One of the most recently described Aspergillus fumigatus CYP51A-mediated azole resistance mechanisms is TR46 Y121F T289A. Clinical A. fumigatus strains harboring these substitutions have been reported worldwide, with the exception of South America.
KEYWORDS: Aspergillus fumigatus, resistance, CYP51A, TR46, South America, TR46 Y121F T289A mutation, azole
ABSTRACT
One of the most recently described Aspergillus fumigatus CYP51A-mediated azole resistance mechanisms is TR46 Y121F T289A. Clinical A. fumigatus strains harboring these substitutions have been reported worldwide, with the exception of South America. We describe the first clinical A. fumigatus strain with this resistance mechanism isolated from an Argentinian patient. The strain was isolated in 2009 (1 year after the first-described mutant in United States), demonstrating that these alleles were scattered worldwide earlier than previously thought.
TEXT
Invasive aspergillosis (IA) is an important cause of mortality in immunocompromised patients, especially those undergoing chemotherapy and those who have received a bone marrow transplant (1, 2). Aspergillus fumigatus is the main causative agent of the mycoses, and triazoles are the drugs of choice for its treatment (3). However, clinical azole resistance may arise in patients exposed to these drugs and in azole-naive individuals (4–6). A. fumigatus azole resistance is mainly linked to CYP51A mutations that may or may not be associated with alterations in its promoter (tandem repeats [TR]) (7, 8). One of the most recently described CYP51A-mediated resistance mechanisms is a double substitution combined with a 46-bp tandem duplication in the CYP51A promoter (named TR46 Y121F T289A). These mutations lead to high levels of voriconazole (VRC) resistance associated with increased MIC values for the other azoles (9). Clinical A. fumigatus strains harboring these substitutions were reported mainly in Europe but also in Asia, Africa, and North America (6, 10–14). Recently, these mutants were found in environmental samples in Colombia (13, 15). In this work, we describe the first clinical A. fumigatus strain harboring the TR46 Y121F T289A mutation found in South America.
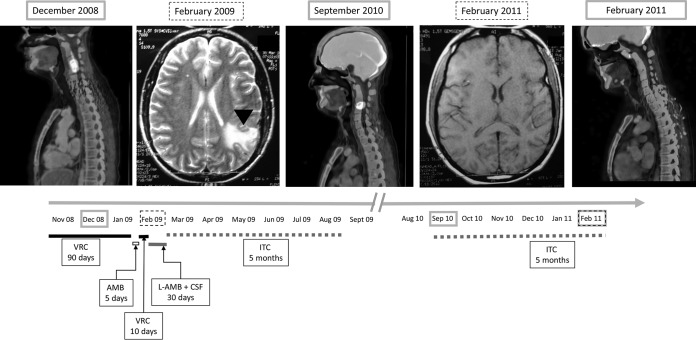
The aforementioned strain was isolated from a 25-year-old patient diagnosed in November 2007 with acute lymphoblastic leukemia. He developed a lung lesion in the course of febrile neutropenia in November 2008. Following the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) definitions, the patient was diagnosed with possible aspergillosis (no culture was obtained and no other mycology tests were performed) (16, 17), and empirical VRC treatment was initiated (two 6-mg/kg intravenous doses followed by 200 mg/day for 90 days). In December 2008, he started to have cervical pain, and a positron emission tomography computed tomography (PET-CT) scan showed augmented metabolic activity in his cervical area (Fig. 1). In February 2009, he developed aphasia, and a left parieto-occipital lesion was observed on magnetic resonance imaging (MRI) (Fig. 1). A brain biopsy was performed, and septated hyaline hyphae were observed. Liposomal amphotericin B (AMB) treatment was started. On the 5th day, the initial antifungal treatment was shifted to VRC because an A. fumigatus isolate was identified in the cultured specimens. Antifungal susceptibility testing was performed by agar diffusion (VRC) and microdilution (VRC, itraconazole [ITC], posaconazole [POS], AMB, caspofungin [CAS], and anidulafungin [AFG]) following CLSI documents M51A and M38, respectively (18, 19). No inhibition halo was obtained when VRC susceptibility was assessed by agar diffusion. VRC resistance was confirmed by microdilution (VRC MIC, >16 μg/ml). The POS MIC was also elevated (0.50 μg/ml,). On the other hand, the strain showed low AMB and ITC MICs and CAS and AFG minimum effective concentrations (1, 1, 0.03, and 0.06 μg/ml, respectively). Based on the susceptibility testing results, treatment was shifted to liposomal AMB combined with 50 mg/day CAS. Treatment was changed again after a month to ITC (400 mg/day), which was discontinued after 5 months because of liver toxicity. Twelve months after this last azole treatment (September 2010), the neck pain reappeared. On the PET-CT scan, a significant metabolic increase was observed (similar to the one observed in December 2008) (Fig. 1). The ITC treatment was repeated for 5 months, after which the symptoms disappeared and metabolic activity, according to PET-CT scan, returned to basal values (Fig. 1). During the described antifungal treatment, the patient′s underlying disease was treated and considered clinically cured. In April 2018, 9 years after treatment of the neck and brain aspergillosis and almost 8 years after completing his last antifungal treatment, the patient was asymptomatic and had not relapsed. The patient provided written informed consent for inclusion in this study.
FIG 1.
Gray boxes, positron emission tomography scans showing the patient's neck lesion as of December 2008, September 2010, and February 2011 (cured). Dotted boxes, brain magnetic resonance images as of February 2009 and February 2011 (no lesions). Arrowhead, brain lesion in February 2009 MRI. Lines represent antifungal treatment: black, initial 90-day (November 2008 to January 2009) voriconazole (VRC) treatment; white, 5-day amphotericin B (AMB) treatment; short black line, 10-day VRC treatment; gray line indicates 30-day liposomal AMB and caspofungin (CSF) combined treatment and dotted gray lines indicate two 5-month itraconazole (ITC) treatments between the end of February 2009 and August 2009 and between September 2010 and February 2011.
The azole-resistant strain was identified as A. fumigatus sensu stricto by β-tubulin and calmodulin gene sequencing and by matrix-assisted laser desorption ionization–time of flight mass spectrometry (20, 21). It is deposited and conserved at the Culture Collection of the Departamento Micologia (Instituto Nacional de Enfermedades Infecciosas [INEI] “Dr. C. G. Malbrán,” Buenos Aires, Argentina) under number DMIC-093515. A PCR designed to amplify a fragment of the CYP51A promoter (A7, 5′-TCATATGTTGCTCAGCGG-3′; A5R, 5′-TCTCTGCACGCAAAGAAGAAC-3′) was used to assess the presence of CYP51A promoter alterations. This PCR was performed in an Applied Biosystems thermocycler (Tecnolab-AB, Argentina) using the Pegasus DNA polymerase (PBL, Argentina) following the manufacturer′s protocol. The PCR bands were resolved in 2% agarose gel. In the studied clinical strain, the amplified promoter was bigger than its wild-type counterpart (Fig. 2), demonstrating that an alteration in this region would be responsible for the resistance phenotype (9, 22).
FIG 2.
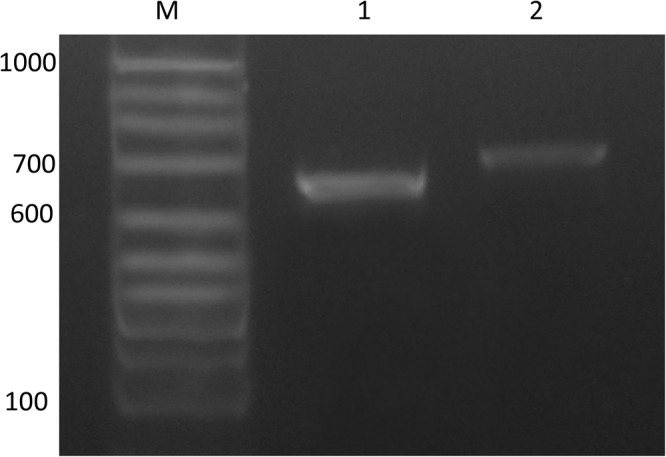
Electrophoresis of the A7-A5R PCR fragment of the CYP51A gene of the A. fumigatus strain resolved with 2% agarose gel. Lane M, 100-bp marker; lane 1, A. fumigatus LMDM-31 (wild type, azole susceptible); lane 2, TR46 Y121F T289A (DMIC-093515, VRC resistant).
The CYP51A open reading frame (ORF) and promoter (5′-UTR, 1,000 bp upstream of the start codon) were sequenced by Sanger methodology as described previously using ABI genetic analyzer 3500 (Applied Biosystems, CA) (22, 23). Compared with the wild-type A. fumigatus CYP51A sequence (GenBank accession no. AF338659.1), a tandem duplication of a 46-bp sequence (5′-GTCTAGAATCACGCGGTCCGGATGTGTGCTGAGCCGAATGAAAGTT-3′) was found between 282 and 327 nt downstream of the start codon. In addition, sequence analysis of the CYP51A ORF showed two substitutions (A433T and A936G), resulting in the amino acid changes at residues 121 and 289 (Y121F and T289A) (Fig. 2).
In this work, we report the first case in Argentina of an azole-resistant A. fumigatus strain harboring the CYP51A TR46 Y121F T289A mutation. Although these A. fumigatus mutants are considered to have an environmental origin (15, 24–26), the source of our strain is not clear because it was isolated 1 year after finishing 90 days of VRC treatment.
Interestingly, the studied strain was isolated in February 2009. Thus, it was isolated in the same year as the first European isolate harboring these mutations and only 1 year after the first reported strain harboring this CYP51A allele (14, 26), demonstrating that these mutations were scattered worldwide earlier than previously thought. In addition, the data presented here suggest that ITC treatment would be a plausible option for infections caused by this particular A. fumigatus mutant. However, ITC reaches low levels of concentration in cerebrospinal fluid, and it is not a treatment option for central nervous system infections (27). This azole is also not recommended as first-line treatment for any type of IA due to A. fumigatus (27, 28). The observed microbiological cure seems more likely to be due to recuperation of the patient's immune status than to the specific antifungal treatment. The finding of this mutant allele in South America is important clinically and epidemiologically, considering that antifungal susceptibility testing is not routinely performed and VRC is used as empirical therapy for pulmonary aspergillosis (3). Moreover, our finding highlights the need to acknowledge the extent of azole resistance in A. fumigatus worldwide.
ACKNOWLEDGMENTS
This study was supported in part by Science, Technology and Productive Innovation Ministry (MinCyT; Argentina) grant PICT2016/1985 to G.G.E. and by ANLIS “Carlos G Malbrán” (Argentina) grant FOCANLIS 2014, NRU 1422 to G.I. F.L. has a fellowship from CONICET (Argentina).
We thank R. Abrantes and J. Fernandez (ANLIS, INEI) for technical support.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 3.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dauchy C, Bautin N, Nseir S, Reboux G, Wintjens R, Le RO, Sendid B, Viscogliosi E, Le PP, Arendrup MC, Gosset P, Fry S, Frealle E. 2017. Emergence of Aspergillus fumigatus azole resistance in azole-naive patients with chronic obstructive pulmonary disease and their homes. Indoor Air 28:298–306. doi: 10.1111/ina. [DOI] [PubMed] [Google Scholar]
- 5.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelaez T, Monteiro MC, Garcia-Rubio R, Bouza E, Gomez-Lopez A, Mellado E. 2015. First detection of Aspergillus fumigatus azole-resistant strain due to Cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New Microbes New Infect 6:33–34. doi: 10.1016/j.nmni.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudakova A, Spiess B, Tangwattanachuleeporn M, Sasse C, Buchheidt D, Weig M, Gross U, Bader O. 2017. Molecular tools for the detection and deduction of azole antifungal drug resistance phenotypes in Aspergillus species. Clin Microbiol Rev 30:1065–1091. doi: 10.1128/CMR.00095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M. 2016. Triazole resistance in Aspergillus spp.: a worldwide problem? J Fungi (Basel) 2:E21. doi: 10.3390/jof2030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snelders E, Camps SM, Karawajczyk A, Rijs AJ, Zoll J, Verweij PE, Melchers WJ. 2015. Genotype-phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet Biol 82:129–135. doi: 10.1016/j.fgb.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Wang H, Lu Z, Li P, Zhang Q, Jia T, Zhao J, Tian S, Han X, Chen F, Zhang C, Jia X, Huang L, Qu F, Han L. 2015. Emergence of TR46/Y121F/T289A in an Aspergillus fumigatus isolate from a Chinese patient. Antimicrob Agents Chemother 59:7148–7150. doi: 10.1128/AAC.00887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2014. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J Antimicrob Chemother 69:555–557. doi: 10.1093/jac/dkt397. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 69:2979–2983. doi: 10.1093/jac/dku259. [DOI] [PubMed] [Google Scholar]
- 13.Goncalves SS. 2017. Global aspects of triazole resistance in Aspergillus fumigatus with focus on Latin American countries. J Fungi (Basel) 3:E5. doi: 10.3390/jof3010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, Sanders C, Fan H, Fothergill AW, Sutton DA. 2016. First detection of TR34 L98H and TR46 Y121F T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol 54:168–171. doi: 10.1128/JCM.02478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Moreno C, Lavergne RA, Hagen F, Morio F, Meis JF, Le PP. 2017. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci Rep 7:45631. doi: 10.1038/srep45631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascioglu S, Rex JH, De PB, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 17.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2008. Reference method for broth diution antifungal susceptibility testing of filamentous fungi; approved standard— 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2010. Method for antifungal disk diffusion susceptibility testing of filamentous fungi; proposed guideline. CLSI document M51-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Samson R, Hong S, Frisvad J. 2006. Old and new concepts of species differentiation in Aspergillus. Med Mycol 44:S133–S148. [DOI] [PubMed] [Google Scholar]
- 21.Vidal-Acuna MR, Ruiz-Perez de PM, Torres-Sanchez MJ, Aznar J. 2017. Identification of clinical isolates of Aspergillus, including cryptic species, by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Med Mycol. doi: 10.1093/mmy/myx115. [DOI] [PubMed] [Google Scholar]
- 22.Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Melchers WJ, Verweij PE, Cuenca-Estrella M, Rodriguez-Tudela JL. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother 51:1897–1904. doi: 10.1128/AAC.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL. 2003. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 47:1120–1124. doi: 10.1128/AAC.47.3.1120-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurst SF, Berkow EL, Stevenson KL, Litvintseva AP, Lockhart SR. 2017. Isolation of azole-resistant Aspergillus fumigatus from the environment in the south-eastern USA. J Antimicrob Chemother 72:2443–2446. doi: 10.1093/jac/dkx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snelders E, Huis In 't Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 27.Nett JE, Andes DR. 2016. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am 30:51–83. doi: 10.1016/j.idc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Florl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Bruggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Loffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinko J, Skiada A, Vehreschild MJGT, Viscoli C, Cornely OA. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]