Major gaps exist in our knowledge of antimicrobial pharmacokinetics in critically ill neonates and infants that require validated microsampling and bioanalysis methods to support therapeutic drug monitoring. We compared serially collected intravenous (i.v.) and heel stick capillary (HSC)-sampled plasma concentrations of micafungin (8 mg/kg) in eight infants born preterm with systemic candidiasis.
KEYWORDS: micafungin, neonates, pharmacokinetics, systemic candidiasis, microsampling, pediatric, antifungal agents, antimicrobial agents, candidiasis, echinocandin, heel stick, pharmacodynamics
ABSTRACT
Major gaps exist in our knowledge of antimicrobial pharmacokinetics in critically ill neonates and infants that require validated microsampling and bioanalysis methods to support therapeutic drug monitoring. We compared serially collected intravenous (i.v.) and heel stick capillary (HSC)-sampled plasma concentrations of micafungin (8 mg/kg) in eight infants born preterm with systemic candidiasis. The mean (standard deviation) micafungin area under the plasma concentration-time curve to infinity (AUCinf) was 316 (65.0) h · mg/liter based on HSC concentrations that strongly correlated (R2 = 0.92) with i.v. values to support dose adjustment.
TEXT
Sepsis due to invasive candidiasis is a major cause of mortality in preterm neonates that requires effective antifungal therapy, vasoactive and inotropic agents, and intravenous fluids (1). Optimization of antifungal doses is challenging in neonates and young infants born preterm because of the marked intraindividual variability caused by developmental, pathological, and therapeutic interventions that can dramatically modify drug distribution and clearance (2). Our current therapeutic approach often relies on drug dose extrapolation from adults and adolescents that are scaled to the neonatal body size, which does not sufficiently account for this between- and within-subject variability (2). Improved bioanalytical approaches can support precision dosing in this special population. However, the acquisition of useful pharmacological information requires multiple plasma measurements, which are difficult to collect in very-low-weight infants due to difficulties in finding accessible vessels and the limited volume of blood that can be collected (2). Several microsampling techniques have been developed, such as dried blood spot, capillary tube, and volumetric absorptive microsampling; however, validation of these methods is critical to ensure that the results are true (3). We compared four serial plasma levels of micafungin measured in blood samples collected simultaneously via central venous catheter and heel stick in eight at-term and preterm infants. The median gestational and postnatal ages at drug administration were 28.5 and 9.6 weeks, respectively. Our aim was to ascertain whether heel stick capillary (HSC) samples can be a valid alternative for intravenous blood sampling in very preterm and at-term neonates for micafungin dose optimization.
This investigation was carried out in a subset of eight babies during a phase 2 prospective study that was performed to evaluate plasma concentrations of micafungin administered to 30 infants and children up to 180 days of life who were diagnosed with systemic candidiasis. The study took place in the neonatal intensive care unit of Bambino Gesù Children's Hospital during a period of 30 months, from June 2015 to December 2017 (clinical study protocol no. 800_OPBG_2014, EUDRACT no. 2014-003087-20), after ethics committee approval. All patients' parents or legal guardians gave their consent to enrollment of the babies before study procedures. Micafungin 8 mg/kg daily was diluted in 0.9% saline solution at a concentration of 2 mg/ml and infused intravenously by central venous catheter in 1 h for a minimum of 14 days of therapy. Blood samples were collected from the neonates 1 h before infusion of the drug and 1, 2, and 8 h after the end of the intravenous infusion between days 3 and 10 of therapy through the central venous catheter and by a simultaneous HSC draw. A warm (40°C) pack was applied to the heel for 5 min before a 0.65- to 0.85-mm puncture using a Gentleheel incision device (Cardinal Health, Dublin, OH) at alternate sites per time point. The HSC blood was collected using a 0.3-ml capillary tube and transferred into microtubes (both contained EDTA). Plasma was separated by centrifugation at 3,000 × g and stored at 4°C until analysis within 6 h or frozen at −20°C and assayed within 7 days.
A high-performance liquid chromatographic method with a diode array detector was validated to ensure selectivity, calibration range and linearity, precision, accuracy, lower limit of detection (LLOD), lower limit of quantification (LLOQ), and extraction recovery (4). In addition, micafungin and the internal standard (anidulafungin) stock solution stability studies were performed under variable temperature and time storage conditions. For the analysis, micafungin and anidulafungin were extracted using protein precipitation and chromatographic separation on a reversed-phase column. The effluents were monitored at UV wavelengths of 273 nm (micafungin) and 306 nm (anidulafungin). The method was found to have high selectivity for micafungin, since no interfering peaks from endogenous or environmental compounds were detected at the expected retention time for micafungin (8.911 min) in any six independent drug-free plasma extracts evaluated. Micafungin showed an excellent (R2 = 0.93) linear response in the range 1 to 30 mg/liter with a relative error of <15% of the nominal drug concentrations (±20% for the lowest point of the standard curve). The LLOQ of micafungin was 1 mg/liter, and LLOD was 0.5 mg/liter. The means (standard deviations [SD]) extraction recovery of micafungin from plasma spiked with 10 and 30 mg/liter were 108% (2%) and 104% (2%), respectively. No carryover was observed for either compound. Methodological details and analytical results are detailed in the supplemental material.
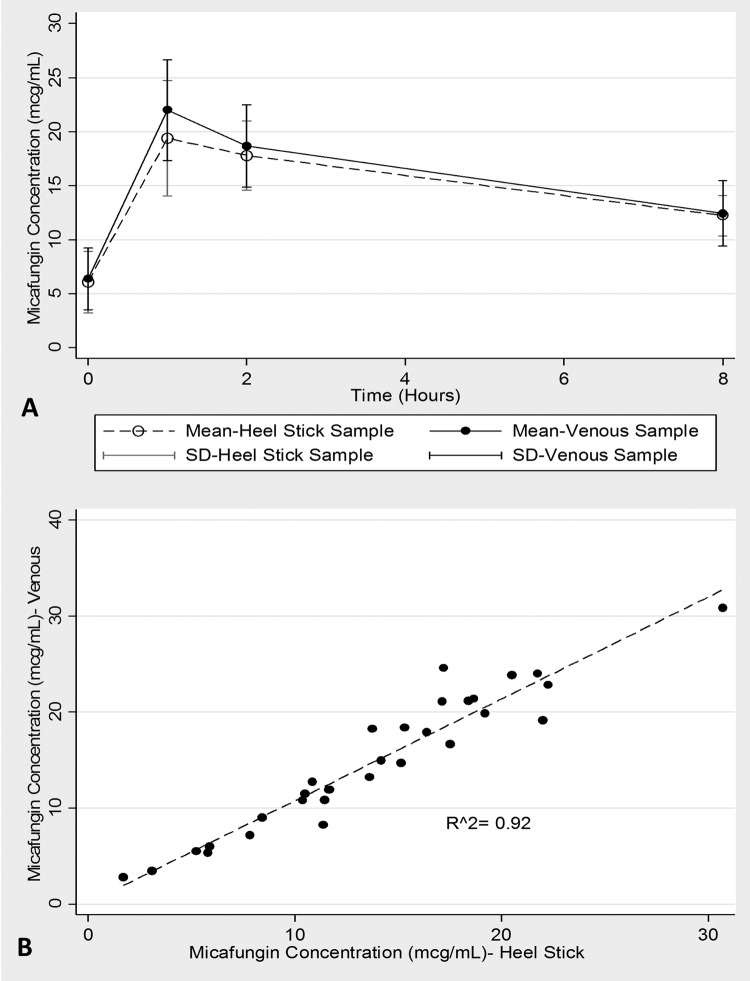
The median (min, max) estimated gestational age, birth weight, and weight at micafungin administration of the neonates were 28.5 weeks (26.0, 40.0 weeks), 1.05 kg (0.60, 3.10 kg), and 2.65 kg (1.50, 5.20 kg), respectively. The median (min, max) 1-h predose and postdose concentrations were 5.76 mg/liter (2.85, 11.5 mg/liter) and (18.5 mg/liter 13.8, 30.7 mg/liter), respectively. Figure 1A illustrates the mean (SD) micafungin concentration time plots that show near-superimposable profiles by HSC compared with venous samples. The mean (95% confidence interval [CI]) coefficients for the slope and constant were 1.06 (0.94, 1.18) and 0.13(−1.68, 1.95), with an R2 of 0.92, demonstrating a very strong linear correlation and proportional measurement between sampling methods (Fig. 1B). The mean (SD) micafungin areas under the plasma concentration-time curve to infinity (AUCinf) were 316 (65.0) and 291 (79.5) h · mg/liter for data generated by HSC versus venous samples, with a geometric mean ratio (90% CI) of 1.10 (0.99, 1.22). No significant (P > 0.05) correlation was observed between micafungin AUCinf and any individual concentration-time measurement from either the HSC or the venous sample.
FIG 1.
(A) Mean ± SD micafungin concentration-time plots by heel capillary and venous blood samples. (B) Scatter and linear regression fit plot of micafungin concentrations by the two sampling methods.
These results serve to validate the role of HSC as a tool to assay echinocandins such as micafungin when used to treat neonates and young infants with invasive candidiasis. Although our sample size may be regarded small, the head-to-head comparison of 32 prospectively collected samples by two strategies is actually quite difficult to study in this delicate population. Our findings are clinically relevant given that AUC estimates matched by both methods permitted translatability of the pharmacodynamic target linked with the decline in central nervous system fungal burden (5–7). Our findings create an opportunity to improve micafungin dosing in preterm and at-term critically ill neonates and give credence to a strategy that should be explored for other comparable agents.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01199-18.
REFERENCES
- 1.Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R, National Institute of Child Health and Human Development Neonatal Research Network. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 2.Mian P, Flint RB, Tibboel D, van den Anker JN, Allegaert K, Koch BCP. 2017. Therapeutic drug monitoring in neonates: what makes them unique? Curr Pharm Des 23:5790–5800. [DOI] [PubMed] [Google Scholar]
- 3.Dorofaeff T, Bandini RM, Lipman J, Ballot DE, Roberts JA, Parker SL. 2016. Uncertainty in antibiotic dosing in critically ill neonate and pediatric patients: can microsampling provide the answers? Clin Ther 38:1961–1975. doi: 10.1016/j.clinthera.2016.07.093. [DOI] [PubMed] [Google Scholar]
- 4.Martens-Lobenhoffer J, Rupprecht V, Bode-Böger SM. 2011. Determination of micafungin and anidulafungin in human plasma: UV- or mass spectrometric quantification? J Chromatogr B Analyt Technol Biomed Life Sci 879:2051–2056. doi: 10.1016/j.jchromb.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 5.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Herbrecht R, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 18(Suppl 7):38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 6.Leroux S, Jacqz Aigrain E, Elie V, Legrand F, Barin-Le Guellec C, Aurich B, Biran V, Dusang B, Goudjil S, Coopman S, Garcia Sanchez R, Zhao W, Manzoni P. FP7 TINN (Treat Infections in NeoNates) consortium. 2018. Pharmacokinetics and safety of fluconazole and micafungin in neonates with systemic candidiasis: a randomized open-label clinical trial. Br J Clin Pharmacol. doi: 10.1111/bcp.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope WW, Mickiene D, Petraitis V, Petraitiene R, Kelaher AM, Hughes JE, Cotton MP, Bacher J, Keirns JJ, Buell D, Heresi G, Benjamin DK Jr, Groll AH, Drusano GL, Walsh TJ. 2008. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis 197:163–171. doi: 10.1086/524063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.