Practitioners commonly use amikacin in patients with cystic fibrosis. Establishment of the pharmacokinetics of amikacin in adults with cystic fibrosis may increase the efficacy and safety of therapy.
KEYWORDS: amikacin, aminoglycosides, cystic fibrosis, population pharmacokinetics
ABSTRACT
Practitioners commonly use amikacin in patients with cystic fibrosis. Establishment of the pharmacokinetics of amikacin in adults with cystic fibrosis may increase the efficacy and safety of therapy. This study was aimed to establish the population pharmacokinetics of amikacin in adults with cystic fibrosis. We used serum concentration data obtained during routine therapeutic drug monitoring and explored the influence of patient covariates on drug disposition. We performed a retrospective chart review to collect the amikacin dosing regimens, serum amikacin concentrations, blood sampling times, and patient characteristics for adults with cystic fibrosis admitted for treatment of acute pulmonary exacerbations. Amikacin concentrations were retrospectively collected for 49 adults with cystic fibrosis, and 192 serum concentrations were available for analysis. A population pharmacokinetic model was developed using nonlinear mixed-effects modeling with the first-order conditional estimation method. A two-compartment model with first-order elimination best described amikacin pharmacokinetics. Creatinine clearance and weight were identified as significant covariates for clearance and the volume of distribution, respectively, in the final model. Residual variability was modeled using a proportional error model. Typical estimates for clearance, central and peripheral volumes of distribution, and intercompartmental clearance were 3.06 liters/h, 14.4 liters, 17.1 liters, and 0.925 liters/h, respectively. The pharmacokinetics of amikacin in individuals with cystic fibrosis seems to differ from those in individuals without cystic fibrosis. However, further investigations are needed to confirm these results and, thus, the need for variations in amikacin dosing. Future pharmacodynamic studies will potentially establish the optimal amikacin dosing regimens for the treatment of acute pulmonary exacerbations in adult patients with CF.
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive genetic disorder caused by a mutation of the cystic fibrosis transmembrane regulator (CFTR) gene (1), affecting multiple organ systems (2, 3). Abnormal secretions due to the dysfunction or absence of the CFTR protein lead to inflammation, microbial colonization and infection, and progressive lung disease. Staphylococcus aureus often appears in the airway in infancy, and infections with Pseudomonas aeruginosa start somewhat later in life (4, 5).
Current Cystic Fibrosis Foundation (CFF) guidelines recommend treating acute pulmonary exacerbations (APE) of CF with two antipseudomonal agents, such as a beta-lactam plus an aminoglycoside (6). Tobramycin has been the most commonly used aminoglycoside, but antimicrobial resistance has increased over time (7). Therefore, amikacin is an increasingly used alternative for those individuals with CF who are not able to tolerate tobramycin or who have infective organisms resistant to tobramycin. However, selecting the optimal dosage of amikacin is challenging due to its narrow therapeutic index and wide intra- and interindividual variability (IIV). These factors reinforce the role of therapeutic drug monitoring (TDM) for these medications. The importance of TDM during amikacin treatment has been demonstrated in other populations. That is the case for septic patients, where TDM proved to be of benefit for the adjustment of amikacin therapy in most of the individuals (8).
Amikacin population pharmacokinetics (popPK) have been extensively studied in pediatric (9–16) and adult (17–29) populations. However, the pharmacokinetics (PK) of aminoglycosides change with the disease being treated (30). Indeed, the pathophysiology of CF may contribute to changes in clearance (CL) and the volume of distribution (V) (31). To improve efficacy and safety, the amikacin dosing regimens utilized in individuals with CF should consider potential PK modifications due to individual and population-specific pathophysiological changes in the population of patients with CF. Unfortunately, there are sparse PK data for amikacin in adults with CF, and the optimal dosing regimen remains unclear.
Although monitoring is crucial to achieve therapeutic concentrations while maximizing efficacy and reducing toxicity (32), treatment optimization through TDM can be implemented only after initial treatment has been started. The identification of patient-associated predictors of IIV in PK parameters allows the subsequent development of a priori optimized dosing regimens by using popPK and simulation techniques.
The aims of this study were (i) to develop a popPK model for amikacin in adult patients with CF and (ii) to evaluate the impact of several covariates on amikacin PK parameters.
RESULTS
Patients and data collection.
A total of 192 blood serum concentrations, obtained from samples collected from 49 patients (26 men, 23 women), were available for amikacin PK evaluation. Patient demographic characteristics are summarized in Table 1.
TABLE 1.
Demographic and biochemical characteristics of the study population
| Characteristic | Valuesa |
|---|---|
| No. of males/no. of females | 26/23 |
| Age (yr) | 30 (18–50) |
| Weight (kg) | 63 (42–83) |
| Height (cm) | 164.6 (152.4–195.1) |
| Serum creatinine concn (mg/dl) | 0.78 (0.44–1.50) |
| Creatinine clearance (ml/min) | 110 (59.85–199.9) |
Values represent the median (range).
A total of 496 doses were administered to the 49 individuals. Twenty-seven individuals received 191 doses in a 24-h interval, 5 individuals received 51 doses in a 12-h interval, and 17 individuals received 254 doses in an 8-h interval. Patients received a median dose of 940.8 mg (range, 500 to 2,000 mg) at 8-h, 12-h, or 24-h intervals. The median number of doses administered per patient was 10.1 (range, 1 to 19). All collected samples had detectable amikacin above the limit of quantification. The median number of concentrations per patient was 3.9 (range, 2 to 6), with the concentrations ranging from 1.1 to 54 μg/ml.
Population pharmacokinetic analysis.
A two-compartment model with first-order elimination kinetics best described the PK of amikacin. A proportional error model best described residual variability. All fixed-effect PK parameters were estimated with adequate precision. IIV was described by an exponential error model and was estimated only on CL.
Whereas the introduction of the serum creatinine concentration (SCR) on intercompartmental clearance (Q) and sex on the central distribution volume (V1) produced some of the sharpest decreases of the objective function value (OFV) (−23.946 and −19.625 points, respectively), they did not significantly reduce the IIV (SCR reduced IIV by −1.1%, and sex increased IIV by +1.3%). In contrast, the introduction of creatinine clearance (CLCR) on CL (which were highly correlated in the studied population; see Fig. S1 in the supplemental material) decreased the OFV (−19.672 points), while it also reduced the IIV significantly (−4.4%). Therefore, CLCR was kept in the univariate screen. In the multivariate analysis, sex and weight (WT) were the most impactful covariates on V1. Although the model incorporating CLCR on CL and sex on V1 had a slightly lower OFV than the model incorporating CLCR on CL and WT on V1 (592.092 versus 593.972), the prediction-corrected visual predictive check (pc-VPC) indicated a slight underprediction for low concentrations in the model incorporating sex on V1. Therefore, the model including CLCR on CL and WT on V1 was selected as the final model. Table 2 presents a summary of those significant steps in the sequential covariate model development. All model parameters could be estimated with good precision (Table 3). The precision of the parameter estimates was further confirmed by the results of the nonparametric bootstrap (Table 3).
TABLE 2.
Sequential covariate model developmenta
| Model | Hypothesis | OFV | ΔOFV | Basis of model | % IIV for CL (% RSE) |
|---|---|---|---|---|---|
| 0 | Base model | 631.909 | Base model | 23.6 (26.4) | |
| 1 | TVCL = θ1 + [θ2 · (CLCR/110)] | 612.237 | −19.672 | Base model | 18.5 (36.2) |
| 2 | TVCL = θ1 · (age/30)θ2 | 614.46 | −17.449 | Base model | 19.5 (44.1) |
| 3 | TVCL = θ1 · (SCR/0.78)θ2 | 618.743 | −13.166 | Base model | 21.3 (33.5) |
| 4 | TVV1 = θ1 + θ2 · sex | 612.284 | −19.625 | Base model | 24.9 (26.4) |
| 5 | TVV1 = θ1 · (WT/63)θ2 | 613.321 | −18.588 | Base model | 25.5 (25.3) |
| 6 | TVQ = θ1 · (SCR/0.78)θ2 | 607.963 | −23.946 | Base model | 22.5 (29.1) |
| 7 | TVQ = θ1 + θ2 · sex | 617.805 | −14.104 | Base model | 24.5 (26.5) |
| 8 | TVQ = θ1 · (WT/63)θ2 | 620.045 | −11.834 | Base model | 24.5 (27.0) |
| 9 | TVCL = θ1 + [θ2 · (CLCR/110)], TVV1 = θ3 + θ4 · sex | 592.092 | −20.145 | 1 | 19.2 (40.2) |
| 10 | TVCL = θ1 + [θ2 · (CLCR/110)], TVV1 = θ3 · (WT/63)θ4 | 593.972 | −18.265 | 1 | 20 (37.8) |
OFV, objective function value; ΔOFV, change in the objective function value; IIV, interindividual variability; RSE, relative standard error; TV, typical value; CL, clearance; θi, typical value of the PK parameter; Q, intercompartmental clearance; V1, central compartment volume; SCR, serum creatinine concentration; CLCR, creatinine clearance; WT, weight.
TABLE 3.
Pharmacokinetic parameter estimates for the final population modela
| Parameter | Final model |
Bootstrap (n = 1,000) |
||
|---|---|---|---|---|
| Estimate | % RSE | Median | 95% PI | |
| CL (liters h−1) | 3.06 | 18.3 | 3.02 | 2.2–4.2 |
| ∼CLCR | 1.55 | 33.9 | 1.58 | 0.5–2.4 |
| V1 (liters) | 14.4 | 2.9 | 14.5 | 13.7–15.4 |
| ∼WT | 0.918 | 15.5 | 0.919 | 0.6–1.3 |
| V2 (liters) | 17.1 | 23.3 | 17.5 | 10.4–28 |
| Q (liter h−1) | 0.925 | 14.9 | 0.913 | 0.6–1.2 |
| % CV for IIV for CL | 20 | 37.8 | 19.5 | 12.7–27.1 |
| Proportional residual variability (%) | 16.61 | 12.3 | 16.2 | 14.1–18.2 |
CL, clearance; CLCR, creatinine clearance; CV, coefficient of variation; IIV, interindividual variability; PI, prediction interval; Q, intercompartmental clearance; RSE, relative standard error; V1, central compartment volume; V2, peripheral compartment volume; WT, weight; ~, covariate associated to the PK parameter.
Model evaluation.
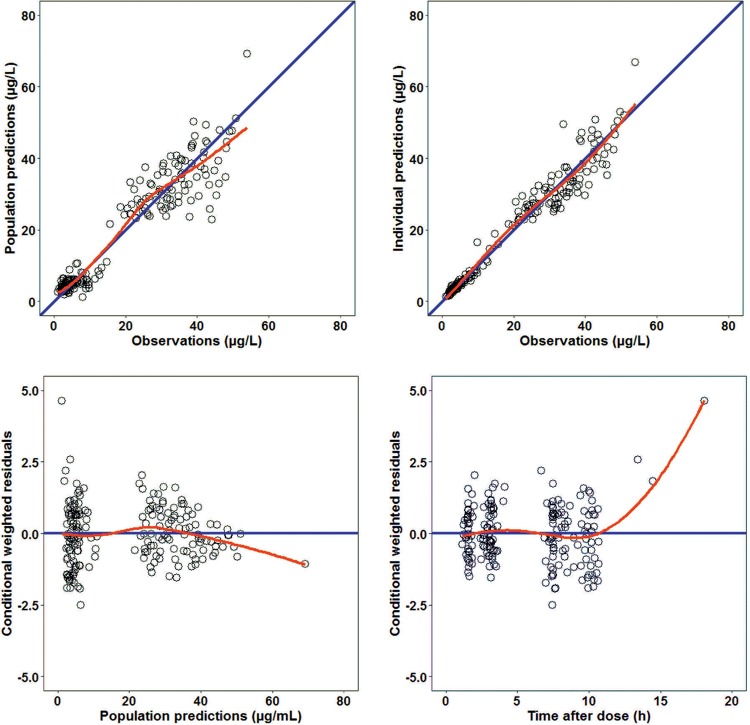
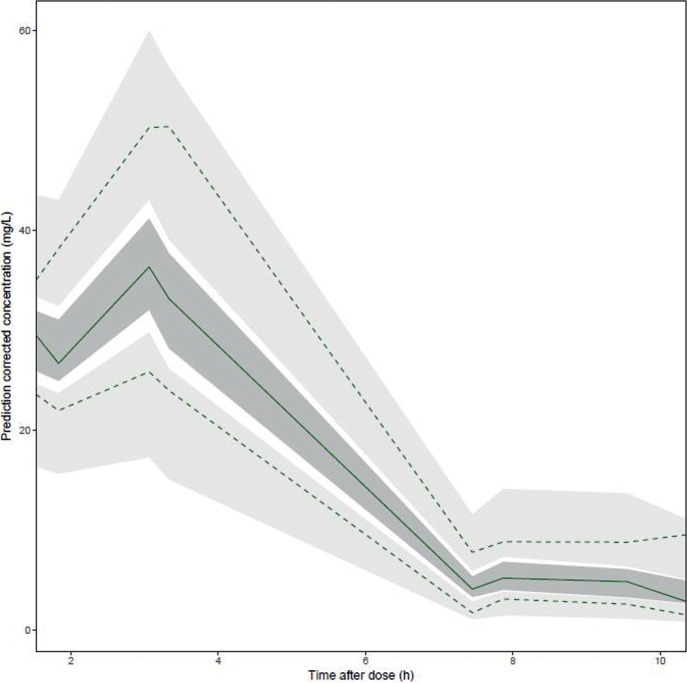
Goodness-of-fit diagnostic plots (Fig. 1) did not show any relevant trends. The pc-VPC indicated that the median and the 5th and 95th percentiles of the observed data fell within the 95% prediction intervals (PI) of the corresponding model-predicted percentiles (Fig. 2).
FIG 1.
Goodness-of-fit plots of the final model. Population predictions (left top) and individual predictions (right top) versus observations and conditional weighted residuals (WRES) versus population predictions (left bottom) and time after dose (right bottom) are shown. The red lines represent loess smoothers.
FIG 2.
Prediction-corrected visual predictive check for the final population pharmacokinetic model. The dashed lines and light gray areas represent the 2.5th and 97.5th percentiles for observed and simulated concentration-time profiles, respectively. The solid line and a dark gray area represent the median observed and simulated concentration-time profiles, respectively.
DISCUSSION
This work presents the results of a popPK analysis of amikacin in a population of adults diagnosed with CF. Although the amikacin popPK have been extensively studied in both general pediatric (9–16) and adult (17–29) populations, our work extends the knowledge to include adult patients with CF specifically.
The PK of several antibacterial agents in individuals with CF differ from those in healthy volunteers and other patients (33, 34). Consequently, selecting the optimal dosage regimen in patients with CF is a significant challenge (35). Specifically for aminoglycosides, PK properties differ between individuals with and without CF (35–37). These PK differences may reflect variations in body composition, particularly a reduction of adipose tissue due to the malnutrition present in individuals with CF compared with individuals without CF (38).
The PK of amikacin in adults has been previously described by either a one-compartment model (17, 19, 20, 23, 26, 29) or a two-compartment model (21, 22, 25, 27, 28). Although data from this study were collected during routine TDM, they still support a two-compartment model, with a significant improvement of the OFV compared to the one-compartment model.
The population CL and V estimates for amikacin in our study (CL, 3.06 liters/h; V, 14.4 liters) were similar to those previously described for others aminoglycosides in adults with CF. A popPK model of tobramycin developed in adults with CF reported a typical CL estimate of 4.65 liters/h and an estimate of V of 13.4 liters (39).
Previous popPK studies evaluating the PK of amikacin among adults without CF reported PK parameter estimates of 4.3 liters/h and 15.9 liters for CL and V, respectively (28). This shows that both the CL and V of amikacin are comparable to those for our CF population (3.06 liters/h and 14.4 liters, respectively). These findings would be in accordance with those of previous studies performed with other antibacterial drugs. However, Kearns et al. (35) and Leeder et al. (37) reported a larger V of gentamicin and ceftazidime, respectively, in patients with CF than in a similar population without the disease. The same authors found that the CL of gentamicin and ceftazidime in individuals diagnosed with CF was higher than that in individuals without the disease (35, 37). This divergence in CL values could be due to the renal function of the patients included in this study, with a minimum CLCR estimate of 59.85 ml/min. Similarly, Levy et al. (36) found that the tobramycin renal CL was also not significantly different between pediatric individuals with CF and those without CF. In this case, the authors postulated that an extrarenal CL pathway could play a significant role in the elimination of tobramycin from the serum of patients with CF (36). A similar mechanism may occur with amikacin.
In our study, CLCR was an important covariate that influenced amikacin CL among adult individuals with CF, consistent with the findings of previous popPK studies of amikacin in adults without the disease (20–25, 27). On the other hand, WT proved to be the most influential covariate on V, an association also reported in adults without CF (20, 23, 26, 28). Nonetheless, for hydrophilic drugs such as aminoglycosides, patients with CF should be dosed on the basis of lean body mass (LBM) rather than actual WT or body surface area (40). Other covariates also identified to be significant in V in adult individuals without CF are sepsis (20) and hypoalbuminemia (23) in intensive care individuals and in hematological patients, respectively. Those covariates were not available in this analysis.
The main limitation of this analysis is that data were collected during routine TDM and a limited number of amikacin concentrations were measured for each patient. Future simulation studies of different dosing regimens and intervals in adults with CF could evaluate potential optimized dosing regimens in this population. Additionally, a potential concern arises over the introduction of WT instead of LBM as a covariate in V, which could be useful for hydrophilic drugs, such as aminoglycosides. However, WT is more readily available in clinical settings, and a full comparison between WT and LBM has not been done. Such a comparison could be done in a future prospective study.
In conclusion, this study presents the results of an amikacin popPK analysis for adult individuals with CF. Amikacin PK were adequately described with a two-compartment model. Our model suggests that CLCR and WT are predictors of CL and V, respectively. The amikacin CL value was similar to the values reported by other studies performed in populations of adults without CF, while the V value was greater than the values reported for adult individuals without CF. As very limited data about amikacin PK in adults with CF are available, further investigations are needed to assess a potential dosing regimen and dosing adjustments in this population. Future pharmacodynamic studies could establish markers of efficacy and toxicity that could be used to develop an optimal amikacin dosing regimen for the treatment of acute pulmonary exacerbations in adults with CF.
MATERIALS AND METHODS
Study population.
All data for adult patients diagnosed with CF who received intravenous (i.v.) amikacin as part of the treatment for an acute pulmonary exacerbation (6) at the University of Utah Hospital between 1 January 2008 and 31 May 2016 were collected retrospectively. Individuals were included if at least two amikacin serum concentrations were recorded. Patient demographics, including gender, age, weight (WT), height (HT), and the serum creatinine concentration (SCR) within 1 day of amikacin TDM sampling, were collected from the electronic data warehouse. Creatinine clearance (CLCR) was computed according to the Cockcroft-Gault formula (41). This study was reviewed and approved by the University of Utah Institutional Review Board.
Dosing and sampling schedule.
Amikacin was administered by i.v. infusion over 30 min or 1 h at intervals of 8, 12, or 24 h. The difference in the dosing interval was a result of a practice change at the institution during the study time period. In most of the cases, the amikacin regimen was based on the traditional interval of dosing (i.e., every 8 h, every 12 h) prior to 2008, whereas extended-interval dosing (i.e., every 24 h) was generally used after 2008. Blood samples for serum amikacin concentration measurements were generally collected as part of routine medical care on day 1 or 2 after initiating the treatment and then again on day 7. Blood serum for drug concentration measurements was collected once between 45 min and 3 h after the end of the infusion and again prior to the next infusion. For each patient, the time that had elapsed between amikacin administration and sampling times was recorded. The treatment duration was typically 14 days, based on clinical status and pulmonary function testing results.
Bioanalysis.
Serum amikacin concentrations were measured using a fluorescence polarization immunoassay (TDx; Abbott, Abbott Park, IL) (42). The assay was linear from 0.8 to 50 μg/ml, with the intraday and interday variability being lower than 5%.
Pharmacokinetic model development.
The popPK analysis for amikacin plasma concentrations was performed using a nonlinear mixed-effect modeling approach implemented in the software package NONMEM (version 7.2) (43). The first-order conditional estimation method with interaction was used throughout the analysis.
For the structural pharmacokinetic model, one- and two-compartment PK models with zero-order input and first-order elimination were tested. Estimates for IIV on PK parameter estimates were modeled according to a log-normal distribution as follows for typical parameter P and individual i (equation 1):
| (1) |
where ηi was distributed according to . Additive, proportional, and combined (additive plus proportional) models were considered for the quantification of residual unexplained variability (RUV).
Model development was guided by the decrease in the −2 log likelihood (−2LL) using a statistical significance criterion of a P value of <0.01 (likelihood ratio test) and the precision of the parameter estimates.
Investigation of potential relationships between covariates and PK parameters was assessed by plotting covariates independently against the individual post hoc parameter estimates and the weighted residuals. However, due to the reduced number of covariates to study, all covariate-parameter relationships were tested. Therefore, the following covariates were evaluated for inclusion: WT, HT, SCR, and CLCR as continuous variables and age and sex as categorical variables. For continuous covariates, both linear (equation 2) and power (equation 3) relationships were considered.
| (2) |
| (3) |
where Pi is the typical parameter of the individual, θi is the typical value of the PK parameter, COVi is the individual covariate value, COVmedian is the median covariate value in the population, and θCOV is the estimated covariate effect parameter.
Covariate effects were tested by incorporating covariates into the base model using stepwise forward addition followed by stepwise backward elimination procedures. The significance of a covariate was statistically tested by use of the objective function value (OFV). In the forward inclusion, a P value of <0.05 was applied (a decrease in the OFV of at least 3.84 points), while a more stringent P value of <0.01 was used in the backward deletion (a decrease in the OFV of at least 6.63 points). Additionally to these statistical criteria, a decrease of the IIV was considered a clinically relevant threshold for covariate inclusion in the model, as well as its physiological plausibility.
Model evaluation.
Diagnostic graphics were used for the evaluation of the goodness of fit of our model, including observed concentrations versus population and individual predicted concentrations, conditional weighted residuals versus population predicted concentrations, and time after the dose. The suitability of the selected model was evaluated using a prediction-corrected visual predictive check (pc-VPC) in which 1,000 individual profiles as those of the original data set were simulated from the final model. Then, the 95% prediction interval for the median (50th percentile) and the 5th and 95th percentiles of the predicted concentrations were calculated and plotted together with the median and the 5th and 95th percentiles of the observed concentrations.
The precision of the parameter estimates of the final population PK model was evaluated using a nonparametric bootstrap analysis and the Perl-speaks-NONMEM (PsN) (version 3.5.2) program. Random resampling with replacement from the original data set generated 1,000 bootstrap data sets. Parameters estimates were summarized regarding median values and the 95% prediction intervals (PI) and compared with those obtained from the model-building data set.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00877-18.
REFERENCES
- 1.Lubamba B, Dhooghe B, Noel S, Leal T. 2012. Cystic fibrosis: insight into CFTR pathophysiology and pharmacotherapy. Clin Biochem 45:1132–1144. doi: 10.1016/j.clinbiochem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan BP, Freedman SD. 2009. Cystic fibrosis. Lancet 373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheimer EH, Esterly JR. 1975. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol 2:241–278. [PubMed] [Google Scholar]
- 4.Flume PA. 2009. Pulmonary complications of cystic fibrosis. Respir Care 54:618–627. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey BW. 1996. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med 335:179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 6.Flume PA, Mogayzel PJ Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC, Clinical Practice Guidelines for Pulmonary Therapies Committee. 2009. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med 180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 7.Emerson J, McNamara S, Buccat AM, Worrell K, Burns JL. 2010. Changes in cystic fibrosis sputum microbiology in the United States between 1995 and 2008. Pediatr Pulmonol 45:363–370. doi: 10.1002/ppul.21198. [DOI] [PubMed] [Google Scholar]
- 8.Duszynska W, Taccone FS, Hurkacz M, Kowalska-Krochmal B, Wiela-Hojenska A, Kubler A. 2013. Therapeutic drug monitoring of amikacin in septic patients. Crit Care 17:R165. doi: 10.1186/cc12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botha JH, du Preez M, Miller R, Adhikari M. 1998. Determination of population pharmacokinetic parameters for amikacin in neonates using mixed-effect models. Eur J Clin Pharmacol 53:337–341. doi: 10.1007/s002280050389. [DOI] [PubMed] [Google Scholar]
- 10.Allegaert K, Anderson BJ, Cossey V, Holford NHG. 2006. Limited predictability of amikacin clearance in extreme premature neonates at birth. Br J Clin Pharmacol 61:39–48. doi: 10.1111/j.1365-2125.2005.02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allegaert K, Scheers I, Cossey V, Anderson BJ. 2008. Covariates of amikacin clearance in neonates: the impact of postnatal age on predictability. Drug Metab Lett 2:286–289. doi: 10.2174/187231208786734157. [DOI] [PubMed] [Google Scholar]
- 12.Sherwin CM, Svahn S, Van der Linden A, Broadbent RS, Medlicott NJ, Reith DM. 2009. Individualised dosing of amikacin in neonates: a pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol 65:705–713. doi: 10.1007/s00228-009-0637-4. [DOI] [PubMed] [Google Scholar]
- 13.De Cock RFW, Allegaert K, Schreuder MF, Sherwin CMT, de Hoog M, van den Anker JN, Danhof M, Knibbe CAJ. 2012. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet 51:105–117. doi: 10.2165/11595640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Sherwin CMT, Wead S, Stockmann C, Healy D, Spigarelli MG, Neely A, Kagan R. 2014. Amikacin population pharmacokinetics among paediatric burn patients. Burns 40:311–318. doi: 10.1016/j.burns.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Yu T, Stockmann C, Healy DP, Olson J, Wead S, Neely AN, Kagan RJ, Spigarelli MG, Sherwin CMT. 2015. Determination of optimal amikacin dosing regimens for pediatric patients with burn wound sepsis. J Burn Care Res 36:E244–E252. doi: 10.1097/BCR.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 16.Illamola SM, Colom H, van Hasselt JGC. 2016. Evaluating renal function and age as predictors of amikacin clearance in neonates: model-based analysis and optimal dosing strategies. Br J Clin Pharmacol 82:793–805. doi: 10.1111/bcp.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debord J, Pessis C, Voultoury JC, Marquet P, Lotfi H, Merle L, Lachatre G. 1995. Population pharmacokinetics of amikacin in intensive care unit patients studied by NPEM algorithm. Fundam Clin Pharmacol 9:57–61. doi: 10.1111/j.1472-8206.1995.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 18.Debord J, Charmes JP, Marquet P, Merle L, Lachatre G. 1997. Population pharmacokinetics of amikacin in geriatric patients studied with the NPEM-2 algorithm. Int J Clin Pharmacol Ther 35:24–27. [PubMed] [Google Scholar]
- 19.Lugo G, Castaneda-Hernandez G. 1997. Amikacin Bayesian forecasting in critically ill patients with sepsis and cirrhosis. Ther Drug Monit 19:271–276. doi: 10.1097/00007691-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Romano S, Del Mar Fdez de Gatta M, Calvo V, Mendez E, Dominguez-Gil A, Lanao JM. 1998. Influence of clinical diagnosis in the population pharmacokinetics of amikacin in intensive care unit patients. Clin Drug Invest 15:435–444. doi: 10.2165/00044011-199815050-00008. [DOI] [PubMed] [Google Scholar]
- 21.Tod M, Lortholary O, Seytre D, Semaoun R, Uzzan B, Guillevin L, Casassus P, Petitjean O. 1998. Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults. Antimicrob Agents Chemother 42:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joubert P, Bressolle F, Gouby A, Doucot PY, Saissi G, Gomeni R. 1999. A population approach to the forecasting of amikacin plasma and urinary levels using a prescribed dosage regimen. Eur J Drug Metab Pharmacokinet 24:39–46. doi: 10.1007/BF03190009. [DOI] [PubMed] [Google Scholar]
- 23.Romano S, Fdez de Gatta MM, Calvo MV, Caballero D, Dominguez-Gil A, Lanao JM. 1999. Population pharmacokinetics of amikacin in patients with haematological malignancies. J Antimicrob Chemother 44:235–242. doi: 10.1093/jac/44.2.235. [DOI] [PubMed] [Google Scholar]
- 24.Lugo-Goytia G, Castaneda-Hernandez G. 2000. Bayesian approach to control of amikacin serum concentrations in critically ill patients with sepsis. Ann Pharmacother 34:1389–1394. [DOI] [PubMed] [Google Scholar]
- 25.Delattre IK, Musuamba FT, Nyberg J, Taccone FS, Laterre PF, Verbeeck RK, Jacobs F, Wallemacq PE. 2010. Population pharmacokinetic modeling and optimal sampling strategy for Bayesian estimation of amikacin exposure in critically ill septic patients. Ther Drug Monit 32:749–756. doi: 10.1097/FTD.0b013e3181f675c2. [DOI] [PubMed] [Google Scholar]
- 26.Jang SB, Lee YJ, Park MS, Song YG, Kim JH, Kim HK, Ahn BS, Park K. 2011. Population pharmacokinetics of amikacin in a Korean clinical population. Int J Clin Pharmacol Ther 49:371–381. doi: 10.5414/CP201520. [DOI] [PubMed] [Google Scholar]
- 27.Matar KM, Al-lanqawi Y, Abdul-Malek K, Jelliffe R. 2013. Amikacin population pharmacokinetics in critically ill Kuwaiti patients. Biomed Res Int 2013:202818. doi: 10.1155/2013/202818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdet C, Pajot O, Couffignal C, Armand-Lefevre L, Foucrier A, Laouenan C, Wolff M, Massias L, Mentre F. 2015. Population pharmacokinetics of single-dose amikacin in critically ill patients with suspected ventilator-associated pneumonia. Eur J Clin Pharmacol 71:75–83. doi: 10.1007/s00228-014-1766-y. [DOI] [PubMed] [Google Scholar]
- 29.Dijkstra JA, van Altena R, Akkerman OW, de Lange WC, Proost JH, van der Werf TS, Kosterink JG, Alffenaar JW. 2015. Limited sampling strategies for therapeutic drug monitoring of amikacin and kanamycin in patients with multidrug-resistant tuberculosis. Int J Antimicrob Agents 46:332–337. doi: 10.1016/j.ijantimicag.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Begg EJ, Barclay ML, Kirkpatrick CM. 2001. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol 52(Suppl 1):35S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rey E, Treluyer JM, Pons G. 1998. Drug disposition in cystic fibrosis. Clin Pharmacokinet 35:313–329. doi: 10.2165/00003088-199835040-00004. [DOI] [PubMed] [Google Scholar]
- 32.Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. 1984. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med 100:352–357. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 33.Zobell JT, Young DC, Waters CD, Stockmann C, Ampofo K, Sherwin CM, Spigarelli MG. 2012. Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations. I. Aztreonam and carbapenems. Pediatr Pulmonol 47:1147–1158. doi: 10.1002/ppul.22655. [DOI] [PubMed] [Google Scholar]
- 34.Zobell JT, Waters CD, Young DC, Stockmann C, Ampofo K, Sherwin CM, Spigarelli MG. 2013. Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations. II. Cephalosporins and penicillins. Pediatr Pulmonol 48:107–122. doi: 10.1002/ppul.22669. [DOI] [PubMed] [Google Scholar]
- 35.Kearns GL, Hilman BC, Wilson JT. 1982. Dosing implications of altered gentamicin disposition in patients with cystic fibrosis. J Pediatr 100:312–318. doi: 10.1016/S0022-3476(82)80663-X. [DOI] [PubMed] [Google Scholar]
- 36.Levy J, Smith AL, Koup JR, Williams-Warren J, Ramsey B. 1984. Disposition of tobramycin in patients with cystic fibrosis: a prospective controlled study. J Pediatr 105:117–124. doi: 10.1016/S0022-3476(84)80375-3. [DOI] [PubMed] [Google Scholar]
- 37.Leeder JS, Spino M, Isles AF, Tesoro AM, Gold R, MacLeod SM. 1984. Ceftazidime disposition in acute and stable cystic fibrosis. Clin Pharmacol Ther 36:355–362. doi: 10.1038/clpt.1984.187. [DOI] [PubMed] [Google Scholar]
- 38.Bulitta JB, Duffull SB, Kinzig-Schippers M, Holzgrabe U, Stephan U, Drusano GL, Sorgel F. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother 51:2497–2507. doi: 10.1128/AAC.01477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alghanem S, Paterson I, Touw DJ, Thomson AH. 2013. Influence of multiple courses of therapy on aminoglycoside clearance in adult patients with cystic fibrosis. J Antimicrob Chemother 68:1338–1347. doi: 10.1093/jac/dkt035. [DOI] [PubMed] [Google Scholar]
- 40.Touw DJ, Vinks AA, Heijerman HG, Hermans J, Bakker W. 1994. Suggestions for the optimization of the initial tobramycin dose in adolescent and adult patients with cystic fibrosis. Ther Drug Monit 16:125–131. doi: 10.1097/00007691-199404000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 42.Jolley ME, Stroupe SD, Schwenzer KS, Wang CJ, Lu-Steffes M, Hill HD, Popelka SR, Holen JT, Kelso DM. 1981. Fluorescence polarization immunoassay. iii. An automated system for therapeutic drug determination. Clin Chem 27:1575–1579. [PubMed] [Google Scholar]
- 43.Beal SL, Boeckman AJ, Sheiner LB. 1998. NONMEM user guides. Division of Pharmacology, University of California, San Francisco, San Francisco, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.