nosocomial infections (references 8 and 9; see also https://www.cdc.gov/fungal/diseases/candidiasis/candida-auris.html). We found that APX001A was highly active against both yeast bloodstream isolates and C. auris isolates, but also demonstrated an unexplained correlation between APX001A and fluconazole MICs across and within the yeast species.
RESULTS
APX001A against quality control strains.
C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were tested 46 and 84 times, respectively, during the study period. The MIC results (mg/liter) for C. parapsilosis ATCC 22019 were as follows: modal MIC, 0.03; MIC50, 0.016; range, 0.008 to 0.03. A total of 44/46 (95.7%) MICs were within the range of 0.016 to 0.03 mg/liter. The MICs against C. krusei ATCC 6258 were 0.5 mg/liter on one occasion and >0.5 mg/liter (outside the tested concentration range) for the rest (n = 83).
APX001A activity against contemporary Danish blood isolates and C. auris.
APX001A displayed in vitro activity with MICs of <0.5 mg/liter against all Candida bloodstream isolates except those of C. krusei and Candida norvegensis (Table 1). C. albicans, Candida dubliniensis and C. tropicalis were the most susceptible species among the 6 most common species, with MIC50 values of 0.004 to 0.008 mg/liter, followed by C. parapsilosis (MIC50, 0.016 mg/liter) and C. glabrata (MIC50, 0.06 mg/liter). APX001A MICs varied considerably against the rare Candida and yeast species, with Candida pelliculosa being the most susceptible organism (MIC, ≤0.0005 mg/liter), followed by Candida fermentati, Candida guilliermondii, Candida lusitaniae, Candida metapsilosis, Candida utilis, Candida orthopsilosis, Candida nivariensis, and Saccharomyces cerevisiae, with MICs in the range of 0.004 to 0.03 mg/liter; Magnusiomyces capitatus, Candida kefyr, and Cryptococcus neoformans, with MICs in the range of 0.125 to 0.5 mg/liter; and C. norvegensis, with MICs of >0.5 mg/liter. Finally, APX001A also displayed in vitro activity against Indian clinical C. auris isolates, with MIC50 and modal MIC values of 0.016 mg/liter and a MIC range of 0.001 to 0.125 mg/liter (Table 1).
TABLE 1.
APX001A MICs (mg/liter) for contemporary Danish bloodstream isolates and C. auris isolates
| Species | N | MIC (mg/liter)b |
Range | MIC50 (mg/liter) | MIC90 (mg/liter) | WT-ULa |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.0005 | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | >0.5 | Visual | Statistical |
||||||
| 97.50% | 99% | ||||||||||||||||||
| Danish blood isolates | |||||||||||||||||||
| Candida albicans | 218 | 1 | 9 | 24 | 108 | 69 | 7 | 0.001–0.03 | 0.008 | 0.016 | 0.03 | 0.03 | 0.03 | ||||||
| Candida dubliniensis | 29 | 5 | 14 | 9 | 1 | 0.002–0.03 | 0.004 | 0.008 | 0.016 | 0.016 | 0.016 | ||||||||
| Candida glabrata | 179 | 1 | 6 | 52 | 102 | 16 | 2 | 0.008–0.25 | 0.06 | 0.06 | 0.125 | 0.125 | 0.125 | ||||||
| Candida krusei | 31 | 1 | 30 | 0.5–>0.5 | >0.5 | >0.5 | ND | ND | ND | ||||||||||
| Candida parapsilosis | 19 | 2 | 8 | 9 | 0.008–0.03 | 0.016 | 0.03 | 0.06 | NP | NP | |||||||||
| Candida tropicalis | 33 | 10 | 13 | 9 | 1 | 0.004–0.125 | 0.008 | 0.016 | 0.03 | 0.03 | 0.03 | ||||||||
| Other Candida spp.c | 25 | 1 | 4 | 10 | 1 | 5 | 1 | 2 | 1 | ≤0.0005–>0.5 | 0.008 | 0.25 | ND | ND | ND | ||||
| Other yeast spp.d | 6 | 1 | 1 | 3 | 1 | 0.3–0.5 | 0.25 | 0.25 | ND | ND | ND | ||||||||
| Indian clinical isolate | |||||||||||||||||||
| Candida auris | 122 | 4 | 6 | 11 | 16 | 41 | 38 | 5 | 1 | 0.001–0.125 | 0.016 | 0.03 | 0.06 | 0.125 | 0.125 | ||||
WT-UL, wild-type upper limit determined visually and statistically using the ECOFFinder program, including 97.5% and 99% of the isolates, respectively. The ECOFFinder program requires at least one isolate with MIC at least one step above the modal MIC to calculate an upper limit. NP, not provided; ND, not done.
MIC values above the WT-UL are highlighted in bold and underlined.
Other Candida spp. included Candida fermentati (n = 2), Candida guilliermondii (n = 6), Candida kefyr (n = 3), Candida lusitaniae (n = 7), Candida metapsilosis (n = 1), Candida nivariensis (n = 1), Candida norvegensis (n = 1), Candida orthopsilosis (n = 2), Candida pelliculosa (n = 1), and Candida utilis (n = 1).
Other yeast spp. included Cryptococcus neoformans (n = 4), Magnusiomyces capitatus (n = 1), and Saccharomces cerevisiae (n = 1).
APX001A wild-type upper limit (WT-UL) values were determined visually and statistically using the EUCAST ECOFFinder program for the species for which the MIC distributions were not truncated (Table 1). The WT-UL were as follows: C. albicans, 0.03 mg/liter; C. dubliniensis, 0.016 mg/liter; C. glabrata, 0.125 mg/liter; and C. tropicalis, 0.03 mg/liter, irrespective of which method was used for determination. For C. parapsilosis, a visual WT-UL was set at 0.06 mg/liter, but a statistical WT-UL could not be determined because there were no MICs higher than the modal MIC. Applying these WT-UL values for classification of the blood isolates into WT and non-WT isolates, the following isolates were classified as non-WT for APX001A: C. dubliniensis, 1/29 (3.4%; MIC, 0.03 mg/liter); C. glabrata, 2/179 (1.1%; both with MIC of 0.25 mg/liter); and C. tropicalis 1/33 (3.0%; MIC, 0.125 mg/liter). Overall, 4/540 (0.74%) of yeast blood isolates were classified as non-WT.
For C. auris, the visual (0.06 mg/liter) or statistical (0.125 mg/liter) WT-UL differed by one dilution. Depending on which WT-UL was adopted, 0.8% or none of the C. auris isolates displayed MICs above the WT-UL.
APX001A in vitro activity compared to comparators.
The in vitro susceptibility to APX001A was compared to that for the five other antifungal agents, adopting modal MICs no more than two dilutions apart as the criterion for equal in vitro activity (see Table 2). APX001A was equally or more active than amphotericin B, anidulafungin, micafungin, fluconazole, and voriconazole on a mg/liter basis against all species except C. krusei and C. norvegensis, against which the two echinocandins were superior. Non-WT susceptibility was found for 0 to 1% of the isolates, depending on the species, for APX001A, compared to 0% for amphotericin B, 0 to 1.1% for anidulafungin, 0 to 3.2% for micafungin, 0 to 15.2% for fluconazole, and 0 to 18.2% for voriconazole.
TABLE 2.
Comparison of APX001A MICs (mg/liter) to those for other licensed antifungal agents for contemporary Danish bloodstream isolates and C. auris
| Species | APX001A |
AMB |
ANF |
MFG |
FLC |
VRC |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC range | Modal MIC | % > ECOFF | MIC range | Modal MIC | % > ECOFF | MIC range | Modal MIC | % > ECOFF | MIC range | Modal MIC | % > ECOFF | MIC range | Modal MIC | % > ECOFF | MIC range | Modal MIC | % > ECOFF | |
| Danish blood isolates | ||||||||||||||||||
| C. albicans | 0.001–0.03 | 0.008 | 0 | 0.06–0.5 | 0.25 | 0 | ≤0.004–0.016 | < = 0.004 | 0 | ≤0.004–0.03 | 0.008 | 1.8 | 0.03–4 | 0.25 | 0.5 | ≤0.004–0.06 | ≤0.004 | 0.5 |
| C. dubliniensis | 0.002–0.03 | 0.004 | 3.4 | 0.03–0.125 | 0.06 | 0 | ≤0.004–0.016 | 0.008 | 0 | ≤0.004–0.03 | 0.016 | ND | 0.06–32 | 0.125 | 3.4 | ≤0.004–0.125 | 0.008 | 3.4 |
| C. glabrata | 0.008–0.25 | 0.06 | 1 | 0.06–0.5 | 0.5 | 0 | 0.008–0.25 | 0.016 | 1.1 | ≤0.004–0.125 | 0.016 | 2.2 | 0.5–>32 | 4 | 9.5 | 0.016–>4 | 0.06 | 9.5 |
| C. krusei | 0.5–>0.5 | >0.5 | NDa | 0.5–1 | 1 | 0 | 0.016–0.06 | 0.03 | 0 | 0.06–0.5 | 0.125 | 3.2 | 8–>32 | 32 | ND | 0.125–2 | 0.25 | ND |
| C. parapsilosis | 0.008–0.03 | 0.03 | 0 | 0.25–1 | 0.5 | 0 | 0.5–2 | 0.5 | 0 | 0.5–2 | 2 | 0 | 0.5–2 | 0.5/1 | 0 | 0.008–0.03 | 0.016 | 0 |
| C. tropicalis | 0.004–0.125 | 0.008 | 3.4 | 0.25–0.5 | 0.5 | 0 | ≤0.004–0.03 | 0.016 | 0 | ≤0.004–0.06 | 0.03 | 0 | 0.25–32 | 0.25/0.5 | 15.2 | 0.008–>4 | 0.016 | 18.2 |
| Other Candida spp. | ≤0.0005–>0.5 | 0.008 | ND | 0.125–1 | 0.25 | ND | ≤0.004–2 | 0.03 | ND | 0.016–0.5 | 0.06 | ND | 0.125–32 | 0.5 | ND | 0.008–0.25 | 0.008 | ND |
| Other yeast spp. | 0.3–0.5 | 0.25 | ND | 0.25–1 | 0.25 | ND | 0.06–>4 | >4 | ND | 0.125–>4 | >4 | ND | 2–16 | 16 | ND | 0.016–0.5 | 0.125 | ND |
| Indian clinical isolate | ||||||||||||||||||
| Candida auris | 0.001–0.125 | 0.016 | 0 | 0.5–1 | 1 | 0 | 1–>32 | 0.06 | 8.2 | 0.03–>32 | 0.125 | 6.6% | 1–>256 | >256 | ND | 0.004–4 | 1 | ND |
ND, not done.
For C. auris specifically, the modal MIC was 0.016 mg/liter for APX001A, compared to 1 mg/liter, 0.06 mg/liter, 0.125 mg/liter, >256 mg/liter, and 1 mg/liter for amphotericin B, anidulafungin, micafungin, fluconazole, and voriconazole, respectively. Non-WT susceptibility was observed more often for the echinocandin and azole comparators. Thus, 10/122 (8.2%) and 8/122 (6.6%) of the C. auris isolates were non-WT to anidulafungin and micafungin, respectively, and 121/122 (99.2%) isolates were resistant to fluconazole, adopting the non-species-specific breakpoint of >4 mg/liter for resistance (Table 2 and Table S10 in the supplemental material).
Comparison of APX001A and fluconazole MICs.
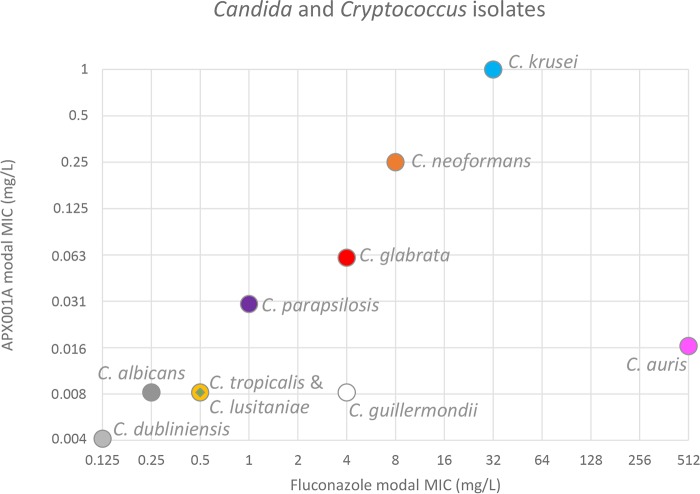
Four of four bloodstream isolates that were non-WT for APX001A were also fluconazole resistant (C. dubliniensis fluconazole MIC, 32 mg/liter; C. glabrata isolate fluconazole MICs, >32 mg/liter; and C. tropicalis fluconazole MIC, ≥16 mg/liter), suggesting a possible correlation between fluconazole and APX001A MICs. Therefore, the APX001A and fluconazole modal MICs were compared across species represented with at least four isolates (Fig. 1). A linear correlation was observed across Candida and Cryptococcus species, with the exception of C. guilliermondii and C. auris. Similarly, when APX001A MICs were examined for C. albicans, C. dubliniensis, C. glabrata, C. tropicalis, and C. auris isolates grouped according to fluconazole MICs, respectively, a correlation was again observed (Table 3).
FIG 1.
Correlation between APX001A and fluconazole modal MICs for bloodstream isolates represented by at least four isolates. C. albicans (dark gray circle), C. auris (pink), C. dubliniensis (light gray circle), C. glabrata (red circle), C. guilliermondii (white circle), C. lusitaniae (green diamond), C. krusei (turquoise circle), C. parapsilosis (purple circle), C. tropicalis (yellow circle), and Cryptococcus neoformans (orange circle).
TABLE 3.
APX001A MICs for isolates with low and high fluconazole MICs
| Species or fluconazole susceptibility | APX001A MIC (mg/liter) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.0005 | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | |
| C. albicans | |||||||||||
| Fluconazole MIC, ≤1 mg/liter | 1 | 9 | 24 | 108 | 69 | 6 | |||||
| Fluconazole MIC, 4 mg/liter | 1 | ||||||||||
| C. dubliniensis | |||||||||||
| Fluconazole MIC, ≤0.5 mg/liter | 5 | 14 | 9 | ||||||||
| Fluconazole MIC, 32 mg/liter | 1 | ||||||||||
| C. glabrata | |||||||||||
| Fluconazole MIC, ≤16 mg/liter | 1 | 6 | 52 | 92 | 7 | ||||||
| Fluconazole MIC, ≥32 mg/liter | 10 | 9 | 2 | ||||||||
| C. tropicalis | |||||||||||
| Fluconazole MIC, ≤8 | 10 | 13 | 8 | ||||||||
| Fluconazole MIC, ≥16 mg/liter | 1 | 1 | |||||||||
| C. auris | |||||||||||
| Fluconazole MIC, ≤64 mg/liter | 4 | 6 | 9 | 2 | |||||||
| Fluconazole MIC, >64 mg/liter | 2 | 14 | 41 | 38 | 5 | 1 | |||||
DISCUSSION
APX001A in vitro activity overall.
This study confirms previous reports that APX001A is a highly active compound on a mg/liter basis against all Candida species except C. krusei and the phylogenetically related species C. norvegensis (2, 3, 5, 10). The MIC50 values for C. albicans and C. glabrata were identical to those presented in the only other study presenting APX001A EUCAST MICs and a single dilution below those for C. parapsilosis and C. tropicalis. This indicates that EUCAST testing of APX001A may be associated with limited interlaboratory variation (3). APX001A also demonstrated activity against a range of rare Candida and other yeast species, with only Magnusiomyces capitatus, C. kefyr, and Cryptococcus neoformans being less susceptible than C. glabrata on a mg/liter basis. Of note, this included C. auris, an organism that is often drug or multidrug resistant, and which has emerged as a significant cause of nosocomial infections over the past 6 years in Asia, South Africa, Latin America, the United States, and in Europe (with cases in Spain, the United Kingdom, Germany, France, Belgium, Norway, and Austria) (8, 11, 12). This extends the findings of two recent reports studying a smaller number of C. auris isolates but confirming the in vitro activity in vivo using immunocompromised murine models of disseminated candidiasis (4, 7).
Single-center WT-UL were suggested for C. albicans, C. dubliniensis, C. glabrata, C. parapsilosis and C. tropicalis using visual inspection of the MIC distributions and statistically using the EUCAST ECOFFinder program. Overall, the WT-UL were in close agreement across the methods for determination and criteria used (97.5 or 99% of the isolates included), although the visual WT-UL was one dilution more restrictive than the statistical one for C. auris. While establishment of formal epidemiologic cutoff (ECOFF) values awaits the generation of multicenter data meeting the EUCAST criteria for aggregation, we believe that the WT-UL estimated here may serve for differentiating non-WT from WT organisms, provided that agreement with the quality control (QC) strain ranges and species-specific modal MIC values is ensured.
APX001A in vitro activity compared to that of licensed compounds.
APX001A was equally or more efficacious in vitro than amphotericin B, anidulafungin, micafungin, fluconazole, and voriconazole on a mg/liter basis. Moreover, only amphotericin B was associated with fewer non-WT organisms than APX001A. This renders APX001A a promising agent, particularly in light of (i) the increasing proportion of bloodstream infections due to C. glabrata, which is intrinsically fluconazole nonsusceptible, (ii) the increasing number of outbreaks involving C. auris, and (iii) the increasing acquired echinocandin resistance in C. glabrata, and at some centers of fluconazole resistance in C. tropicalis (11, 13–16).
APX001A PK/PD targets have recently been explored for C. albicans, C. glabrata, and C. auris for the CLSI method. Pfaller and colleagues performed a head to head comparison of EUCAST and CLSI MICs for C. albicans (n = 21) and C. glabrata (n = 20) isolates and found the EUCAST MIC50 to be one step lower for C. albicans and one step higher for C. glabrata. Subsequently, CLSI APX001A MIC50 values of 0.004 to 0.008 mg/liter have been reported, which are 1 to 2 dilutions lower than the EUCAST MIC50 reported here (4, 7). Translating the proposed CLSI PK/PD targets to EUCAST targets, the doses required to achieve stasis fAUC/MIC targets would be as follows: 41.20 (C. albicans), 0.66 (C. glabrata), 7.34 (C. auris). However, these EUCAST targets need proper validation.
APX001A-fluconazole MIC correlation.
We observed a yet-unrecognized correlation between fluconazole and APX001A susceptibility. Fluconazole inhibits the lanosterol 14-α-demethylase enzyme, thereby inhibiting ergosterol formation and incorporation into the cell membrane. Azole resistance has been linked to target gene mutations, increased expression of the target enzyme, and active efflux mediated by multidrug transporters (17). APX001A, on the other hand, targets fungal inositol acyltransferase enzyme GWT1, thereby preventing GPI-anchored protein maturation and compromising fungal growth. Thus, although the drug targets differ from one another, both are associated with the fungal membrane. We did not find any isolates with elevated APX001A MICs (compared to the WT APX001A population) in the absence of elevated fluconazole MICs. In contrast, we observed isolates with fluconazole resistance but WT APX001A MICs. This suggests that some, but not all, fluconazole resistance mechanisms affect APX001A susceptibility. Further research into the mechanisms of cross-resistance is warranted, as well as exploration of APX001 optimal dosing regimens to determine if non-WT isolates with elevated MICs can be successfully treated.
The MIC distribution for APX001A against C. auris spanned eight dilutions, a range that is broader than that for any of the other Candida species. MIC distributions for WT populations typically span five dilution steps. Broader ranges may reflect technical issues compromising reproducibility of the MIC testing, inclusion of isolates with incorrect species identification, or isolates that are non-WT. The narrower MIC ranges observed for the other species and compliance with the previously published EUCAST MIC data suggest that technical issues are less likely to be the explanation. Broad and multimodal MIC distributions were recently reported for azoles against this C. auris strain collection, and whole-genome sequencing data of C. auris has demonstrated that a considerable proportion of C. auris isolates harbored ERG11 target gene alterations (16, 18). Analysis of ERG11 target gene alterations of Indian C. auris isolates detected the amino acid substitutions Y132 and K143 in 77% (n = 34/44) of strains that were fluconazole resistant, whereas WT genotypes, i.e., without substitutions at these positions, were observed in isolates with low fluconazole MICs (1 to 2 mg/liter), suggesting that these substitutions confer a phenotype of resistance to fluconazole similar to that described for C. albicans (19). We speculate that the presence of azole resistance mechanisms affects APX001A susceptibility and thus explains the broad MIC distributions for both APX001A and the azoles.
In conclusion, we provide here the largest MIC data set so far for APX001A. APX001A showed promising in vitro activity against Candida and other yeasts, including C. auris, on a mg/liter basis and compared to five comparators, with the exception of C. krusei and C. norvegensis. WT-UL were suggested for the common species, and a new and unexplained correlation to fluconazole susceptibility was observed.
MATERIALS AND METHODS
Danish bloodstream isolates.
A total of 540 yeast bloodstream isolates collected during a 15-month study period (1 October 2016 to 31 December 2017) was included. The species distribution (number of isolates) was as follows: C. albicans (218), C. dubliniensis (29), C. glabrata (179), C. krusei (31), C. parapsilosis (19), C. tropicalis (33), other Candida spp. (25), and other yeast spp. (6). The isolates were obtained as part of the nationwide Danish surveillance program and thus represent a contemporary national and population-based isolate collection. During the study period, one Fusarium bloodstream isolate was also collected but not included.
Indian C. auris isolates.
A total of 122 clinical isolates of C. auris were collected from individual patients in 6 tertiary care hospitals in India from 2010 to 2015. The isolates were mainly from patients with candidemia (blood; n = 100), and other specimens (n = 22) from invasive Candida infections included tissue, pleural fluid, and a single isolate from pus.
Susceptibility testing.
EUCAST MICs were determined following E.Def 7.3.1 methodology (20). APX001A (Amplyx Pharmaceuticals, San Diego, CA) pure substance was stored in aliquots at −80°C and stock solutions prepared in dimethyl sulfoxide (DMSO, 5000 mg/liter; Sigma-Aldrich, Brøndby, Denmark). The final drug concentration ranges studied were 0.001 to 0.5 mg/liter for yeast blood isolates and 0.001 to 0.5 mg/liter for C. auris isolates. The following comparator compounds were also investigated (final concentration range and source of compound in parentheses): anidulafungin (0.004 to 4 mg/liter; Pfizer A/S, Ballerup, Denmark), micafungin (0.004 to 4 mg/liter; Astellas Pharma Inc., Tokyo, Japan), amphotericin B (0.004 to 4 mg/liter; Sigma-Aldrich), fluconazole (0.03 to 32 mg/liter for bloodstream isolates and 0.5 to 256 mg/liter for C. auris; Sigma-Aldrich), and voriconazole (0.004 to 4 mg/liter; Pfizer A/S, Ballerup, Denmark). Cell culture-treated microtiter plates (Nunc MicroWell 96-well microplates, catalog no. 167008; Thermo Fisher Scientific) were used throughout. Microtiter plates were prepared with 2-fold drug dilutions in double-concentration medium according to the EUCAST methodology and frozen at −80°C prior to use. The EUCAST quality control (QC) strains C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were tested in parallel.
Data management.
MIC ranges, modal MIC (the most common MIC), MIC50, and MIC90 values were calculated. Wild-type upper limits (WT-UL), defined as the upper MIC value where the wild-type distribution ends, were determined following principles for setting EUCAST ECOFFs. However, as the values reported here are not formally accepted EUCAST APX001A ECOFFs, we used the term “WT-UL” to avoid confusion. The conventional method for determining ECOFF is a visual inspection of histograms of the MICs for single species (the eyeball method) (21). Additionally, WT-UL were determined statistically using 97.5% and 99% endpoints and the EUCAST ECOFF finder program (21).
Supplementary Material
ACKNOWLEDGMENTS
We thank research technician Birgit Brandt for excellent technical assistance.
This study was supported by an unrestricted grant from Amplyx Pharmaceuticals. The funder had no influence on the study design nor on the analysis of the results.
Outside of this work, the authors have the following potential conflicts to declare. M.C.A. has received personal speaker's honoraria from Astellas, Basilea, Gilead, MSD, Pfizer, T2Candida, and Novartis. She has received research grants and contract work paid to the Statens Serum Institute from Astellas, Basilea, Gilead, MSD, Pfizer, T2Candida, F2G, Cidara, and Amplyx. A.C. has nothing to declare. K.M.T.A. has received travel grants from Gilead and Pfizer. K.M.J. has received meeting travel grants from Amplyx, MSD, and F2G.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01225-18.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2008. Approved standard M27-A3. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.Pfaller MA, Hata K, Jones RN, Messer SA, Moet GJ, Castanheira M. 2011. In vitro activity of a novel broad-spectrum antifungal, e1210, tested against Candida spp. as determined by CLSI broth microdilution method. Diagn Microbiol Infect Dis 71:167–170. doi: 10.1016/j.diagmicrobio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Watanabe N, Castanheira M, Messer SA, Jones RN. 2011. Pre-clinical development of antifungal susceptibility test methods for the testing of the novel antifungal agent E1210 versus Candida: comparison of CLSI and European Committee on Antimicrobial Susceptibility Testing methods. J Antimicrob Chemother 66:2581–2584. doi: 10.1093/jac/dkr342. [DOI] [PubMed] [Google Scholar]
- 4.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiederhold NP, Najvar LK, Fothergill AW, McCarthy DI, Bocanegra R, Olivo M, Kirkpatrick WR, Everson MP, Duncanson FP, Pattersona TF, Patterson TF, Pattersona TF. 2015. The investigational agent E1210 is effective in treatment of experimental invasive candidiasis caused by resistant Candida albicans. Antimicrob Agents Chemother 59:690–692. doi: 10.1128/AAC.03944-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hata K, Horii T, Miyazaki M, Watanabe N-AA, Okubo M, Sonoda J, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother 55:4543–4551. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, Lepak AJ, VanScoy B, Bader JC, Marchillo K, Vanhecker J, Ambrose PG, Andes DR. 2018. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother 62:e02542-17. doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohlenberg A, Struelens MJ, Monnet DL, Plachouras D, The Candida auris Survey Collaborative Group. 2018. Candida auris: epidemiological situation, laboratory capacity and preparedness in European Union and European Economic Area countries, 2013 to 2017. Eurosurveillance 23(13):pii=18-00136 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2018.23.13.18-00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki M, Horii T, Hata K, Watanabe N-AA, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 55:4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhary A, Voss A, Meis JF. 2016. Multidrug-resistant Candida auris: “new kid on the block” in hospital-associated infections? J Hosp Infect 94:209–212. doi: 10.1016/j.jhin.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Sharma C, Kumar N, Pandey R, Meis JF, Chowdhary A. 2016. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect 13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astvad KMTT, Johansen HK, Røder BL, Rosenvinge FS, Knudsen JD, Lemming L, Schønheyder HC, Hare RK, Kristensen L, Nielsen L, Gertsen JB, Dzajic E, Pedersen M, Østergård C, Olesen B, Søndergaard TS, Arendrup MC. 2018. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 56:e01564-17. doi: 10.1128/JCM.01564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. 2010. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009). Diagn Microbiol Infect Dis 68:278–283. doi: 10.1016/j.diagmicrobio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Castanheira M, Deshpande LM, Davis AP, Rhomberg PR, Pfaller MA. 2017. Monitoring antifungal resistance in a global collection of invasive yeasts and molds: application of CLSI epidemiological cutoff values and whole-genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob Agents Chemother 61:e00906-17. doi: 10.1128/AAC.00906-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morio F, Jensen RH, Le Pape P, Arendrup MC. 2017. Molecular basis of antifungal drug resistance in yeasts. Int J Antimicrob Agents 50:599–606. doi: 10.1016/j.ijantimicag.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. 2017. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother 61:AAC.00485-17. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, Tarai B, Singh A, Upadhyaya G, Upadhyay S, Yadav P, Singh PK, Khillan V, Sachdeva N, Perlin DS, Meis JF. 2018. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother 73:891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 20.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ. 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.e1–571.e4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.