Antibiotic disruption of the intestinal microbiota favors colonization by Clostridium difficile. Using a charcoal-based adsorbent to decrease intestinal antibiotic concentrations, we studied the relationship between antibiotic concentrations in feces and the intensity of dysbiosis and quantified the link between this intensity and mortality.
KEYWORDS: antibiotics, dysbiosis, C. difficile infection, hamster animal model, mortality, prevention
ABSTRACT
Antibiotic disruption of the intestinal microbiota favors colonization by Clostridium difficile. Using a charcoal-based adsorbent to decrease intestinal antibiotic concentrations, we studied the relationship between antibiotic concentrations in feces and the intensity of dysbiosis and quantified the link between this intensity and mortality. We administered either moxifloxacin (n = 70) or clindamycin (n = 60) to hamsters by subcutaneous injection from day 1 (D1) to D5 and challenged them with a C. difficile toxigenic strain at D3. Hamsters received various doses of a charcoal-based adsorbent, DAV131A, to modulate intestinal antibiotic concentrations. Gut dysbiosis was evaluated at D0 and D3 using diversity indices determined from 16S rRNA gene profiling. Survival was monitored until D16. We analyzed the relationship between fecal antibiotic concentrations and dysbiosis at the time of C. difficile challenge and studied their capacity to predict subsequent death of the animals. Increasing doses of DAV131A reduced fecal concentrations of both antibiotics, lowered dysbiosis, and increased survival from 0% to 100%. Mortality was related to the level of dysbiosis (P < 10−5 for the change of Shannon index in moxifloxacin-treated animals and P < 10−9 in clindamycin-treated animals). The Shannon diversity index and unweighted UniFrac distance best predicted death, with areas under the receiver operating curve (ROC) of 0.89 (95% confidence interval [CI], 0.82, 0.95) and 0.95 (0.90, 0.98), respectively. Altogether, moxifloxacin and clindamycin disrupted the diversity of the intestinal microbiota with a dependency on the DAV131A dose; mortality after C. difficile challenge was related to the intensity of dysbiosis in similar manners with the two antibiotics.
INTRODUCTION
Antibiotics disrupt the structure and composition of the intestinal microbiota and alter metabolic processes occurring in the gut, with possible acute and long-term consequences (1–5). Short-term effects include diarrhea in 5 to 25% of antibiotic-treated patients, and antibiotics are the main risk factor of Clostridium difficile infection (6), which causes a wide range of symptoms from mild diarrhea to toxic megacolon and an annual mortality estimated at 29,000 deaths in the United States (7, 8). The lincosamide antibiotic clindamycin and fluoroquinolones are among the main antibiotic classes associated with C. difficile infection (9).
The burden of C. difficile infection is increasing (10), and C. difficile is considered an urgent threat by the U.S. CDC (11). C. difficile pathophysiology is related to the perturbation of the intestinal microbiota and its metabolism, which allows C. difficile spores to germinate and colonize the gut and cytotoxic toxins to be released. Various animal models have been developed to delineate the pathophysiology of C. difficile infection (12), including the golden Syrian hamster model (13). In this model, hamsters treated with antibiotics and colonized by C. difficile are highly susceptible to lethal infection, and the degree of susceptibility to develop infection varies between classes of antibiotics (14, 15).
There is, however, no precise and quantitative analysis of the relationship between the effects of antibiotics on global bacterial diversity within the intestinal microbiota and the development of a C. difficile infection. Yet, diversity is the first descriptor of the structure of a community and is believed to be a major determinant of its dynamics. The analysis of complex bacterial communities was made possible by the development of efficient sequencing technologies applied to 16S rRNA genes (16). These genes are found in all bacterial species and contain regions which are highly conserved and others which are highly variable in sequence and can be used as molecular fingerprints. Several metrics are available for measuring diversity in bacterial communities. Alpha diversity refers to within-sample diversity and is usually analyzed using the number (richness) and the distribution (evenness) of bacterial taxa observed within a single population, e.g., with the Shannon diversity index, the number of observed operational taxonomic units (OTUs), and the Chao1 index (17, 18). Beta diversity, which refers to diversity between samples, measures the distance between pairs of samples, e.g., with UniFrac distances based on bacterial taxonomy and the Bray-Curtis dissimilarity index (19, 20).
However, whether the intensity of the dysbiosis, as can be reflected by the variations of these global indices, is quantitatively related to the occurrence of C. difficile infection has not be explored so far. This is important for a better understanding of the pathophysiology of C. difficile infection and to determine whether various degrees of dysbiosis are associated with various degrees of risk of C. difficile infection. Here we explored this relationship in an animal model of C. difficile infection in hamsters. We had previously showed that DAV131A, a charcoal-based adsorbent with the same principle of action and based on the same adsorbent as the DAV132 product, which has recently proven to be highly effective to reduce fecal antibiotic concentrations and dysbiosis in human volunteers treated with moxifloxacin (21), reduced mortality through a decrease of fecal antibiotic concentrations in a hamster model of lethal moxifloxacin-induced C. difficile colitis (22). In this study, we induced various degrees of dysbiosis by treating hamsters with either clindamycin or moxifloxacin, which have different antibacterial spectra but are both highly associated with the occurrence of C. difficile infection, and modulating intestinal antibiotic concentrations by using various doses of DAV131A.
RESULTS
In order to further analyze the pathophysiology of severe C. difficile colitis, we treated individually housed hamsters with either moxifloxacin (total of 70 animals) or clindamycin (total of 60 animals) for 5 days in 2 separate studies with similar designs (Fig. 1). Some groups received various doses of DAV131A given orally twice a day (b.i.d.) concomitantly with the antibiotic and for an additional 3 days after antibiotic administration (corresponding to a total of 8 days). All hamsters were challenged with 104 spores of a toxigenic C. difficile strain on the third day of antibiotic treatment. We analyzed the quantitative relationship between antibiotic-induced dysbiosis at the time of the C. difficile challenge and the subsequent occurrence of death from infection.
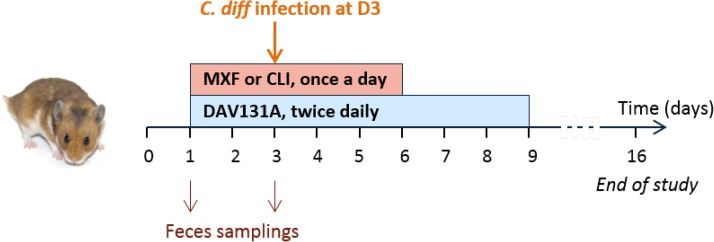
FIG 1.
Experimental design of the studies. Male Syrian golden hamsters were treated with moxifloxacin (MXF) (n = 70) or clindamycin (CLI) (n = 60) once a day by the subcutaneous route for 5 days and received various doses of DAV131A twice a day (b.i.d.) by the oral route for 8 days, which would result in the exposure of the microbiota to various antibiotic concentrations and different bacterial environments. The toxigenic C. difficile strain UNT103-1 was inoculated at D3. Fecal samples were obtained just before the beginning of treatment and at the third treatment day. Microbiota analysis was performed by 16S rRNA gene sequencing on both samples, and the fecal concentration of active antibiotic was determined at D3 by microbiological assay. Survival was monitored up to D16.
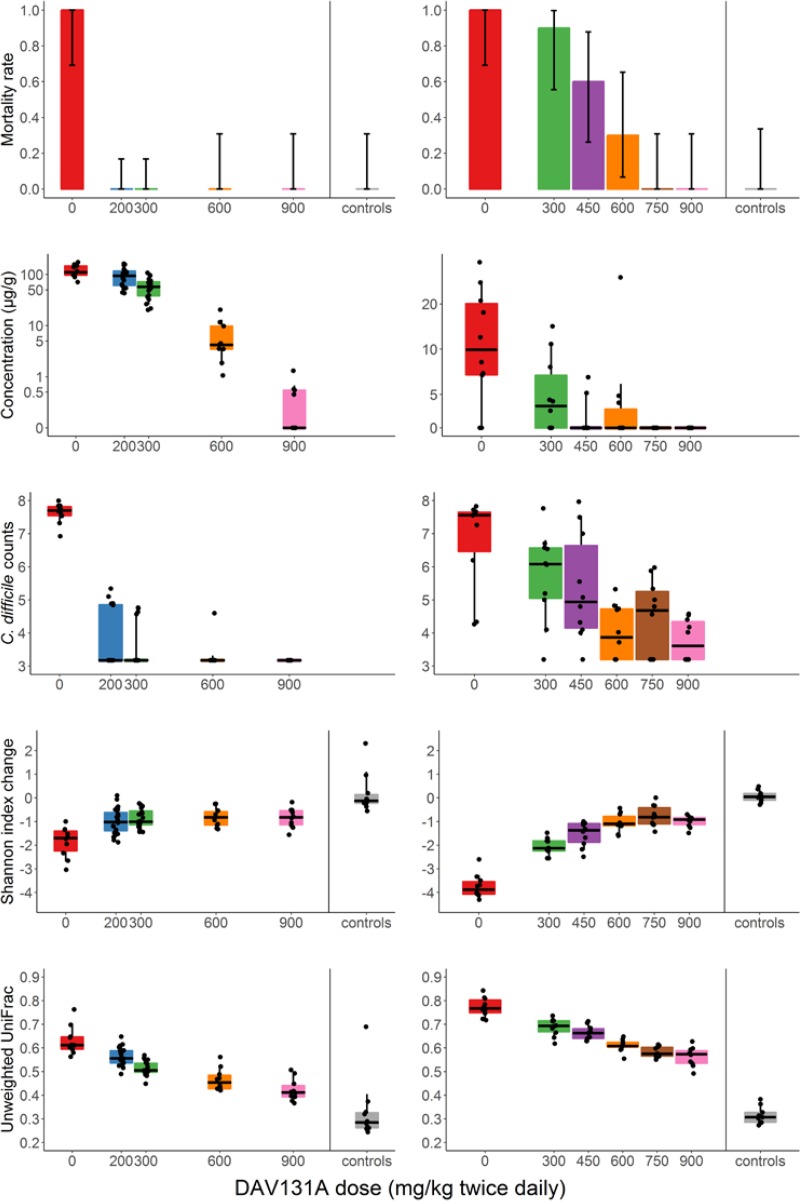
Among antibiotic-treated animals, 10 (12.5%) died in the moxifloxacin study and 28 (40.6%) in the clindamycin study. One hamster from one of the control groups died during the acclimation period, but none did so after the beginning of antibiotic treatment. Significant differences in mortality rates were observed between groups which received various doses of DAV131A in addition to the antibiotic (P < 10−11 in the moxifloxacin study and P < 10−10 in the clindamycin study). In both studies, all hamsters treated with antibiotic plus DAV131A placebo died. In the moxifloxacin study, all hamsters receiving 200 mg/kg DAV131A b.i.d. and greater survived, whereas in the clindamycin study, there was a dose-dependent reduction of mortality from 90% at 300 mg/kg DAV131 b.i.d. to 100% survival reached at 750 mg/kg DAV131 b.i.d. and above. Full results are presented in Table 1 and Fig. 2.
TABLE 1.
Mortality rates, fecal counts of C. difficile at D3, fecal concentrations of active antibiotic at D3, change of Shannon index between D0 and D3, and unweighted UniFrac distances between D0 and D3 according to treatment groups in the moxifloxacin and clindamycin studies
| Antibiotic | Treatment group | n | Mortality |
C. difficile counts |
Concnb |
Shannon index |
UniFrac |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | q or P valuea | Log10 CFU/g, median (minimum, maximum) | q or P value | μg/g, median (minimum, maximum) | q or P value | Change, median (minimum, maximum) | q or P value | Unweighted distance, median (minimum, maximum) | q or P value | |||
| Moxifloxacin | Controls | 10 | 0 (0) | 3.2 (3.2, 3.2) | −0.1 (−0.6, 2.3) | 0.28 (0.24, 0.69) | ||||||
| MXF/0 | 10 | 10 (100) | 7.7 (6.9, 8.0) | 110.0 (70.5, 172.7) | −1.7 (−3.0, −1.0) | 0.61 (0.56, 0.76) | ||||||
| MXF/200 | 20 | 0 (0) | <10−7 | 3.2 (3.2, 5.3) | <10−4 | 94.6 (43.0, 162.1) | 0.14 | −1 (−1.9, 0.1) | 0.00065 | 0.56 (0.49, 0.65) | 0.00075 | |
| MXF/300 | 20 | 0 (0) | <10−7 | 3.2 (3.2, 4.8) | <10−4 | 57.2 (20.2, 107.4) | <10−4 | −1 (−1.5, −0.2) | 0.00026 | 0.51 (0.45, 0.57) | <10−6 | |
| MXF/600 | 10 | 0 (0) | <10−4 | 3.2 (3.2, 4.6) | <10−5 | 4.2 (1.1, 20.3) | <10−4 | −0.8 (−1.3, −0.3) | 0.00023 | 0.45 (0.42, 0.56) | <10−4 | |
| MXF/900 | 10 | 0 (0) | <10−4 | 3.2 (3.2, 3.2) | <10−5 | 0.0 (0.0, 1.3) | 0.00033 | −0.8 (−1.6, −0.2) | 0.0022 | 0.41 (0.37, 0.51) | <10−4 | |
| All groups | 80 | 10 (12.5) | <10−11 | 3.2 (3.2, 8.0) | <10−8 | 59.4 (0.0, 172.7) | <10−9 | −1 (−3, 2.3) | <10−5 | 0.51 (0.24, 0.76) | <10−9 | |
| Clindamycin | Controls | 9 | 0 (0) | 3.2 (3.2, 3.2) | 0.0 (−0.3, 0.5) | 0.31 (0.27, 0.38) | ||||||
| CLI/0 | 10 | 10 (100) | 7.6 (4.3, 7.8) | 10.1 (0.0, 37.8) | −3.9 (−4.3, −2.6) | 0.77 (0.72, 0.84) | ||||||
| CLI/300 | 10 | 9 (90) | >0.99 | 6.1 (3.2, 7.8) | 0.075 | 4.2 (0.0, 14.2) | 0.061 | −2.1 (−2.6, −1.5) | <10−4 | 0.69 (0.62, 0.74) | <10−4 | |
| CLI/450 | 10 | 6 (60) | 0.11 | 4.9 (3.2, 8.0) | 0.066 | 0.0 (0.0, 6.5) | 0.0041 | −1.4 (−2.5, −1.0) | <10−4 | 0.66 (0.63, 0.71) | <10−4 | |
| CLI/600 | 10 | 3 (30) | 0.0052 | 3.9 (3.2, 5.3) | 0.0041 | 0.0 (0.0, 30.0) | 0.024 | −1.1 (−1.6, −0.4) | <10−4 | 0.61 (0.55, 0.65) | <10−4 | |
| CLI/750 | 10 | 0 (0) | <10−4 | 4.7 (3.2, 6.0) | 0.0074 | 0.0 (0.0, 0.0) | 0.0019 | −0.8 (−1.4, 0.0) | <10−4 | 0.57 (0.55, 0.61) | <10−4 | |
| CLI/900 | 10 | 0 (0) | <10−4 | 3.6 (3.2, 4.6) | 0.0041 | 0.0 (0.0, 0.0) | 0.0019 | −0.9 (−1.5, 0.7) | <10−4 | 0.57 (0.49, 0.63) | <10−4 | |
| All groups | 69 | 28 (40.6) | <10−10 | 4.4 (3.2, 8.0) | <10−5 | 0.0 (0.0, 37.8) | <10−4 | −1.1 (−4.3, 0.5) | <10−9 | 0.62 (0.27, 0.84) | <10−10 | |
q values refer to the comparison of the corresponding treatment group with the antibiotic-plus-DAV131A placebo treatment group (MXF/0 or CLI/0), after Benjamini-Hochberg correction. The P values for the comparison of all treatment groups using the Fisher exact test or Kruskal-Wallis test are reported in the “All groups” lines.
In the analysis of concentrations, only antibiotic-treated groups were included.
FIG 2.
Mortality rate, fecal concentration of free antibiotic at D3, C. difficile counts at D3, and change of Shannon index and unweighted UniFrac distance between D0 and D3 according to treatment group in the moxifloxacin (left panels) and clindamycin (right panels) studies. Bar plots of the mortality rates are presented with their 95% binomial confidence intervals. For concentrations, Shannon index, and unweighted UniFrac distances, the boxes present the 25th and 75th percentiles and the horizontal black bars report the median value, while whiskers report the 5th and 95th percentiles.
Fecal counts of C. difficile decreased with increasing doses of DAV131A (P < 10−7 in the moxifloxacin study and P = 0.00018 in the clindamycin study).
Fecal concentrations of free and active antibiotics, as measured by a microbiological assay, also decreased as expected with increasing doses of DAV131A (P < 10−9 in the moxifloxacin study and P < 10−4 in the clindamycin study). These concentrations were significantly lower in hamsters that survived than in those that died during the study (P = 0.00025 in the moxifloxacin study and P < 10−6 in the clindamycin study) (Table 2).
TABLE 2.
Values of free antibiotic concentrations and changes of α- and β-diversity indices between D0 and D3 according to vital status at D16 in antibiotic-treated groups for each study and their respective areas under the ROC curves for predicting occurrence of death by D16
| Antibiotic and parameter | Median (minimum, maximum) value for mice that: |
P valuec | AUROC (95% CI) | |
|---|---|---|---|---|
| Dieda | Survivedb | |||
| Moxifloxacin | ||||
| Concn | 110.0 (70.5, 172.7) | 52.9 (0.0, 162.1) | 0.00025 | 0.87 (0.76, 0.95) |
| α diversity | ||||
| Change of Shannon index | −1.7 (−3.0, −1.0) | −1.0 (−1.9, 0.1) | <10−4 | 0.91 (0.80, 0.98) |
| Change of no. of OTUs | −135.9 (−207.3, −52.2) | −72.9 (−201.0, 74.5) | 0.001 | 0.83 (0.67, 0.95) |
| Change of Chao1 index | −137.9 (−213.4, −46.2) | −75.5 (−229.3, 83.5) | 0.002 | 0.81 (0.64, 0.93) |
| β diversity | ||||
| UniFrac distance | ||||
| Unweighted | 0.61 (0.56, 0.76) | 0.51 (0.37, 0.65) | <10−5 | 0.95 (0.90, 0.99) |
| Weighted | 0.33 (0.24, 0.48) | 0.26 (0.13, 0.57) | 0.02 | 0.73 (0.58, 0.87) |
| Bray-Curtis dissimilarity | 0.78 (0.63, 0.86) | 0.60 (0.31, 0.87) | <10−4 | 0.91 (0.81, 0.99) |
| Clindamycin | ||||
| Concn | 5.0 (0.0, 37.8) | 0.0 (0.0, 4.4) | <10−6 | 0.81 (0.72, 0.91) |
| α diversity | ||||
| Change of Shannon index | −2.2 (−4.3, −0.4) | −1.1 (−2.6, 0.0) | <10−6 | 0.88 (0.78, 0.96) |
| Change of no. of OTUs | −223.9 (−344.6, −75.8) | −106.8 (−202.2, −22.0) | <10−5 | 0.86 (0.75, 0.95) |
| Change of Chao1 index | −227.7 (−358.2, −73.8) | −110.0 (−209.0, −22.7) | <10−6 | 0.86 (0.76, 0.95) |
| β diversity | ||||
| UniFrac distance | ||||
| Unweighted | 0.71 (0.59, 0.84) | 0.60 (0.49, 0.68) | <10−10 | 0.94 (0.88, 0.99) |
| Weighted | 0.42 (0.24, 0.62) | 0.30 (0.24, 0.59) | <10−6 | 0.87 (0.76, 0.96) |
| Bray-Curtis dissimilarity | 0.86 (0.71, 0.98) | 0.70 (0.61, 0.87) | <10−9 | 0.92 (0.84, 0.97) |
n = 10 for the moxifloxacin study and 28 for the clindamycin study.
n = 60 for the moxifloxacin study and 32 for the clindamycin study.
By the nonparametric Wilcoxon test.
The structure and composition of the bacterial intestinal microbiota were studied by 16S rRNA gene profiling using Illumina sequencing technology. The two antibiotics exhibited different effects on the taxonomic composition of the intestinal microbiota (see Fig. S1 in the supplemental material). Moxifloxacin administration had a relatively modest effect, consisting in a decrease of the mean relative abundance of Actinobacteria from 2.9% to 1.4%, and of Proteobacteria from 1.9% to 0.4% and an increase of the mean relative abundance of Bacteroidetes from 11.8% to 17.0%, while Firmicutes remained stable (changed from 79.4% to 80.3%). In contrast, clindamycin administration resulted in a very pronounced decrease of the mean relative abundance of Firmicutes from 87.4% to 14.0% and of Bacteroidetes from 5.1% to 0.1%. The mean relative abundance of Proteobacteria increased from 2.5% to 84.4%, and that of Actinobacteria remained quite stable (changed from 2.4% to 1.3%). For both antibiotics, the effect on the composition of the intestinal microbiota varied with the dose of DAV131A, with a much larger amplitude of variation in the case of clindamycin.
Several α-diversity (within sample) and β-diversity (between samples) metrics were computed for investigating the individual-specific change of diversity between the time of antibiotic initiation and the time of C. difficile inoculation. The loss of diversity between the beginning of antibiotic treatment and C. difficile inoculation was lower in DAV131A-treated hamsters than in those treated with an antibiotic and DAV131A placebo (Table 1; see Table S1 in the supplemental material). Indeed, loss of diversity increased with increasing concentrations of free antibiotic in feces, attesting to a direct relationship between exposure of the microbiota to an antibiotic and the extent of dysbiosis (in the moxifloxacin study, Spearman r = −0.25 [P = 0.043] for the change of Shannon index between day 0 [D0] and D3 and r = 0.71 [P < 10−10] for the unweighted UniFrac distance between D0 and D3; in the clindamycin study, r = −0.49 [P < 10−4] for the change of Shannon index between D0 and D3 and r = 0.57 [P < 10−5] for the unweighted UniFrac distance between D0 and D3 [see Fig. S2 and Table S2 in the supplemental material]).
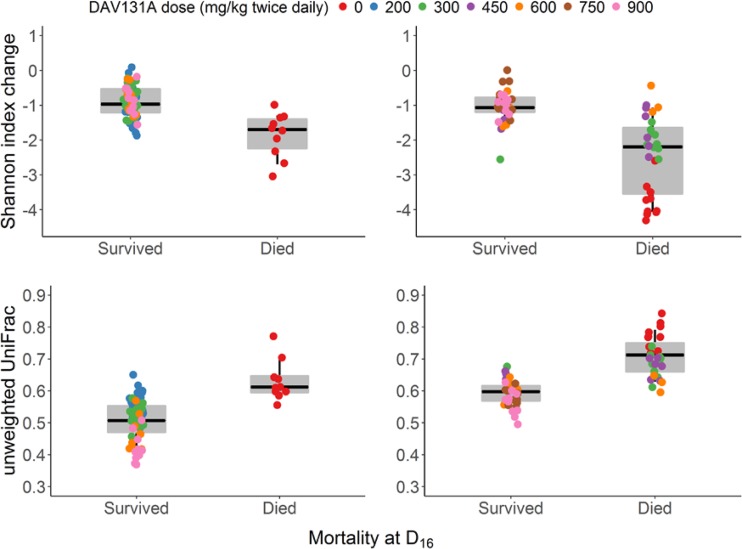
We also compared the changes in diversity within the intestinal microbiota between D0 and D3 according to the vital status at D16. The diversity at the time of C. difficile challenge was significantly less affected in hamsters that survived (Table 2 and Fig. 3). In the moxifloxacin study, the median (minimum, maximum) change of the Shannon index was −1.7 (−3.0, −1.0) in hamsters that died by D16, versus −1.0 (−1.9, −0.1) in those that survived (P < 10−4). In the clindamycin study, the median (minimum, maximum) change of the Shannon index was −2.2 (−4.3, −0.4) in hamsters that died by D16, versus −1.1 (−2.6, 0.0) in those that survived (P < 10−7). Interestingly, the median change of the Shannon index in hamsters that died was rather similar for the 2 antibiotics, in spite of their different spectra of activity and modes of action. In order to further assess the ability of diversity indices to predict death by D16, we computed for each diversity index the area under the receiver operating curve (AUROC), which can be interpreted as the probability that the index correctly ranks 2 randomly chosen animals. AUROCs were above 0.8 for all diversity indices studied (Table 2), indicating that they are highly predictive of the outcome (23). Each index also exhibited a similar predictability of death for both antibiotics. Changes in the Shannon index at the time of challenge had the best predictability of death by D16 among α-diversity indices, with AUROCs (95% confidence intervals [CI]) of 0.91 (0.80, 0.98) for moxifloxacin and 0.88 (0.78, 0.96) for clindamycin, whereas the unweighted UniFrac was the most predictive β-diversity index, with AUROCs of 0.95 (0.90, 0.99) for moxifloxacin and 0.94 (0.88, 0.99) for clindamycin. These two indices were further studied after pooling data from the two different antibiotic treatments. Overall, data from 130 antibiotic-treated animals were available, among which 38 animals (29.2%) died by D16. Logistic models of mortality by D16 for both diversity indices are presented in Fig. 4. The AUROC of the Shannon index change was 0.89 (0.82, 0.95), and that of the unweighted UniFrac distance was 0.95 (0.90, 0.98) (see Fig. S3 in the supplemental material), which also is indicative of their high predictive value. The difference between the two AUROCs was not significant (P = 0.10).
FIG 3.
Change of Shannon index (top panels) and unweighted UniFrac distance (bottom panels) between D0 and D3 according to the occurrence of death by D16 in the moxifloxacin (left panels) and clindamycin (right panels) studies. The boxes present the 25th and 75th percentiles and the horizontal black bars report the median value, while whiskers report the 5th and 95th percentiles.
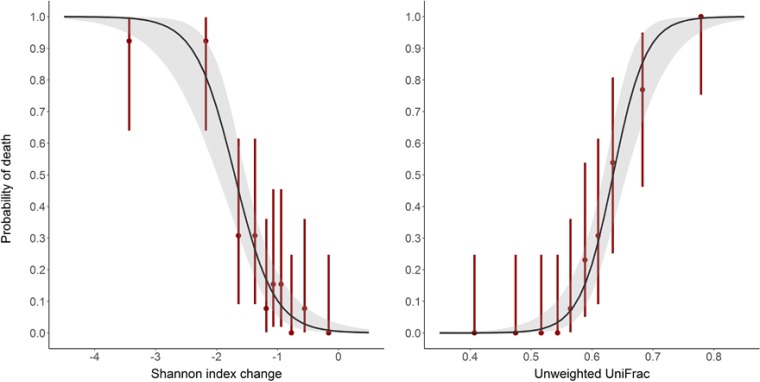
FIG 4.
Logistic models of mortality according to the change of Shannon index (left panel) (P < 10−15) and unweighted UniFrac distance (right panel) (P < 10−15) between D0 and D3 after pooling data from antibiotic-treated animals in the moxifloxacin and clindamycin studies. Red bars represent the mortality rates and their 95% confidence intervals of deciles of the observed diversity indices. The shaded areas represent the 95% confidence interval of the predicted probability of death.
As these two indices were highly predictive of mortality, we further studied them by determining their optimal cutoff values best discriminating between death and survival by D16 using the Youden index. The value of the Shannon index change best discriminating between death and survival at D16 was −1.7 (−1.8, −1.2) (see Fig. S4 in the supplemental material). The probability of observing a loss of diversity higher than −1.7 in hamsters that would die by D16 (sensitivity) was 0.71 (0.63, 0.95), and the probability of observing a loss of diversity lower than −1.7 in hamsters that survived at D16 (specificity) was 0.96 (0.76, 0.99). The best cutoff value of the unweighted UniFrac distance was 0.61 (0.58, 0.64) (Fig. S4). The associated sensitivity and specificity (95% CI) were 0.87 (0.79, 1.00) and 0.88 (0.72, 0.97), respectively. These values of sensitivity and specificity further illustrate the high predictability of these two diversity indices for the occurrence of the death of hamsters in these experiments.
Finally, in order to quantify the relationship between the loss of diversity and mortality and to determine the maximal change of diversity required to limit the mortality rate to predefined values, we developed a logistic model of the probability of death according to the diversity observed in the intestinal microbiota. The model showed that small losses of diversity were sufficient to allow the development of severe colitis and death in a substantial number of animals. For instance, a reduction of the Shannon index (95% CI) between D0 and D3 of as little as 0.7 (0.4, 1.1) was predicted to result in the death of 5% of the animals. The same mortality rate was predicted by an unweighted UniFrac distance of 0.51 (0.47, 0.55) between D0 and D3. Results for other mortality rates are presented in Table 3.
TABLE 3.
Estimated change of Shannon index and unweighted UniFrac distances between D0 and D3 needed to decrease the mortality rate to various levels in the hamster model of moxifloxacin-induced Clostridium difficile infection
| Mortality rate (%) | Change of Shannon index (95% CI) | Unweighted UniFrac distance (95% CI) |
|---|---|---|
| 10 | −1.0 (−1.2, −0.8) | 0.58 (0.55, 0.60) |
| 5 | −0.7 (−1.1, −0.4) | 0.55 (0.53, 0.58) |
| 1 | −0.2 (−0.7, 0.3) | 0.51 (0.47, 0.55) |
DISCUSSION
Our main result was the evidence of an association between the probability of hamster death and the antibiotic-induced loss of diversity of the intestinal microbiota at the time of C. difficile inoculation. Seemingly, such a quantitative relationship had never been described. In this animal model, antibiotics perturb the structure and function of the intestinal microbiota, allowing the germination and growth of C. difficile spores and the production of cytotoxic toxins, leading to death of the animals (12). The protection provided by DAV131A through lowering the fecal concentration of active antibiotic, previously shown for moxifloxacin (22), was extended here to clindamycin, an antibiotic from a different class with a very different mode of action and spectrum of activity.
Despite the fact that the two antibiotics studied had different impacts on the taxonomic composition of the intestinal microbiota, global indices of intestinal bacterial diversity exhibited similar variation patterns of change with antibiotic concentrations. Our observations showed a clear relation between the loss of intestinal microbiota diversity and the development of C. difficile infection-associated death in this model. Both the α- and β-diversity indices studied had high predictive capacities for the ability of C. difficile spores to generate a lethal infection, with the change of Shannon index between the beginning of antibiotic treatment and the time of C. difficile inoculation (for α diversity) and the unweighted UniFrac distance (for β diversity) appearing as the most predictive metrics. A link between the reduction of intestinal microbiota diversity after treatment with the glycylcycline antibiotic tigecycline had previously been reported in mice (24), but the precise quantitative relationship was not analyzed. In humans, the occurrence of C. difficile infection has been associated with a reduced diversity within the intestinal microbiota as assessed from the diarrheal feces (25, 26). Here, we extended these observations to hamsters treated with either of two very different antibiotics, moxifloxacin and clindamycin. We showed, using various metrics, that individual-specific loss of diversity within the intestinal microbiota induced by antibiotics prior to C. difficile inoculation was highly predictive of the animal's susceptibility to C. difficile infection, thus providing further insight into C. difficile pathophysiology. Furthermore, we were able to quantify this link, with even a small loss of diversity significantly increasing the risk of mortality. Indeed, a 0.7 reduction of the Shannon diversity index was associated with a 5% risk of death. Transposition of our results to humans, however, is challenging, in particular due to the differences in physiology between rodents and humans. The ANTICIPATE European observational study (NCT02896244) was undertaken to evaluate the incidence of Clostridium difficile infections in hospitalized patients aged over 50 who were treated with various antibiotics; evaluation of the associated states of the intestinal microbiota by 16S rRNA gene profiling in those patients should shed further light on their link to the risk of Clostridium difficile infection.
We observed that the loss of diversity was correlated with the concentration of free antibiotic in the fecal content. By adsorbing antibiotic residues reaching the colon after subcutaneous administration, DAV131A protected the microbiota against antibiotic-induced dysbiosis and reduced mortality in a dose-dependent manner. This approach appears to be promising, as it might be extended to most classes of antibiotics, in addition to the two tested here, due to the wide adsorbing capacities of the product (21). Transposition to humans is ongoing. In a phase 1 clinical trial, DAV132, the human counterpart of DAV131A containing the same adsorbent, was shown to reduce by more than 99% the fecal exposure to moxifloxacin in healthy volunteers, while the plasma concentration of the antibiotic remained unaffected; in subjects cotreated with moxifloxacin and DAV132, the diversity of the microbiota was protected from moxifloxacin-induced disruption (21). Further developments of this strategy to protect patients from the deleterious effects of antibiotic treatments on the microbiota are ongoing.
MATERIALS AND METHODS
Hamster model of antibiotic-induced C. difficile infection.
A previously developed hamster model of antibiotic-induced C. difficile infection was adapted to moxifloxacin (a fluoroquinolone antibiotic) and clindamycin (a lincosamide antibiotic) (27). After an 8-day acclimation period, male golden Syrian hamsters (80 to 120 g) received the antibiotic by subcutaneous injection at a time designated H0 once a day from day 1 (D1) to day 5 (D5). Administered doses were 30 mg/kg for moxifloxacin and 5 mg/kg for clindamycin. These doses were chosen as the lowest dose resulting in a 100% mortality rate in treated hamsters infected with C. difficile spores.
Animals were infected orally on day 3 (D3), 4 h after antibiotic administration (H4), with 104 spores of the nonepidemic C. difficile strain UNT103-1 (VA-11, REA J strain) (TcdA+ TcdB+ CdtB−; vancomycin MIC, 2 μg/ml; moxifloxacin MIC, 16 μg/ml; clindamycin MIC, >256 μg/ml; ceftriaxone MIC, 128 μg/ml), obtained from Curtis Donskey, Ohio VA Medical Center.
Hamsters were individually housed during all experiments, with no contact between animals. The vital status of the animals was evaluated daily until the end of the study at D16. Animals judged to be in a moribund stated were euthanized. All surviving hamsters were euthanized at D16.
Ethics statement.
Animals were housed in conformity with NIH guidelines (28). All procedures were conducted at the University of North Texas Health Science Center in Fort Worth, TX, USA, in accordance with Protocol IACUC-2016-0015 approved by the local Institutional Animal Care and Use Committee.
DAV131A.
DAV131A is an activated charcoal-based adsorbent with high adsorption capacity (29). It was administered to hamsters by oral gavage after mixing with 0.25% (wt/vol) Natrosol 250 hydroxyethylcellulose. Hamsters in placebo groups received Natrosol alone.
Study design.
Two studies with rather similar designs were performed, each with one antibiotic, moxifloxacin or clindamycin, in order to assess the protection provided by DAV131A against lethal antibiotic-induced C. difficile infection. DAV31A was administered twice a day (b.i.d.) to hamsters for 8 days, at H0 and H5 on D1 and then at H−4 and H1 from D2 to D8.
In the moxifloxacin study, 70 animals were treated with moxifloxacin and 10 animals were left untreated. Groups of 10 or 20 antibiotic-treated animals were constituted according to the DAV131A unit dose administered b.i.d.: DAV131A placebo (MXF/0, n = 10), 200 mg/kg (MXF/200, n = 20), 300 mg/kg (MXF/300, n = 20), 600 mg/kg (MXF/600, n = 10), or 900 mg/kg (MXF/900, n = 10). The control group was not treated with antibiotic and received the DAV131A placebo.
In the clindamycin study, 60 animals were treated with clindamycin and 10 were left untreated. Groups of 10 antibiotic-treated animals were constituted according to the DAV131A unit dose administered b.i.d.: DAV131A placebo (CLI/0, n = 10), 300 mg/kg (CLI/300, n = 10), 450 mg/kg (CLI/450, n = 10), 600 mg/kg (CLI/600, n = 10), 750 mg/kg (CLI/750, n = 10), or 900 mg/kg (CLI/900, n = 10). The control group was not treated with antibiotic and received the DAV131A placebo.
Sample collection.
For each animal, fecal samples were collected at D0 and D3. On D0, the fecal sample comprised pellets emitted in the 12 h preceding the first antibiotic administration. On D3, 2 pools of feces were collected. The first was constituted of pellets emitted in the 12 h following antibiotic administration (H0 to H12); this surrounds the time at which animals were challenged by gavage with C. difficile spores (at 4 h after antibiotic administration). The second was constituted by pellets emitted in the period between 12 and 24 h after antibiotic administration (H12 to H24). Coprophagy of hamsters was not controlled, as this is a natural behavior in rodents. Fecal samples were stored at −80°C until further analysis.
Determination of C. difficile counts in feces.
Fecal counts of C. difficile were determined extemporaneously at D3 (on the pool H12 to H24) by plating serial dilutions of the samples on CDSA selective medium (BBL C. difficile selective agar; BD). Counts were read after anaerobic incubation at 37°C for 48 h. Fecal counts below the limit of quantification (LOQ) (3.2 log10 CFU/g of feces) were assigned the LOQ value.
Measurement of antibiotic concentrations.
Fecal concentrations of free and active antibiotic were determined on fecal samples collected at D0 and D3 (on the pool H0 to H12) by a microbiological bioassay. On the day of the assay, feces were weighted and homogenized in sterile saline, and debris were eliminated by centrifugation. Fecal active moxifloxacin concentrations were measured using Bacillus subtilis ATCC 6633 after incubation at 37°C for 24 h (30). Fecal concentrations of active clindamycin were measured using Micrococcus luteus ATCC 9341 after incubation at 37°C for 24 h (31). Data below the limit of quantification were assigned the value 0.
16S rRNA gene bacterial community profiling.
Both D0 and D3 (pool H0 to H12) samples were analyzed using 16S rRNA gene profiling. Microbial DNA was extracted using an extraction protocol optimized at GenoScreen, based partially on commercially available extraction kits (QIAamp DNA stool kit; Qiagen, Germany) with the addition of chemical and mechanical lysis steps.
The V3-V4 region of the 16S rRNA gene was then amplified using an optimized and standardized amplicon library preparation protocol (Metabiote; GenoScreen, Lille, France). Positive (artificial bacterial community comprising 17 different bacteria [ABCv2]) and negative (sterile water) controls were also included. Briefly, PCRs were performed using 5 ng of genomic DNA and in-house fusion barcoded primers (final concentrations of 0.2 μM), with an annealing temperature of 50°C for 30 cycles. PCR products were purified using Agencourt AMPure XP magnetic beads (Beckman Coulter, Brea, CA, USA), quantified according to GenoScreen's protocol, and mixed in an equimolar amount. Sequencing was performed using 250-bp paired-end sequencing chemistry on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) at GenoScreen.
For the moxifloxacin study, a total of 25,284,850 raw sequences were obtained (16,421 to 57,650 sequences per sample), and for the clindamycin study, a total of 24,159,124 raw sequences were obtained (22,027 to 84,598 sequences per sample).
Raw paired-end reads were then demultiplexed per sample and subjected to the following process: (i) search for and removal of both forward and reverse primers using CutAdapt, with no mismatches allowed in the primers sequences; (ii) quality filtering using the PRINSEQ-lite PERL script (32) by truncating bases at the 3′ end with Phred quality scores of <30; and (iii) paired-end read assembly using FLASH (33) with a minimum overlap of 30 bases and >97% overlap identity. After preprocessing, 17,735,465 and 18,429,753 reads were available for the moxifloxacin and clindamycin studies, respectively.
Taxonomic and diversity analyses were performed using the Metabiote Online v2.0 pipeline (GenoScreen, Lille, France), which is based partially on the QIIME software v1.9.1 (34). Following the preprocessing steps, chimera sequences were detected and eliminated (by an in-house method based on the use of Usearch 6.1). Clustering of similar sequences (97% identity threshold for an affiliation at the genus level on the V3-V4 regions of the 16S rRNA gene) was then performed with Uclust v1.2.22q (35) through an open-reference OTU picking process and complete-linkage method, finally creating groups of sequences or “operational taxonomic units” (OTUs). An OTU cleaning step corresponding to the elimination of singletons was performed. For each OTU, the most abundant sequence was considered the reference sequence and was taxonomically compared to the Greengenes database, release 13_8 (http://greengenes.secondgenome.com/), by the RDP classifier method v2.2 (36).
Various diversity indices were computed using QIIME (34). α-diversity metrics included the Shannon diversity index, the number of observed OTUs, and the Chao1 index. These indices were computed for each sample after rarefaction of the data (9,217 and 13,938 sequences allowed an exhaustive description of the bacterial diversity in the moxifloxacin and the clindamycin studies, respectively). In order to study the evolution of the bacterial diversity after the beginning of antibiotic treatment, we computed for each animal the difference between the values of these indices at D3 and D0. For β-diversity metrics, we computed the unweighted and weighted UniFrac distances, as well as Bray-Curtis dissimilarity for each animal between the samples collected at D3 and D0.
Statistical analysis.
For each study, we compared mortality rates at D16 and diversity indices across groups using nonparametric Fisher exact or Kruskal-Wallis tests, as appropriate. Fecal free antibiotic concentrations and fecal counts of C. difficile at D3 were compared according to DAV131A unit dose in antibiotic-treated hamsters using the Kruskal-Wallis test. In case of a significant difference, post hoc comparisons of each of the antibiotic-treated groups to the control group were performed using the nonparametric Fisher exact test or Wilcoxon test with Benjamini-Hochberg's correction for multiple testing. The correlations between active moxifloxacin or clindamycin fecal concentrations and diversity indices were studied using the Spearman rank correlation coefficient among antibiotic-treated hamsters.
We then compared for each study the fecal free antibiotic concentrations or diversity indices at D3 according to the vital status at D16 in antibiotic-treated hamsters, using the nonparametric Wilcoxon test. The predictability of death by D16 using the fecal free antibiotic concentration and each studied diversity index was evaluated using the area under the receiving operator curve (AUROC) and its 95% confidence interval, computed using 2,000 paired bootstrap replicates. In the context of the present work, the AUROC can be interpreted as the probability that the diversity index will correctly rank two randomly chosen animals, one which would die by D16 and 1 which would survive (37).
In order to further analyze the link between microbial diversity and mortality by D16, we pooled the data from the two studies and performed a logistic regression of mortality by D16 according to diversity index in all antibiotic-treated hamsters. The diversity indices studied were those with the best predictive capacity among α- and β-diversity indices. Predictability was estimated using the AUROC and its 95% confidence interval. AUROCs of the two indices were compared using 2,000 paired bootstrap replicates. The best cutoff value for discriminating between hamsters that died and those that survived at D16 was determined as the value allowing the maximization of both sensitivity and specificity, using the Youden index (38) and its 95% confidence interval. In the framework of the present study, sensitivity represents the probability of change of diversity between D0 and D3 being higher than a cutoff value in hamsters that will die by D16, and specificity is the probability of the change of diversity being lower than a cutoff value in hamsters that will survive until D16. The Youden index is computed as sensitivity plus specificity minus 1 and ranges between −1 and 1. A logistic model was then used to determine the diversity index values required to reduce mortality to various rates ranging from 1% to 10%.
Data are presented as number of observations n (%) or median (minimum, maximum). All tests were 2 sided with a type I error of 0.05. All analyses were performed using R software v3.2.2.
Accession number(s).
Sequence data have been submitted to the NCBI database under accession number PRJNA478191.
Supplementary Material
ACKNOWLEDGMENTS
S.S.-J., F.S.-G., N.S.-L., T.C., A.A., and J.D.G. designed the research. M.P., W.W., and F.S.-G. performed the research. S.F. performed the metagenomic analysis. C.B., T.T.N., and F.M. performed the statistical analysis of the data. C.B., S.S.-J., F.M., A.A., and J.D.G. wrote the paper. All authors agreed on the final version of the manuscript.
P.H., F.S.-G., N.S.-L., T.C., and S.S.-J. are employees of the Da Volterra Company. A.A., C.B., J.D.G., and F.M. are consultants for the Da Volterra Company.
This work was supported by Da Volterra, Paris, France. T.T.N. performed statistical work for the Da Volterra Company through a contract with INSERM UMR 1137.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00925-18.
REFERENCES
- 1.Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108(Suppl 1):S4554–S4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtman JS, Ferreyra JA, Ng KM, Smits SA, Sonnenburg JL, Elias JE. 2016. Host-microbiota interactions in the pathogenesis of antibiotic-associated diseases. Cell Rep 14:1049–1061. doi: 10.1016/j.celrep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, Neulinger SC, Daumer C, Heinsen FA, Latorre A, Barbas C, Seifert J, dos Santos VM, Ott SJ, Ferrer M, Moya A. 2013. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62:1591–1601. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theriot CM, Bowman AA, Young VB. 2016. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere 1:e00045-15. doi: 10.1128/mSphere.00045-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jernberg C, Lofmark S, Edlund C, Jansson JK. 2010. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 6.Bergogne-Berezin E. 2000. Treatment and prevention of antibiotic associated diarrhea. Int J Antimicrob Agents 16:521–526. doi: 10.1016/S0924-8579(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 7.Theriot CM, Young VB. 2015. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol 69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubberke ER, Olsen MA. 2012. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 55(Suppl 2):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slimings C, Riley TV. 2014. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 69:881–891. doi: 10.1093/jac/dkt477. [DOI] [PubMed] [Google Scholar]
- 10.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. 2017. Biggest threats. https://www.cdc.gov/drugresistance/biggest_threats.html. Accessed 4 May 2018.
- 12.Best EL, Freeman J, Wilcox MH. 2012. Models for the study of Clostridium difficile infection. Gut Microbes 3:145–167. doi: 10.4161/gmic.19526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson KH, Silva J, Fekety FR. 1981. Suppression of Clostridium difficile by normal hamster cecal flora and prevention of antibiotic-associated cecitis. Infect Immun 34:626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson HE, Borriello SP. 1990. Quantitative study of antibiotic-induced susceptibility to Clostridium difficile enterocecitis in hamsters. Antimicrob Agents Chemother 34:1348–1353. doi: 10.1128/AAC.34.7.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmer GW, Vega R, Mohutsky MA, McFarland LV. 1999. Variable time of onset of Clostridium difficile disease initiated by antimicrobial treatment in hamsters. Microb Ecol Health Dis 11:163–168. doi: 10.1080/089106099435745. [DOI] [Google Scholar]
- 16.Metzker ML. 2010. Sequencing technologies—the next generation. Nat Rev Genet 11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 17.Shannon C. 1948. A mathematical theory of communication. Bell Syst Tech J 27:623–656. doi: 10.1002/j.1538-7305.1948.tb00917.x. [DOI] [Google Scholar]
- 18.Chao A. 1984. Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270. [Google Scholar]
- 19.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray J, Curtis J. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol Monog 27:325–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 21.de Gunzburg J, Ghozlane A, Ducher A, Le Chatelier E, Duval X, Ruppé E, Armand-Lefèvre L, Sablier-Gallis F, Burdet C, Alavoine L, Chachaty E, Augustin V, Varastet M, Levenez F, Kennedy S, Pons N, Mentré F, Andremont A. 2018. Protection of the human gut microbiome from antibiotics. J Infect Dis 217:628–636. doi: 10.1093/infdis/jix604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdet C, Sayah-Jeanne S, Nguyen TT, Miossec C, Saint-Lu N, Pulse M, Weiss W, Andremont A, Mentre F, de Gunzburg J. 2017. Protection of hamsters from mortality by reducing fecal moxifloxacin concentration with DAV131A in a model of moxifloxacin-induced Clostridium difficile colitis. Antimicrob Agents Chemother 61:e00543-17. doi: 10.1128/AAC.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S. 2000. Applied logistic regression, 2nd ed Wiley Interscience, Hoboken, NJ, USA. [Google Scholar]
- 24.Bassis CM, Theriot CM, Young VB. 2014. Alteration of the murine gastrointestinal microbiota by tigecycline leads to increased susceptibility to Clostridium difficile infection. Antimicrob Agents Chemother 58:2767–2774. doi: 10.1128/AAC.02262-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert AM, Rogers MA, Ring C, Mogle J, Petrosino JP, Young VB, Aronoff DM, Schloss PD. 2014. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. mBio 5:e01021-14. doi: 10.1128/mBio.01021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Dong D, Jiang C, Li Z, Wang X, Peng Y. 2015. Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe 34:1–7. doi: 10.1016/j.anaerobe.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Phillips ST, Nagaro K, Sambol SP, Johnson S, Gerding DN. 2011. Susceptibility of hamsters to infection by historic and epidemic BI Clostridium difficile strains during daily administration of three fluoroquinolones. Anaerobe 17:166–169. doi: 10.1016/j.anaerobe.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 28.National Research Council. 2011. Guide for the care and use of laboratory animals. The National Academy Press, Washington, DC, USA. [Google Scholar]
- 29.Grall N, Massias L, Nguyen TT, Sayah-Jeanne S, Ducrot N, Chachaty E, de Gunzburg J, Andremont A. 2013. Oral DAV131, a charcoal-based adsorbent, inhibits intestinal colonization by beta-lactam-resistant Klebsiella pneumoniae in cefotaxime-treated mice. Antimicrob Agents Chemother 57:5423–5425. doi: 10.1128/AAC.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampougeris G, Antoniadou A, Kavouklis E, Chryssouli Z, Giamarellou H. 2005. Penetration of moxifloxacin into the human aqueous humour after oral administration. Br J Ophthalmol 89:628–631. doi: 10.1136/bjo.2004.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courvalin O, Leclercq R, Rice L. 2010. Antibiogram. ASM Press, Washington, DC. [Google Scholar]
- 32.Schmieder R, Edwards R. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fawcett T. 2006. An introduction to ROC analysis. Pattern Recog Lett 27:861–874. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- 38.Youden WJ. 1950. Index for rating diagnostic tests. Cancer 3:32–35. doi:. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.