We examined the antiviral activity of the integrase inhibitor (INI) cabotegravir against HIV-2 isolates from INI-naive individuals. HIV-2 was sensitive to cabotegravir in single-cycle and spreading-infection assays, with 50% effective concentrations (EC50s) in the low to subnanomolar range; comparable results were obtained for HIV-1 in both assay formats.
KEYWORDS: HIV-2, PrEP, West Africa, antiretroviral therapy, cabotegravir, human immunodeficiency virus, treatment
ABSTRACT
We examined the antiviral activity of the integrase inhibitor (INI) cabotegravir against HIV-2 isolates from INI-naive individuals. HIV-2 was sensitive to cabotegravir in single-cycle and spreading-infection assays, with 50% effective concentrations (EC50s) in the low to subnanomolar range; comparable results were obtained for HIV-1 in both assay formats. Our findings suggest that cabotegravir should be evaluated in clinical trials as a potential option for antiretroviral therapy and preexposure prophylaxis in HIV-2-prevalent settings.
TEXT
Human immunodeficiency virus type 2 (HIV-2) is endemic in West Africa and has spread to other locales with socioeconomic ties to the region (1, 2). Relative to HIV-1, HIV-2 infection involves a slower rate of CD4 cell decline, lower plasma viral loads, and slower disease progression (3–7). Nevertheless, significant numbers of HIV-2 and HIV-1/2 dually infected individuals eventually progress to AIDS and can benefit from antiretroviral therapy (ART) (7–11).
There are important differences between HIV-1 and HIV-2 with regard to antiretroviral (ARV) drug sensitivity (12, 13). HIV-2 is intrinsically resistant to nonnucleoside reverse transcriptase inhibitors (NNRTIs) (14, 15) and shows relatively poor sensitivity to several HIV-1-active protease inhibitors (PIs); saquinavir, darunavir, and lopinavir appear to be the only PIs with clinically effective potency against HIV-2 (16–20). These distinctions complicate HIV treatment in West Africa and other regions where HIV-1 and HIV-2 cocirculate. Difficulties in differentiating HIV-2 or HIV-1/2 dual infection from HIV-1 infection can lead to the inappropriate use of NNRTI-based regimens in HIV-2-infected patients and to premature use of PI-based regimens as first-line ART in patients infected solely with HIV-1 (21–23). Efforts are needed to simplify ART in areas where HIV-1/HIV-2 discriminatory testing is unreliable and stockouts of HIV-2-active antivirals are commonplace (24).
ARV regimens containing two nucleoside reverse transcriptase inhibitors (NRTIs) plus an integrase inhibitor (INI) or an NNRTI are currently recommended by the World Health Organization for first-line treatment of HIV-1 infection (25). A growing body of evidence suggests that INI-based regimens might fulfill the need for universally active first-line ART in settings where HIV-2 is endemic. HIV-2 is susceptible to the INIs raltegravir, elvitegravir, and dolutegravir, with 50% effective concentrations (EC50s) in the low-nanomolar to picomolar range (26–31). Data from case studies and small case series indicate that raltegravir- and elvitegravir-based regimens can suppress HIV-2 viral loads in ART-naive individuals (32, 33) and in ART-experienced patients whose treatment history does not include an INI (32, 34–39). More recently, two groups conducting clinical trials in ART-naive HIV-2-infected patients reported favorable immunovirologic outcomes in response to INI-based regimens (40, 41). In addition, some evidence suggests that dolutegravir might be effective in a subset of HIV-2-infected patients who have developed resistance to raltegravir (42–44).
Cabotegravir (S/GSK1265744; Shionogi/GlaxoSmithKline) is an investigational INI currently in development for the prevention and treatment of HIV-1 infection (45, 46). The antiviral potency and pharmacokinetic properties of cabotegravir render the drug amenable to once-daily oral dosing, and long-acting injectable formulations of the drug have been evaluated in nonhuman primate models of HIV-1 infection and in clinical trials (47–58). In contrast, there are no published data regarding the activity of cabotegravir against HIV-2, although one group reported a mean EC50 of 0.12 nM for four HIV-2 isolates at an international meeting (59).
In the current study, we tested the susceptibility of 15 different HIV-2 isolates (8 from group A, 6 from group B, and 1 A/B intergroup recombinant) to cabotegravir in single-cycle infections of MAGIC-5A indicator cells. A detailed description of the single-cycle assay has been published elsewhere (60). We further tested a subset of our HIV-2 library in 6-day spreading infections of an immortalized T-cell line (CEMss) as described below. In both assay formats, HIV-1 isolates from ART-naive individuals were included for comparison. The 50% cytotoxic concentrations (CC50) of cabotegravir in MAGIC-5A and CEMss cells were >1 and >10 μM, respectively, as assessed by CellTiter-Glo luminescent cell viability assay (Promega) (see Fig. S1 in the supplemental material).
Single-cycle assays: HIV-1NL4-3 and HIV-2ROD9.
We initially compared the susceptibility of two prototypic HIV strains to cabotegravir, i.e., HIV-1NL4-3 (group M, subtype B) and HIV-2ROD9 (group A). These viruses were derived from 293T/17 cultures that were transfected with corresponding full-length plasmid molecular clones as previously described (61). Both strains were tested against cabotegravir from two sources, GlaxoSmithKline (GSK) and Selleck Chemicals, Inc. All dilutions of the drug were prepared in 10% vol/vol dimethyl sulfoxide (DMSO); the final concentration of DMSO in the assay wells was 1%.
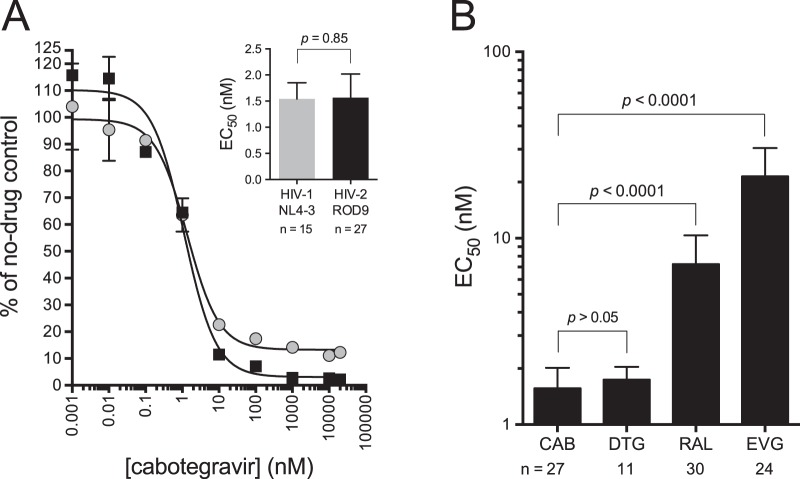
Cabotegravir from both suppliers was highly active against HIV-1NL4-3 and HIV-2ROD9, with EC50s ranging from 1.2 to 1.7 nM (see Table S1 in the supplemental material). These values are consistent with the EC50s reported for HIV-1NL4-3-based vectors in a single round of replication (EC50s of 0.5 nM [47] and 1.6 nM [62]). Altogether, HIV-1NL4-3 and HIV-2ROD9 were similar in their susceptibility to cabotegravir; after 15 and 27 independent determinations, respectively, the mean EC50s for these two strains differed by <1.1-fold (Fig. 1A and Table 1). For HIV-2ROD9, the antiviral potency of cabotegravir was comparable to that of dolutegravir but greater than that of raltegravir and elvitegravir, as determined in the single-cycle assay (Fig. 1B).
FIG 1.
Antiviral activity of cabotegravir against HIV-1NL4-3 and HIV-2ROD9. All data in the figure are from single-cycle infections of MAGIC-5A cells. Error bars indicate ±1 SD and, when not visible, are smaller than the symbols. (A) Results from a single assay in which HIV-1NL4-3 (gray circles) and HIV-2ROD9 (black boxes) were tested head-to-head. Data points represent the amount of β-galactosidase activity produced in HIV-infected cabotegravir-treated cultures relative to HIV-infected solvent-only (i.e., no-drug) controls. Each point is the mean of two cultures that were maintained in parallel. Curves were generated using a sigmoidal regression equation (GraphPad Prism 6.0 software). Mean EC50s from multiple assay runs with HIV-1NL4-3 and HIV-2ROD9 are shown in the inset. Values below the x axis indicate the total number of assay runs that were performed for each strain. The P value was calculated using Welch's t test. (B) EC50s obtained for HIV-2ROD9 tested against cabotegravir (CAB), dolutegravir (DTG), raltegravir (RAL), and elvitegravir (EVG). Data for raltegravir and dolutegravir are contemporaneous with the cabotegravir data set; data for elvitegravir were previously published (71). P values are from analysis of variance with Tukey's posttest.
TABLE 1.
Susceptibility of HIV-1 and HIV-2 isolates to cabotegravir in single-cycle infections of MAGIC-5A cells
| Isolate by HIV type | Group/subtype | EC50 (nM)a for: |
|
|---|---|---|---|
| Cabotegravirb | Efavirenzc | ||
| HIV-1 | |||
| 92UG029 | M/A | 2.0 ± 0.43 | 2.9 ± 0.35 |
| NL4-3 | M/B | 1.5 ± 0.31 | 2.2 ± 0.46 |
| LAI | M/B | 1.4 ± 0.45 | 1.7 ± 0.073 |
| MJ4 | M/C | 1.3 ± 0.061 | 1.4 ± 0.16 |
| 92UG001 | M/D | 2.2 ± 1.3 | 2.0 ± 0.21 |
| MVP5180-91 | O | 2.0 ± 0.11 | 54 ± 6.5 |
| HIV-2 | |||
| ROD9 | A | 1.6 ± 0.45 | >1,000 |
| 7924A | A | 1.0 ± 0.15 | >1,000 |
| MVP15132 | A | 2.5 ± 1.2 | >1,000 |
| 60415K | A | 2.7 ± 0.70 | >1,000 |
| CBL-20 | A | 0.92 ± 0.048 | >1,000 |
| CBL-23 | A | 2.1 ± 0.79 | >1,000 |
| CDC77618 | A | 2.3 ± 0.81 | >1,000 |
| ST | A | 1.6 ± 0.30 | >1,000 |
| CDC310072 | B | 1.0 ± 0.23 | >1,000 |
| CDC310319 | B | 4.0 ± 0.84 | >1,000 |
| EHO | B | 4.1 ± 1.4 | >1,000 |
| DIL | B | 2.5 ± 0.33 | >1,000 |
| COU | B | 2.3 ± 0.46 | >1,000 |
| BER | B | 2.7 ± 0.82 | >1,000 |
| 7312A | CRF01_ABd | 1.6 ± 0.46 | >1,000 |
EC50, 50% effective concentration (mean ± SD).
All EC50s for cabotegravir were calculated from three or more independent assay runs. EC50s for NL4-3 and ROD9 were obtained using cabotegravir from GlaxoSmithKline, Inc., and Selleck Chemicals, Inc. (see also Table S1 in the supplemental material). The remaining isolates listed above were tested against cabotegravir from Selleck Chemicals.
The NNRTI efavirenz serves as a non-INI control. EC50s for efavirenz are the results of 3 determinations for each HIV-1 isolate and ≥2 determinations for each HIV-2 isolate.
Intergroup (A/B) recombinant. The integrase-encoding sequence of HIV-27312A is monophyletic with that of other isolates belonging to HIV-2 group B (72).
Single-cycle assays: other HIV-1 and HIV-2 isolates.
Next, we tested other HIV isolates from ARV-naive individuals in single-cycle infections. Cabotegravir inhibited group M HIV-1 strains from subtypes A, B, C, and D, as well as the group O isolate HIV-1MVP5180-91, with EC50s ranging from 1.3 to 2.2 nM (Table 1). A similar range of EC50s was observed for eight group A HIV-2 strains (0.92 to 2.7 nM) (Table 1). Slight reductions in cabotegravir sensitivity relative to HIV-2ROD9 were apparent for group B isolates HIV-2CDC310319 and HIV-2EHO (EC50s, 4.0 ± 0.84 and 4.1 ± 1.4 nM, respectively; P < 0.0001, analysis of variance with Sidak's posttest). However, four other HIV-2 group B isolates yielded EC50s that were similar to those seen for HIV-1 and HIV-2 group A (range, 1.0 to 2.7 nM) (Table 1). In addition, the A/B intergroup recombinant HIV-27312A (CRF01_AB), which contains a group B integrase sequence, was fully susceptible to the drug (EC50, 1.6 nM) (Table 1). Altogether, the average EC50s (±1 standard deviation) for HIV-1, group A HIV-2, and group B HIV-2 were 1.7 ± 0.38, 1.8 ± 1.0, and 2.6 ± 1.3 nM, respectively.
As an additional control, we determined the susceptibility of each of the HIV-1 and HIV-2 isolates discussed above to the NNRTI efavirenz. All HIV-2 strains were highly resistant to efavirenz in single-cycle infections, whereas all HIV-1 group M strains were susceptible to the drug (Table 1; see also Fig. S1 in the supplemental material). HIV-1MVP5180-91 (group O) also showed a reduction in efavirenz susceptibility relative to HIV-1 group M (EC50, 54 ± 6.5 nM); this result is consistent with a previous report showing that HIV-1MVP5180-91 is intrinsically resistant to the NNRTIs delavirdine and nevirapine in culture (63).
To ensure that our single-cycle assay could detect subtle differences in cabotegravir susceptibility, we constructed and tested HIV-1 and HIV-2 variants that contained site-directed mutations in the integrase-encoding region of pol; these mutations encode amino acid changes that are known to confer low- to intermediate-level resistance to cabotegravir and/or other INIs in vitro (27–31, 62, 64–67). The combination of replacements E92Q and N155H in HIV-1NL4-3 integrase conferred a 3.9-fold increase in the EC50 for cabotegravir relative to the parental wild-type clone. In contrast, the Y143C mutation alone or in combination with T97A had no impact on cabotegravir susceptibility (Table 2). These results are concordant with previous findings for Y143C and E92Q+N155H mutants of HIV-1 in single-cycle assays (47, 62). In addition, the E138K+G140S+Q148R mutant of HIV-1NL4-3 was 10-fold resistant to cabotegravir. For HIV-2ROD9, mutants E92Q+Y143C, E92Q+N155H, and G140A+Q148R were 1.5-, 7.5-, and 6.9-fold resistant to cabotegravir, respectively, relative to wild-type HIV-2ROD9 (Table 2). Collectively, these data show that the single-cycle assay can reliably detect low-level cabotegravir resistance in both HIV-1 and HIV-2.
TABLE 2.
Antiviral activity of cabotegravir against site-directed mutants of HIV-1 and HIV-2 integrase
| Genotypea by HIV type | EC50 (nM)b | Fold changec |
|---|---|---|
| HIV-1 | ||
| Wild type | 1.5 ± 0.31 | |
| Y143C | 1.2 ± 0.069 | 0.80 |
| T97A+Y143C | 1.2 ± 0.75 | 0.80 |
| E92Q+N155H | 5.9 ± 0.81 | 3.9 |
| E138K+G140S+Q148R | 15 ± 3.1 | 10 |
| HIV-2 | ||
| Wild type | 1.6 ± 0.45 | |
| E92Q+Y143C | 2.4 ± 0.48 | 1.5 |
| E92Q+N155H | 12 ± 5.4 | 7.5 |
| G140A+Q148R | 11 ± 5.5 | 6.9 |
Amino acid changes in HIV-1 and HIV-2 integrase were engineered via site-directed mutagenesis of plasmid molecular clones pNL4-3 and pROD9, respectively. Wild type indicates virus stocks produced from the parental (nonmutated) copies of pNL4-3 and pROD9. The integrase-encoding region of each plasmid clone was confirmed by automated Sanger DNA sequencing.
EC50 determined in the MAGIC-5A single-cycle assay (means ± SD from ≥3 independent assay runs). Values shown in boldface are significantly different from the corresponding wild-type EC50 (P < 0.0001, analysis of variance of log10-transformed EC50s with Sidak's posttest).
EC50 for the mutant divided by the EC50 for the corresponding wild-type virus.
Spreading-infection assays.
To assess the robustness of our findings with the single-cycle assay, we evaluated the activity of cabotegravir against two HIV-1 and eight HIV-2 isolates (five from group A, three from group B) in spreading infections of CEMss cells (also referred to as the multicycle assay). Briefly, 96-well microcultures of CEMss cells were treated with various concentrations of cabotegravir, followed by infection with HIV-1 or HIV-2 at a multiplicity of 0.01 to 0.04 focus-forming units per cell. Half of the culture volume was removed at days 2 and 4 postinfection and replaced with an equivalent volume of fresh medium and drug. On day 6, the cultures were frozen at −80°C to ablate CEMss viability. Samples from the assay plates were then diluted in complete medium and transferred to MAGIC-5A cells to measure the level of infectious virus; this “scoring” phase utilized our previously described protocol for the MAGIC-5A single-cycle assay (60).
Cabotegravir potently inhibited HIV-2 replication in the multicycle assay; EC50s ranged from 0.14 to 1.0 nM for group A and 0.20 to 1.3 nM for group B HIV-2 isolates, respectively (Table 3). The control/comparator strains HIV-192UG029 and HIV-1NL4-3 were likewise sensitive to the drug (Table 3). Of note, for HIV-192UG029 and HIV-1NL4-3, the EC50s obtained in spreading infections were ∼10-fold lower than those seen in single-cycle infections; a similar fold increase in cabotegravir sensitivity was observed for HIV-2ROD9, HIV-2ST, and HIV-2CBL-23 (compare Tables 1 and 3). EC50s for HIV-2CDC77618, HIV-2CDC310319, and HIV-2DIL were also 2- to 4-fold lower in the spreading assay compared with single-cycle infections, although run-to-run variation for these three strains was relatively high in the spreading-infection assay (Table 3). The tendency toward lower EC50s in spreading infections relative to single-cycle assays is consistent with previous studies of INIs from our group and others (27, 31, 65) and has also been observed with inhibitors belonging to the NRTI drug class (60, 68). Overall, our findings from the single-cycle and spreading-infection assays indicate that HIV-2 is sensitive to cabotegravir in vitro, with EC50s in the low to subnanomolar range.
TABLE 3.
Susceptibility of HIV-1 and HIV-2 isolates to cabotegravir in spreading infections of CEMss cells
| Isolate by HIV type | Group/subtype | EC50 (nM)a | No. of assaysb |
|---|---|---|---|
| HIV-1 | |||
| 92UG029 | M/A | 0.21 ± 0.072 | 3 |
| NL4-3 | M/B | 0.15 ± 0.029 | 4 |
| HIV-2 | |||
| ROD9 | A | 0.14 ± 0.056 | 5 |
| ST | A | 0.25 ± 0.014 | 3 |
| CBL-20 | A | 1.0 ± 0.82 | 2 |
| CBL-23 | A | 0.16 ± 0.059 | 3 |
| CDC77618 | A | 0.85 ± 0.57 | 3 |
| CDC310319 | B | 0.99 ± 0.90 | 6 |
| EHO | B | 0.20 ± 0.027 | 2 |
| DIL | B | 1.3 ± 1.1 | 3 |
Values are means ± SD. These assays were performed using cabotegravir from GlaxoSmithKline, Inc.
Independent dose-response assays performed for each strain.
Implications for HIV-2 prevention and treatment.
The UNAIDS (Joint United Nations Programme on HIV and AIDS)/World Health Organization has set ambitious targets for HIV diagnosis, prevention, and treatment, with the ultimate aim of ending the global AIDS epidemic by 2030 (69). Efforts to attain these goals in West Africa and other areas will require a renewed commitment to clinical care for HIV-2-infected individuals (24). In particular, efforts are needed to improve HIV-2 patient access to fixed-dose, single-tablet formulations in which all antiretroviral components are active against HIV-2.
Cabotegravir is a novel strand transfer inhibitor that could potentially be coformulated with two NRTIs for once-daily oral administration (55, 58). Our findings suggest that such a regimen would be active in HIV-2-infected patients and therefore might simplify first-line treatment of HIV infection in settings in which HIV-2 is endemic.
Long-acting, injectable formulations of cabotegravir (CAB-LA) have been proposed for two modalities: (i) as maintenance therapy (in combination with the NNRTI rilpivirine [RPV-LA]) for HIV-1-infected patients who are virologically suppressed (55, 58) and (ii) as preexposure prophylaxis (PrEP) in individuals with a high risk of HIV acquisition (49, 53, 54, 57). With regard to maintenance therapy, CAB-LA/RPV-LA would likely be precluded in HIV-2-infected patients because of the intrinsic resistance of HIV-2 to rilpivirine and other NNRTIs (14, 15, 70). For PrEP, CAB-LA is currently being compared with daily oral tenofovir disoproxil fumarate-emtricitabine in phase 2b and phase 3 clinical trials (clinicaltrials.gov NCT02720094 and NCT03164564, respectively). Based on the locations of the study sites, participants will be at risk primarily for acquiring HIV-1; risk of HIV-2 acquisition will be minimal. If CAB-LA proves to be effective for PrEP, we believe that an evaluation of the drug should be performed in an HIV-2-prevalent setting, preferably in the context of a controlled clinical trial.
Altogether, our findings suggest that cabotegravir may be useful for HIV prevention and treatment in areas that harbor significant numbers of HIV-2-infected individuals. Clinical studies should be performed to address these possibilities.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported by grants to G.S.G. from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID, 2R01-AI060466 and R01-AI120765), the UW Center For AIDS Research (CFAR, an NIH-funded program, P30 AI027757), and the UW Royalty Research Fund (A92723). These funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank ViiV Healthcare UK, Ltd., for providing cabotegravir.
Additional UW-Dakar HIV-2 Study Group members are as follows: Fatima Sall, Khardiata Diallo Mbaye, Mouhamadou Baïla Diallo, Khadim Faye, Samba Cisse, Marie Pierre Sy, Bintou Diaw, Ousseynou Ndiaye, Babacar Faye, Ndeye Astou Diop, Amadou Bale Diop, and Marianne Fadam Diome (Clinique des Maladies Infectieuses Ibrahima DIOP Mar, Centre Hospitalier Universitaire de Fann, Universite' Cheikh Anta Diop de Dakar, Dakar, Senegal); Jean Jacques Malomar, ElHadji Ibrahima Sall, Ousseynou Cisse, Ibrahima Tito Tamba, Dominique Faye, Jean Philippe Diatta, Raphael Bakhoum, Jacque Francois Sambou, Juliette Gomis, and Therese Dieye (Région Médicale de Ziguinchor, Ziguinchor, Casamance, Senegal); Stephen Hawes, Noelle Benzekri, John Lin, Jennifer Song, Robbie Nixon, Ming Chang, Robert Coombs, James Mullins, and Nancy Kiviat (University of Washington, Seattle, Washington).
G.S.G. has received research grants and support from the U.S. National Institutes of Health, University of Washington, Bill and Melinda Gates Foundation, Gilead Sciences, Alere Technologies, Merck & Co., Inc., Janssen Pharmaceutica, Cerus Corporation, ViiV Healthcare, and Abbott Molecular Diagnostics. V.H.W. received an undergraduate research scholarship from the Mary Gates Endowment for Students (University of Washington). M.S. has received support from the National Institutes of Health/National Institute of Allergy and Infectious Diseases and the France REcherche Nord & Sud Sida-hiv Hépatites (ANRS). We have no other disclosures to report.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01299-18.
Contributor Information
for the University of Washington-Dakar HIV-2 Study Group:
Fatima Sall, Khardiata Diallo Mbaye, Mouhamadou Baïla Diallo, Khadim Faye, Samba Cisse, Marie Pierre Sy, Bintou Diaw, Ousseynou Ndiaye, Babacar Faye, Ndeye Astou Diop, Amadou Bale Diop, Marianne Fadam Diome, Jean Jacques Malomar, ElHadji Ibrahima Sall, Ousseynou Cisse, Ibrahima Tito Tamba, Dominique Faye, Jean Philippe Diatta, Raphael Bakhoum, Jacque Francois Sambou, Juliette Gomis, Therese Dieye, Stephen Hawes, Noelle Benzekri, John Lin, Jennifer Song, Robbie Nixon, Ming Chang, Robert Coombs, James Mullins, and Nancy Kiviat
REFERENCES
- 1.Visseaux B, Damond F, Matheron S, Descamps D, Charpentier C. 2016. HIV-2 molecular epidemiology. Infect Genet Evol 46:233–240. doi: 10.1016/j.meegid.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 2.de Silva TI, Cotten M, Rowland-Jones SL. 2008. HIV-2: the forgotten AIDS virus. Trends Microbiol 16:588–595. doi: 10.1016/j.tim.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, Traore I, Hsieh CC, Dia MC, Gueye EH, Hellinger J, Gueye-Ndiaye A, Sankale JL, Ndoye I, Mboup S, Essex M. 1994. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 4.Lisse IM, Poulsen AG, Aaby P, Knudsen K, Dias F. 1996. Serial CD4 and CD8 T-lymphocyte counts and associated mortality in an HIV-2-infected population in Guinea-Bissau. J Acquir Immune Defic Syndr Hum Retrovirol 13:355–362. doi: 10.1097/00042560-199612010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Jaffar S, Wilkins A, Ngom PT, Sabally S, Corrah T, Bangali JE, Rolfe M, Whittle HC. 1997. Rate of decline of percentage CD4+ cells is faster in HIV-1 than in HIV-2 infection. J Acquir Immune Defic Syndr Hum Retrovirol 16:327–332. doi: 10.1097/00042560-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb GS, Sow PS, Hawes SE, Ndoye I, Redman M, Coll-Seck AM, Faye-Niang MA, Diop A, Kuypers JM, Critchlow CW, Respess R, Mullins JI, Kiviat NB. 2002. Equal plasma viral loads predict a similar rate of CD4+ T cell decline in human immunodeficiency virus (HIV) type 1- and HIV-2-infected individuals from Senegal, West Africa. J Infect Dis 185:905–914. doi: 10.1086/339295. [DOI] [PubMed] [Google Scholar]
- 7.Popper SJ, Sarr AD, Travers KU, Gueye-Ndiaye A, Mboup S, Essex ME, Kanki PJ. 1999. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis 180:1116–1121. doi: 10.1086/315010. [DOI] [PubMed] [Google Scholar]
- 8.Schim van der Loeff MF, Jaffar S, Aveika AA, Sabally S, Corrah T, Harding E, Alabi A, Bayang A, Ariyoshi K, Whittle HC. 2002. Mortality of HIV-1, HIV-2 and HIV-1/HIV-2 dually infected patients in a clinic-based cohort in The Gambia. AIDS 16:1775–1783. doi: 10.1097/00002030-200209060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Matheron S, Pueyo S, Damond F, Simon F, Lepretre A, Campa P, Salamon R, Chene G, Brun-Vezinet F. 2003. Factors associated with clinical progression in HIV-2 infected-patients: the French ANRS cohort. AIDS 17:2593–2601. doi: 10.1097/00002030-200312050-00006. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Steele E, Awasana AA, Corrah T, Sabally S, van der Sande M, Jaye A, Togun T, Sarge-Njie R, McConkey SJ, Whittle H, Schim van der Loeff MF. 2007. Is HIV-2- induced AIDS different from HIV-1-associated AIDS? Data from a West African clinic. AIDS 21:317–324. [DOI] [PubMed] [Google Scholar]
- 11.van Tienen C, Schim van der Loeff M, Peterson I, Cotten M, Andersson S, Holmgren B, Vincent T, de Silva T, Rowland-Jones S, Aaby P, Whittle H. 2011. HTLV-1 and HIV-2 infection are associated with increased mortality in a rural West African community. PLoS One 6:e29026. doi: 10.1371/journal.pone.0029026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menendez-Arias L, Alvarez M. 2014. Antiretroviral therapy and drug resistance in human immunodeficiency virus type 2 infection. Antiviral Res 102:70–86. doi: 10.1016/j.antiviral.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Ntemgwa ML, d'Aquin Toni T, Brenner BG, Camacho RJ, Wainberg MA. 2009. Antiretroviral drug resistance in human immunodeficiency virus type 2. Antimicrob Agents Chemother 53:3611–3619. doi: 10.1128/AAC.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richman D, Rosenthal AS, Skoog M, Eckner RJ, Chou TC, Sabo JP, Merluzzi VJ. 1991. BI-RG-587 is active against zidovudine-resistant human immunodeficiency virus type 1 and synergistic with zidovudine. Antimicrob Agents Chemother 35:305–308. doi: 10.1128/AAC.35.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witvrouw M, Pannecouque C, Van Laethem K, Desmyter J, De Clercq E, Vandamme AM. 1999. Activity of non-nucleoside reverse transcriptase inhibitors against HIV-2 and SIV. AIDS 13:1477–1483. doi: 10.1097/00002030-199908200-00006. [DOI] [PubMed] [Google Scholar]
- 16.Desbois D, Roquebert B, Peytavin G, Damond F, Collin G, Benard A, Campa P, Matheron S, Chene G, Brun-Vezinet F, Descamps D. 2008. In vitro phenotypic susceptibility of human immunodeficiency virus type 2 clinical isolates to protease inhibitors. Antimicrob Agents Chemother 52:1545–1548. doi: 10.1128/AAC.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brower ET, Bacha UM, Kawasaki Y, Freire E. 2008. Inhibition of HIV-2 protease by HIV-1 protease inhibitors in clinical use. Chem Biol Drug Des 71:298–305. doi: 10.1111/j.1747-0285.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 18.Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. 2004. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther 9:57–65. [PubMed] [Google Scholar]
- 19.Raugi DN, Smith RA, Ba S, Toure M, Traore F, Sall F, Pan C, Blankenship L, Montano A, Olson J, Dia Badiane NM, Mullins JI, Kiviat NB, Hawes SE, Sow PS, Gottlieb GS. 2013. Complex patterns of protease inhibitor resistance among antiretroviral treatment-experienced HIV-2 patients from Senegal: implications for second-line therapy. Antimicrob Agents Chemother 57:2751–2760. doi: 10.1128/AAC.00405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodes B, Sheldon J, Toro C, Jimenez V, Alvarez MA, Soriano V. 2006. Susceptibility to protease inhibitors in HIV-2 primary isolates from patients failing antiretroviral therapy. J Antimicrob Chemother 57:709–713. doi: 10.1093/jac/dkl034. [DOI] [PubMed] [Google Scholar]
- 21.Tchounga BK, Inwoley A, Coffie PA, Minta D, Messou E, Bado G, Minga A, Hawerlander D, Kane C, Eholie SP, Dabis F, Ekouevi DK. 2014. Re-testing and misclassification of HIV-2 and HIV-1&2 dually reactive patients among the HIV-2 cohort of the West African Database to evaluate AIDS collaboration. J Int AIDS Soc 17:19064. doi: 10.7448/IAS.17.1.19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchounga BK, Honge BL, Eholie SP, Coffie PA, Jespersen S, Wejse C, Dabis F, Geoffrey GS, Ekouevi DK. 2016. Effect of sex and age on outcomes among HIV-2-infected patients starting antiretroviral therapy in West Africa. AIDS 30:2707–2714. doi: 10.1097/QAD.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honge BL, Jespersen S, Medina C, Te DS, da Silva ZJ, Christiansen M, Kjerulff B, Laursen AL, Wejse C, Krarup H, Erikstrup C. 2018. The challenge of discriminating between HIV-1, HIV-2 and HIV-1/2 dual infections. HIV Med 19:403–410. doi: 10.1111/hiv.12606. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb GS, Raugi DN, Smith RA. 2018. 90-90-90 for HIV-2? Towards ending the HIV-2 epidemic by enhancing care and clinical management of the HIV-2-infected patient. Lancet HIV 5:e390–e399. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2016. Consolidated guidelines on the use of antiretroviral drugs for preventing and treating HIV infection: recommendations for a public health approach, 2nd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 26.Roquebert B, Damond F, Collin G, Matheron S, Peytavin G, Benard A, Campa P, Chene G, Brun-Vezinet F, Descamps D. 2008. HIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvitegravir in vitro. J Antimicrob Chemother 62:914–920. doi: 10.1093/jac/dkn335. [DOI] [PubMed] [Google Scholar]
- 27.Smith RA, Raugi DN, Kiviat NB, Hawes SE, Mullins JI, Sow PS, Gottlieb GS. 2011. Phenotypic susceptibility of HIV-2 to raltegravir: integrase mutations Q148R and N155H confer raltegravir resistance. AIDS 25:2235–2241. doi: 10.1097/QAD.0b013e32834d8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimura K, Kodama E, Sakagami Y, Matsuzaki Y, Watanabe W, Yamataka K, Watanabe Y, Ohata Y, Doi S, Sato M, Kano M, Ikeda S, Matsuoka M. 2008. Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137). J Virol 82:764–774. doi: 10.1128/JVI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charpentier C, Larrouy L, Collin G, Damond F, Matheron S, Chene G, Nie T, Schinazi R, Brun-Vezinet F, Descamps D. 2010. In-vitro phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitor S/GSK1349572. AIDS 24:2753–2755. doi: 10.1097/QAD.0b013e32833f9e36. [DOI] [PubMed] [Google Scholar]
- 30.Andreatta K, Miller MD, White KL. 2013. HIV-2 antiviral potency and selection of drug resistance mutations by the integrase strand transfer inhibitor elvitegravir and NRTIs emtricitabine and tenofovir in vitro. J Acquir Immune Defic Syndr 62:367–374. doi: 10.1097/QAI.0b013e31827b55f1. [DOI] [PubMed] [Google Scholar]
- 31.Smith RA, Raugi DN, Pan C, Sow PS, Seydi M, Mullins JI, Gottlieb GS. 2015. In vitro activity of dolutegravir against wild-type and integrase inhibitor-resistant HIV-2. Retrovirology 12:10. doi: 10.1186/s12977-015-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson K, Ruelle J, Vekemans M, Siegal FP, Deayton JR, Colebunders R. 2012. The role of raltegravir in the treatment of HIV-2 infections: evidence from a case series. Antivir Ther 17:1097–1100. doi: 10.3851/IMP2303. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Lambert C, Arendt V, Seguin-Devaux C. 2014. Virological and immunological outcomes of elvitegravir-based regimen in a treatment-naive HIV-2-infected patient. AIDS 28:2329–2331. doi: 10.1097/QAD.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 34.Garrett N, Xu L, Smit E, Ferns B, El-Gadi S, Anderson J. 2008. Raltegravir treatment response in an HIV-2 infected patient: a case report. AIDS 22:1091–1092. doi: 10.1097/QAD.0b013e3282f9b165. [DOI] [PubMed] [Google Scholar]
- 35.Damond F, Lariven S, Roquebert B, Males S, Peytavin G, Morau G, Toledano D, Descamps D, Brun-Vezinet F, Matheron S. 2008. Virological and immunological response to HAART regimen containing integrase inhibitors in HIV-2-infected patients. AIDS 22:665–666. doi: 10.1097/QAD.0b013e3282f51203. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong-James D, Stebbing J, Scourfield A, Smit E, Ferns B, Pillay D, Nelson M. 2010. Clinical outcome in resistant HIV-2 infection treated with raltegravir and maraviroc. Antiviral Res 86:224–226. doi: 10.1016/j.antiviral.2010.02.324. [DOI] [PubMed] [Google Scholar]
- 37.Francisci D, Martinelli L, Weimer LE, Zazzi M, Floridia M, Masini G, Baldelli F. 2011. HIV-2 infection, end-stage renal disease and protease inhibitor intolerance: which salvage regimen? Clin Drug Investig 31:345–349. doi: 10.1007/BF03256933. [DOI] [PubMed] [Google Scholar]
- 38.Wandeler G, Furrer H, Rauch A. 2011. Sustained virological response to a raltegravir-containing salvage therapy in an HIV-2-infected patient. AIDS 25:2306–2308. doi: 10.1097/QAD.0b013e32834cdb89. [DOI] [PubMed] [Google Scholar]
- 39.Cavaco-Silva J, Abecasis A, Miranda AC, Pocas J, Narciso J, Aguas MJ, Maltez F, Almeida I, Germano I, Diniz A, Goncalves Mde F, Gomes P, Cunha C, Camacho RJ. 2014. HIV-2 integrase polymorphisms and longitudinal genotypic analysis of HIV-2 infected patients failing a raltegravir-containing regimen. PLoS One 9:e92747. doi: 10.1371/journal.pone.0092747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matheron S, Descamps D, Gallien S, Besseghir A, Sellier P, Blum L, Mortier E, Charpentier C, Tubiana R, Damond F, Peytavin G, Ponscarme D, Collin F, Brun-Vezinet F, Chene G. 2018. First line raltegravir/emtricitabine/tenofovir combination in HIV-2 infection: phase 2 non-comparative trial (ANRS 159 HIV-2). Clin Infect Dis doi: 10.1093/cid/ciy245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ba S, Raugi DN, Smith RA, Sall F, Faye K, Hawes SE, Sow PS, Seydi M, Gottlieb GS. 2018. A trial of a single tablet regimen of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate for the initial treatment of HIV-2 infection in a resource-limited setting: 48 week results from Senegal, West Africa. Clin Infect Dis. doi: 10.1093/cid/ciy324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Descamps D, Peytavin G, Visseaux B, Tubiana R, Damond F, Campa P, Charpentier C, Khuong-Josses MA, Duvivier C, Karmochkine M, Lukiana T, Matheron S. 2015. Dolutegravir in HIV-2-infected patients with resistant virus to first-line integrase inhibitors from the French Named Patient Program. Clin Infect Dis 60:1521–1527. [DOI] [PubMed] [Google Scholar]
- 43.Trevino A, Cabezas T, Lozano AB, Garcia-Delgado R, Force L, Fernandez-Montero JM, Mendoza C, Caballero E, Soriano V. 2015. Dolutegravir for the treatment of HIV-2 infection. J Clin Virol 64:12–15. doi: 10.1016/j.jcv.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Requena S, Trevino A, Cabezas T, Garcia-Delgado R, Amengual MJ, Lozano AB, Penaranda M, Fernandez JM, Soriano V, de Mendoza C. 2017. Drug resistance mutations in HIV-2 patients failing raltegravir and influence on dolutegravir response. J Antimicrob Chemother 72:2083–2088. doi: 10.1093/jac/dkx090. [DOI] [PubMed] [Google Scholar]
- 45.McPherson TD, Sobieszczyk ME, Markowitz M. 2018. Cabotegravir in the treatment and prevention of human immunodeficiency virus-1. Expert Opin Investig Drugs 27:413–420. doi: 10.1080/13543784.2018.1460357. [DOI] [PubMed] [Google Scholar]
- 46.Stellbrink HJ, Hoffmann C. 2018. Cabotegravir: its potential for antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS 13:334–340. doi: 10.1097/COH.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 47.Yoshinaga T, Kobayashi M, Seki T, Miki S, Wakasa-Morimoto C, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. 2015. Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob Agents Chemother 59:397–406. doi: 10.1128/AAC.03909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, St Clair M, Piscitelli S, Fujiwara T. 2013. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials 14:192–203. doi: 10.1310/hct1405-192. [DOI] [PubMed] [Google Scholar]
- 49.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, Russell-Lodrigue K, Bohm RP, Cheng-Mayer C, Hong Z, Markowitz M, Ho DD. 2014. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 343:1151–1154. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, Piscitelli S. 2014. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr 67:481–486. doi: 10.1097/QAI.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 51.Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y, Gould E, Stevens M, Piscitelli S. 2014. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 67:487–492. doi: 10.1097/QAI.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 52.Karmon SL, Mohri H, Spreen W, Markowitz M. 2015. GSK1265744 demonstrates robust in vitro activity against various clades of HIV-1. J Acquir Immune Defic Syndr 68:e39–41. doi: 10.1097/QAI.0000000000000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews CD, Yueh YL, Spreen WR, St Bernard L, Boente-Carrera M, Rodriguez K, Gettie A, Russell-Lodrigue K, Blanchard J, Ford S, Mohri H, Cheng-Mayer C, Hong Z, Ho DD, Markowitz M. 2015. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 7:270ra4. doi: 10.1126/scitranslmed.3010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radzio J, Spreen W, Yueh YL, Mitchell J, Jenkins L, Garcia-Lerma JG, Heneine W. 2015. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med 7:270ra5. doi: 10.1126/scitranslmed.3010297. [DOI] [PubMed] [Google Scholar]
- 55.Margolis DA, Brinson CC, Smith GHR, de Vente J, Hagins DP, Eron JJ, Griffith SK, Clair MHS, Stevens MC, Williams PE, Ford SL, Stancil BS, Bomar MM, Hudson KJ, Smith KY, Spreen WR. 2015. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis 15:1145–1155. doi: 10.1016/S1473-3099(15)00152-8. [DOI] [PubMed] [Google Scholar]
- 56.Andrews CD, Bernard LS, Poon AY, Mohri H, Gettie N, Spreen WR, Gettie A, Russell-Lodrigue K, Blanchard J, Hong Z, Ho DD, Markowitz M. 2017. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS 31:461–467. doi: 10.1097/QAD.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markowitz M, Frank I, Grant RM, Mayer KH, Elion R, Goldstein D, Fisher C, Sobieszczyk ME, Gallant JE, Van Tieu H, Weinberg W, Margolis DA, Hudson KJ, Stancil BS, Ford SL, Patel P, Gould E, Rinehart AR, Smith KY, Spreen WR. 2017. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV 4:e331–e340. doi: 10.1016/S2352-3018(17)30068-1. [DOI] [PubMed] [Google Scholar]
- 58.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, Lutz T, Angel JB, Richmond GJ, Clotet B, Gutierrez F, Sloan L, Clair MS, Murray M, Ford SL, Mrus J, Patel P, Crauwels H, Griffith SK, Sutton KC, Dorey D, Smith KY, Williams PE, Spreen WR. 2017. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 390:1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 59.Yoshinaga T, Kobayashi M, Seki T, Kawasuji T, Taishi T, Sato A, Fujiwara T, Johns B, Hazen R, Ferris R, Underwood M. 2013. Antiviral characteristics of S/GSK1265744, an HIV integrase inhibitor (INI) dosed by oral or long-acting parenteral injection, abstr H-550. Abstr 53rd Intersci Conf Antimicrob Agents Chemother, 10 to 13 September 2013, Denver, CO. [Google Scholar]
- 60.Smith RA, Raugi DN, Wu VH, Leong SS, Parker KM, Oakes MK, Sow PS, Ba S, Seydi M, Gottlieb GS. 2015. The nucleoside analog BMS-986001 shows greater in vitro activity against HIV-2 than against HIV-1. Antimicrob Agents Chemother 59:7437–7446. doi: 10.1128/AAC.01326-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith RA, Anderson DJ, Pyrak CL, Preston BD, Gottlieb GS. 2009. Antiretroviral drug resistance in HIV-2: three amino acid changes are sufficient for classwide nucleoside analogue resistance. J Infect Dis 199:1323–1326. doi: 10.1086/597802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith SJ, Zhao XZ, Burke TR Jr, Hughes SH. 2018. Efficacies of cabotegravir and bictegravir against drug-resistant HIV-1 integrase mutants. Retrovirology 15:37. doi: 10.1186/s12977-018-0420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Descamps D, Collin G, Letourneur F, Apetrei C, Damond F, Loussert-Ajaka I, Simon F, Saragosti S, Brun-Vezinet F. 1997. Susceptibility of human immunodeficiency virus type 1 group O isolates to antiretroviral agents: in vitro phenotypic and genotypic analyses. J Virol 71:8893–8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abram ME, Hluhanich RM, Goodman DD, Andreatta KN, Margot NA, Ye L, Niedziela-Majka A, Barnes TL, Novikov N, Chen X, Svarovskaia ES, McColl DJ, White KL, Miller MD. 2013. Impact of primary elvitegravir resistance-associated mutations in HIV-1 integrase on drug susceptibility and viral replication fitness. Antimicrob Agents Chemother 57:2654–2663. doi: 10.1128/AAC.02568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. 2011. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 55:813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salgado M, Toro C, Simon A, Garrido C, Blanco F, Soriano V, Rodes B. 2009. Mutation N155H in HIV-2 integrase confers high phenotypic resistance to raltegravir and impairs replication capacity. J Clin Virol 46:173–175. doi: 10.1016/j.jcv.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 67.Underwood MR, Johns BA, Sato A, Martin JN, Deeks SG, Fujiwara T. 2012. The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. J Acquir Immune Defic Syndr 61:297–301. doi: 10.1097/QAI.0b013e31826bfd02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu VH, Smith RA, Masoum S, Raugi DN, Ba S, Seydi M, Grobler JA, Gottlieb GS. 2017. MK-8591 (4′-ethynyl-2-fluoro-2′-deoxyadenosine) exhibits potent activity against HIV-2 isolates and drug-resistant HIV-2 mutants in culture. Antimicrob Agents Chemother 61:e00744-17. doi: 10.1128/AAC.00744-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Health Organization. 2018. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. World Health Organization, Geneva, Switzerland: www.unaids.org/en/resources/909090. [Google Scholar]
- 70.Azijn H, Tirry I, Vingerhoets J, de Bethune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky LT. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith RA, Raugi DN, Pan C, Coyne M, Hernandez A, Church B, Parker K, Mullins JI, Sow PS, Gottlieb GS. 2012. Three main mutational pathways in HIV-2 lead to high-level raltegravir and elvitegravir resistance: implications for emerging HIV-2 treatment regimens. PLoS One 7:e45372. doi: 10.1371/journal.pone.0045372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao F, Yue L, Robertson DL, Hill SC, Hui H, Biggar RJ, Neequaye AE, Whelan TM, Ho DD, Shaw GM, Sharp PM, Hahn BH. 1994. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J Virol 68:7433–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.