Since its original isolation in 2009, Candida auris has spread across the globe as a causative agent of invasive candidiasis. C. auris is typically intrinsically resistant to fluconazole and can also be resistant to echinocandins and even amphotericin B.
KEYWORDS: Candida auris, antifungals, Prestwick library, repurposing, candidiasis
ABSTRACT
Since its original isolation in 2009, Candida auris has spread across the globe as a causative agent of invasive candidiasis. C. auris is typically intrinsically resistant to fluconazole and can also be resistant to echinocandins and even amphotericin B. Thus, there is an urgent need to find new treatment options against this emerging pathogen. To address this growing problem, we performed a screen of the Prestwick Chemical library, a repurposing library of 1,280 small molecules, consisting mostly of approved off-patent drugs, in search of those with activity against a multidrug-resistant C. auris isolate. Our initial screen, using standardized susceptibility testing methodologies, identified nine miscellaneous compounds with no previous clinical indication as antifungals or antiseptics that displayed activity against C. auris. Confirmation and follow-up studies identified ebselen as the drug displaying the most potent activity, with 100% inhibition of growth detected at concentrations as low as 2.5 μM. We further evaluated the ability of ebselen to inhibit C. auris biofilm formation and examined the effects of combination therapies of ebselen with clinically used antifungals. We extended our studies to different C. auris strains with various susceptibility patterns and also confirmed its antifungal activity against Candida albicans and clinical isolates of multiple other Candida species. Furthermore, ebselen displayed a broad spectrum of antifungal actions on the basis of its activity against a variety of medically important fungi, including yeasts and molds. Overall, our results indicate the promise of ebselen as a repositionable agent for the treatment of candidiasis and possibly other mycoses and, in particular, for the treatment of infections refractory to conventional treatment with current antifungals.
INTRODUCTION
Candidiasis represents a significant challenge to clinicians, as Candida species are currently the third to fourth most common cause of nosocomial bloodstream infections in hospitalized patients (1–3). Invasive candidiasis carries substantial morbidity and mortality, with crude mortality rates approaching 40% (4). Although Candida albicans remains the main causative agent of this infectious disease, non-albicans Candida spp. account for approximately half the cases. Most recently, attention has focused on the emerging pathogen Candida auris. First described in 2009 as an isolate collected from the external ear canal of a patient in Japan (5), C. auris has quickly spread and become a growing threat in hospitals throughout Asia, Europe, South America, and more recently, the United States (6–10). Unlike C. albicans, this emerging pathogen has the ability to live on surfaces outside the human body, further complicating the management of these infections by health care facilities (11, 12). For example, an outbreak of C. auris infections in London from April 2015 to July 2016 had 50 cases, with 44% of patients developing candidemia, and strict infection and prevention control measures had to be implemented to slow hospital transmission (12, 13). In a recent retrospective review of the clinical history of 54 patients, most had multiple risk factors for invasive disease, and candidemia was observed in 61% (8). Strikingly, the mortality rate in this series of patients was 59%. In the United States, as of 31 December 2017, the number of documented cases of infection caused by this species has escalated to a total of 228 (14), which is markedly higher than just a few months before (15, 16). Unfortunately, antifungal therapy against invasive infections caused by this emerging pathogen may be limited, as up to 90% of isolates are resistant to fluconazole and 50% have reduced susceptibility to other azoles. Currently, the echinocandins are recommended for the treatment of C. auris infections, but elevated MICs secondary to hot spot regions in the FKS genes (FKS1 and FKS2) known to cause resistance to the echinocandins in other Candida species and Aspergillus fumigatus have been found in a proportion of C. auris isolates (17, 18). A few isolates have been documented to be resistant to all clinically available classes of antifungal agents, including amphotericin B. In addition, although C. auris does not form hyphae, it has the ability to form biofilms which can also contribute to high levels of resistance (19). To make matters worse, this species is also often misidentified by commercially available automated systems that use biochemical means for species identification (20, 21). This may further negatively affect outcomes if inappropriate therapy is initiated on the basis of an incorrect species identification.
Repurposing or repositioning existing drugs can considerably decrease the effort, time, and expense that it takes to develop novel antifungals (22), which represents a pressing and unmet clinical need, as highlighted by the emergence of multidrug-resistant C. auris, its spread, and the increasing number of infections caused by this recently described pathogen (23, 24). Drug repurposing consists of the evaluation of drugs that are already approved or undergoing evaluation for the treatment of other diseases and, for the purpose of this study, have no previous clinical indications as antifungals (25, 26). To this extent, we screened a repurposing library in search of drugs with antifungal activity against a multidrug-resistant C. auris isolate. The Prestwick Chemical library consists mostly of an FDA-approved off-patent collection of approximately 1,200 small molecules (80 to 1,670 g/mol) with a wide range of functions and mechanisms of action that are used as drugs for a variety of diseases, including infectious, neurodegenerative, psychiatric, and cardiovascular diseases and cancer. Moreover, since the structure, chemical properties, and biological functions of almost all members of this library are known, we would expect any hits obtained in our antifungal screen to be easier to interpret and also facilitate the further analysis of the novel functionality of the established molecule (27). Our results identified ebselen, an organoselenium compound currently undergoing clinical trials for the treatment of different diseases (28, 29), as the leading repositionable candidate with potent antifungal activity against C. auris.
RESULTS AND DISCUSSION
Screening the Prestwick library for inhibitors of C. auris growth.
We performed a primary screen of the Prestwick Chemical library, at a 20 μM fixed concentration, to identify inhibitors of C. auris growth, for which we used a 96-well microtiter plate-based model mostly in accordance with Clinical and Laboratory Standards Institute (CLSI) methodologies with slight modifications. This initial screen was conducted using C. auris 0390 strain from the Centers for Disease Control and Prevention (CDC) panel (30), which is resistant to fluconazole and amphotericin B and also has decreased susceptibility against echinocandins. The screen was conducted in duplicates (using independent plates), and the results are expressed as a percentage inhibition of growth compared to that of untreated controls, estimated from spectrophotometric readings at the end of the 48-h incubation period (Fig. 1A and Table 1). On the basis of this primary screen, we identified 27 initial hit compounds that inhibited growth by >70% (Fig. 1A), resulting in a 2.1% initial hit rate. These 27 hits were initially classified into three major categories, including 12 antiseptics/antimicrobials, 6 known antifungal agents, and 9 other miscellaneous drugs with no previous clinical indication as antifungals (Fig. 1B and Table 1; see also Table S1 in the supplemental material). Because the main emphasis of this work was to find drugs that can be repurposed as antifungals for the treatment of invasive infections, follow-up experiments were focused on these miscellaneous drugs. However, we note that since, unlike C. albicans, C. auris has the ability to live on surfaces outside the human body, the identification of different antiseptics (Table S1) with activity against this emerging nosocomial pathogen can also be important for the disinfection of surfaces that could help curtail microepidemics in health care facilities (12, 31, 32). Regarding the established antifungals (Table S1), as expected and confirming the validity of our screening technique, the results of the primary screen confirmed the lack of activity of both fluconazole and amphotericin B (echinocandins are not represented in the Prestwick library). As expected, most other azole derivatives lacked activity in this screen. A few clinically available antifungals, including voriconazole, were represented among the hits, possibly due to the relatively high concentration at which we performed the initial screen. C. auris strain 0390 is reported to be resistant to 5-flucytosine (30), and so it was somewhat surprising to see this antifungal in the initial list of hits for this primary screen. Another antifungal compound with activity in this screen, ciclopirox ethanolamine, is used for the topical dermatologic treatment of mycoses and could represent an option for the treatment of superficial infections caused by C. auris (33).
FIG 1.
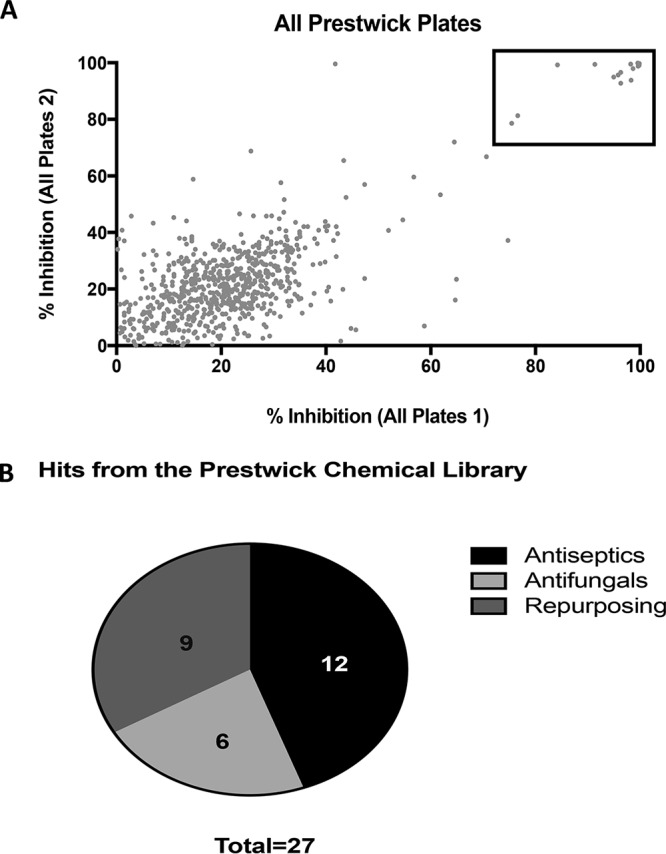
Primary screening of the Prestwick Chemical library. (A) Graphical representation of results of primary screening. The experiment was performed in duplicates and results expressed as percent inhibition according to OD490 spectrophotometric readings. (B) Initial hits were classified into three classes: antiseptics, antifungals, and repositionable agents.
TABLE 1.
Potential repositionable hit compounds identified during the primary screen of the Prestwick library and their corresponding IC50s against C. auris strain 0390 planktonic growth calculated from dose-dependent confirmatory experiments
| Drug Name | Drug type | % inhibition (from primary screen) | IC50 (μM) |
|---|---|---|---|
| R(-)-Apomorphine hydrochloride hemihydrate | Emetic | >90 | 6.928 |
| Suloctidil | Vasodilator | >90 | Not confirmed |
| Ebselen | Anti-inflammatory | >90 | 1.413 |
| Nisoldipine | Antihypertensive | 79 | 29.42 |
| Argatroban | Anticoagulant | >90 (on single plate) | Not confirmed |
| Dimethisoquin hydrochloride | Local anesthetic | 70 | 28.61 |
| Pentetic acid | Chelating agent/radioactive decontaminant | >90 | 11.15 |
| Pentamidine isethionate | Antiparasitic | 87 | 72.94 |
| Pyrvinium pamoate | Antiparasitic | 75 | 7.192 |
Dose-response assays to confirm the activity and establish the potency of initial hit compounds.
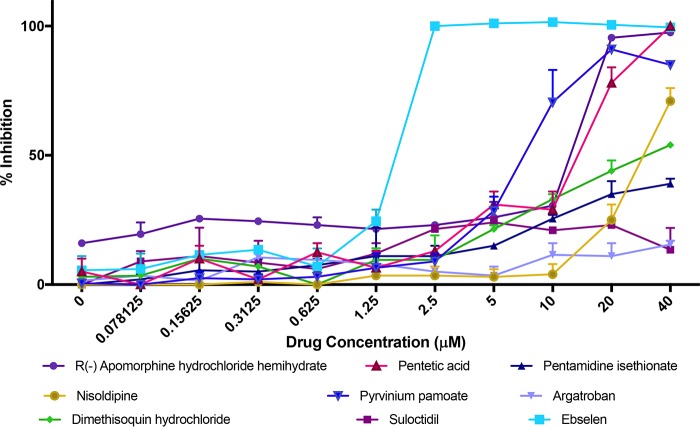
The nine miscellaneous drugs with no previous clinical indication as antifungals identified as hits in the primary screen were apomorphine, suloctidil, ebselen, nisoldipine, argatroban, dimethisoquin, pentetic acid, pentamidine isethionate, and pyrvinium pamoate. These compounds have a variety of original classifications as emetic, antihypertensive, antiparasitic, and anti-inflammatory drugs (Table 1). To confirm their inhibitory effects on C. auris growth, we performed dose-response assays using the same method used for the primary screen but tested their inhibitory activity over a range of drug concentrations, from 40 to 0.078 μM (Fig. 2). From the resulting dose-response curves, we calculated the 50% inhibitory concentration (IC50) for each known drug, a measure of the antifungal potency of each compound against C. auris (Table 1). Although we confirmed the activity of a majority of these initial hit compounds, from these experiments, ebselen clearly emerged as the top drug with potent inhibitory activity against C. auris, with concentrations as low as 2.5 μM completely abrogating the growth of this multidrug-resistant strain (Fig. 2). Notably, this concentration is well within the physiologically achievable levels of ebselen according to previous studies (34, 35). Thus, we decided to focus our attention on this drug.
FIG 2.
Dose-response assays to confirm the inhibitory activity and determine the potency of the repositionable compounds identified as initial hits in the primary screen. Experiments were performed in two independent plates with two duplicate wells per plate. Bars indicate standard errors.
Ebselen [2-phenyl-1,2-benzisoselenazol-3(2H)-one] (see Fig. S1) is a synthetic organoselenium compound that is part of the NIH clinical collection, and despite extensive research, its target molecules and mechanism of action are not completely understood (36–40). Due to its highly electrophilic nature, it interacts with cysteine-rich proteins (including thioredoxin) as well as with nonproteins such as thiols (41, 42). It is generally considered an antioxidant, mimicking glutathione peroxidase activity and catalyzing the reduction of reactive oxidase species (25, 29, 43), leading to the attenuation of damage caused by oxidants and radicals. Although it is not currently FDA approved, it is considered clinically safe and is undergoing clinical trials for different indications, including stroke, arthritis, hearing loss, cardiovascular disease, atherosclerosis, and cancer (28, 44). In addition, the antibacterial activity of ebselen has been demonstrated, which may be related to the inhibition of protein synthesis (45–47). Interestingly, the activity of ebselen against Saccharomyces cerevisiae was recently described, which was associated with the induction of reactive oxygen species (ROS)-mediated cytotoxicity and the membrane H+-ATPase pump (Pma1p) in yeast, already hinting to its potential antifungal activity (48). These observations were subsequently confirmed in elegant studies by the Seleem group, demonstrating the antifungal activity of ebselen against pathogenic yeast species by regulating glutathione and ROS production in fungal cells and depleting intracellular glutathione levels (41). Altogether, these data confirm our observations and provide further credence to the potential of ebselen to be repurposed as an antifungal drug.
Activity of ebselen against several C. auris clinical isolates from the CDC panel.
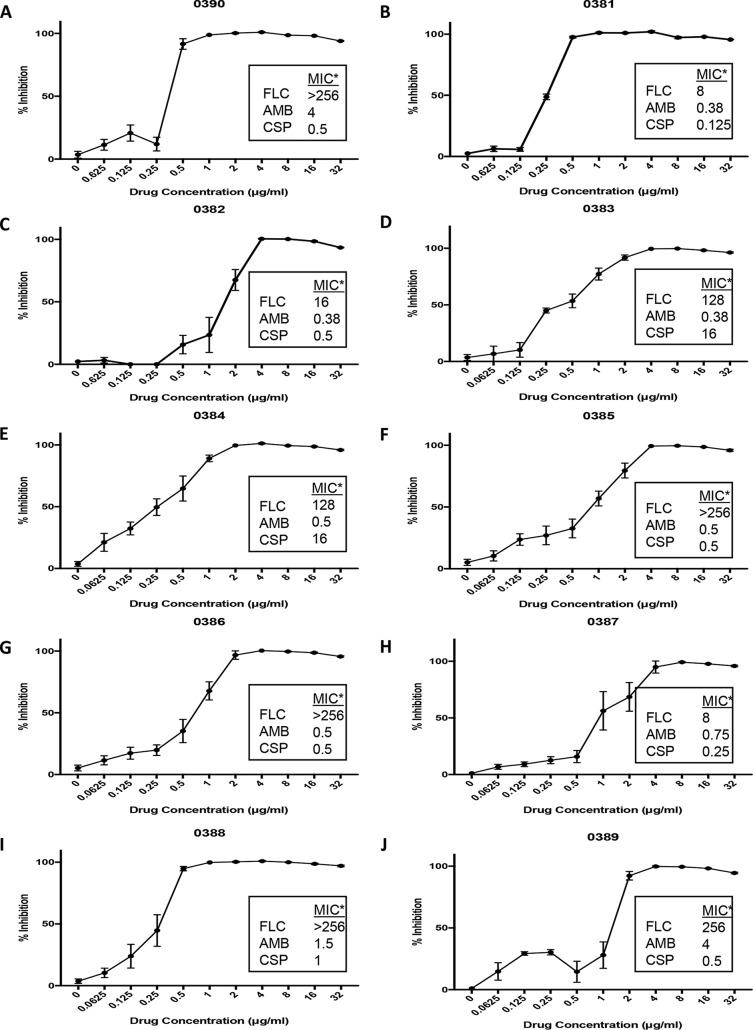
We expanded our experiments to examine the ability of ebselen to inhibit planktonic growth of all 10 C. auris clinical isolates present in the CDC panel (30). For these experiments, we used the same 96-well microtiter plate method as for the dose-response assays described above, a slightly modified version of CLSI susceptibility techniques, with ebselen concentrations ranging from 32 to 0.0625 μg/ml. From the resulting dose-response assays (Fig. 3), we calculated the corresponding IC50s of ebselen against the different C. auris strains, which ranged from 0.2345 to 1.47 μg/ml (see Table S2). The overall results indicated the potent activity of ebselen against all 10 C. auris strains tested, irrespective of their patterns of susceptibility to current antifungals (see also boxes in Fig. 3 for specific information on MIC values for fluconazole, amphotericin B, and caspofungin against each C. auris strain, previously determined at the CDC).
FIG 3.
Inhibition of planktonic growth of 10 C. auris strains by ebselen. Graphs depict dose-response activity of ebselen against the different C. auris strains from the CDC panel. The corresponding MIC values for fluconazole, amphotericin B, and caspofungin are included for comparison purposes (*, provided by the CDC, determined by Etest). Experiments were performed in two independent plates with two duplicate wells per plate. Bars indicate standard errors.
Activity of ebselen against C. auris biofilms.
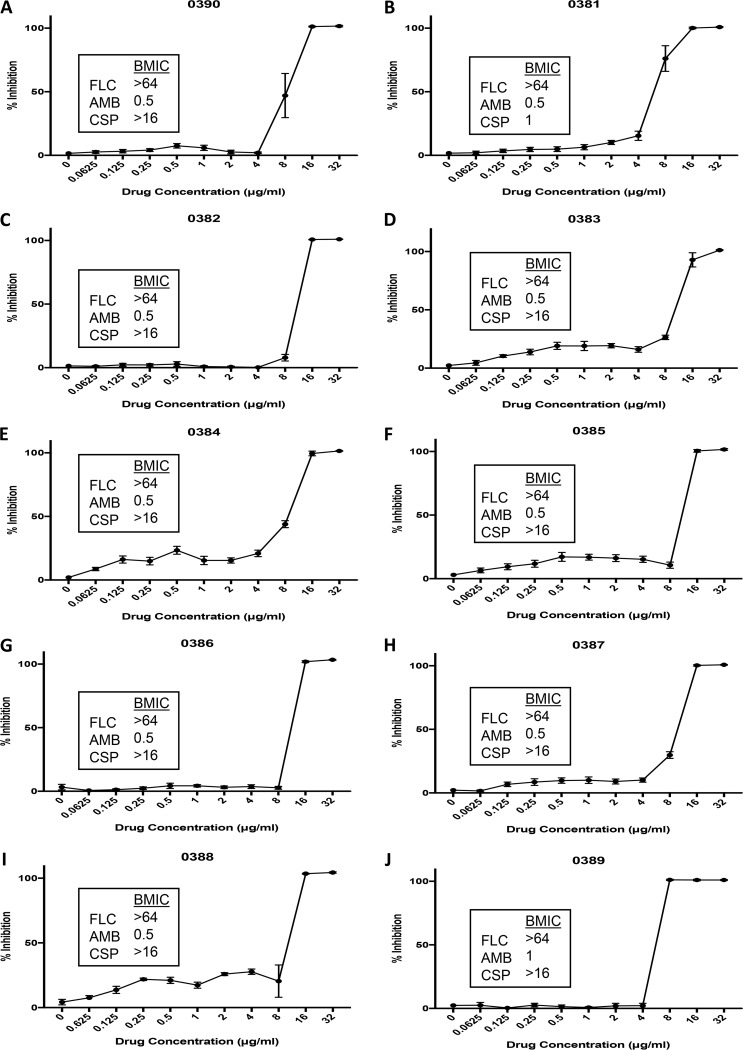
Candida spp. display the ability to form biofilms on inert and biological surfaces, and biofilm formation further complicates treatment due to the high levels of resistance against most clinically used antifungals (49). For example, Candida cells within biofilms are up to 1,000 times more resistant to fluconazole than their planktonic counterparts (50). C. auris is not an exception to this rule; although a poorer biofilm former than C. albicans, its ability to form biofilms has recently been described (19, 51). Particularly worrisome are the facts that the ability of C. auris to form biofilms has been associated with increased virulence and poorer patient outcomes and that C. auris biofilms are intrinsically resistant to all clinically used antifungals, including the echinocandins (19). Thus, there is an urgent need for new antifungals with activity against C. auris biofilms. To examine the ability of ebselen to inhibit C. auris biofilm formation, all 10 C. auris clinical isolates in the CDC panel were grown in flat-bottomed 96-well polystyrene microtiter plates in the presence or absence of different concentrations of the drug. The efficacy of ebselen to inhibit biofilm formation was assessed by the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay that measures the metabolic activity of sessile cells within the biofilms. From the corresponding dose-response assays (Fig. 4), we determined the IC50s at which ebselen inhibits biofilm formation by the different C. auris strains tested, as shown in Table S2. The results indicate a reasonable level of activity of ebselen against C. auris biofilms, with biofilm IC50s ranging from 5.864 to 9.781 μg/ml, as the drug is able to inhibit biofilm formation at concentrations that are only slightly elevated compared to those that inhibit planktonic C. auris populations and are still within the achievable clinical levels (34, 35). In parallel experiments, we also determined the ability of established antifungals to inhibit the formation of C. auris biofilms (see boxes in Fig. 4 showing biofilm MIC [BMIC] values for fluconazole, amphotericin B, and caspofungin against each C. auris isolate tested). The results indicated that amphotericin B was moderately effective; however, both fluconazole and caspofungin were completely ineffective at inhibiting the biofilm formation of all different C. auris isolates tested. This is in agreement with a previous report demonstrating that, contrary to those of C. albicans, C. auris biofilms are intrinsically resistant to echinocandins (19). Thus, the biofilm inhibitory activity of ebselen compares favorably to those obtained with current antifungal drugs, in particular, fluconazole and caspofungin. Figure S2 shows the crystal violet staining of biofilms formed by all 10 strains of C. auris, as well as the dose-response curve for ebselen in this assay. Microscopy was performed for the control biofilms of each strain to show their biofilm-forming capability, while the dose-response curves showed the ability of ebselen to inhibit biofilm formation through the estimation of biofilm biomass compared to that of the untreated control. The results of this assay indicated that, regardless of biofilm-forming ability, ebselen demonstrated inhibitory activity against all C. auris isolates tested, thereby confirming our observations from the XTT colorimetric assay.
FIG 4.
Inhibition of biofilm formation of 10 C. auris strains by ebselen. Graphs depict dose-response biofilm-inhibitory activity of ebselen against the different C. auris strains from the CDC panel. The corresponding BMIC values, established at 80% inhibition, for fluconazole, amphotericin B, and caspofungin against each strain were also determined and are included in the boxes. Experiments were performed in two independent plates with two duplicate wells per plate. Bars indicate standard errors.
Activity of ebselen against different Candida species.
Having demonstrated the activity of ebselen against C. auris, we were interested in determining its spectrum of activities against other Candida species. For these experiments, we used representative isolates of different Candida species, including C. lusitaniae and C. krusei strains from the same CDC panel (30), the laboratory strain C. albicans SC5314, and clinical isolates of C. dubliniensis, C. parapsilosis, C. tropicalis, and C. glabrata from the Fungus Testing Laboratory collection. Susceptibility testing was performed both under planktonic and biofilm growing conditions using the same 96-well microtiter plate methods described above. Table 2 shows the calculated IC50s for the different Candida spp. for the inhibition of both planktonic and biofilm growth, while the corresponding dose-response graphs are shown in Fig. S3 and S4. Although some differences between the species were observed, the results indicate that ebselen is active against all Candida spp. tested, with planktonic IC50s generally in the range of 0.5 to 2 μg/ml and biofilm IC50s only slightly higher, in the range of 2 to 8 μg/ml.
TABLE 2.
Calculated IC50s of ebselen for inhibition of planktonic growth and inhibition of biofilm formation for representative strains of other Candida species
| Candida species | IC50 (μg/ml [95% CIa]) |
|
|---|---|---|
| Planktonic | Biofilm | |
| C. albicans | 2.832 (2.634–3.044) | 8.967 (≤9.514) |
| C. dubliniensis | 0.4012 (0.3728–0.431) | 5.01 (3.514–7.282) |
| C. tropicalis | 0.6979 (0.5217–0.9181) | 10.35 (≤11.4) |
| C. parapsilosis | 0.9341 (0.8296–1.047) | 4.296 (3.106–5.891) |
| C. glabrata | 0.537 (undetermined) | 4.705 (undetermined) |
| C. lusitaniae | 0.2913 (≤0.3043) | 6.867 (undetermined) |
| C. krusei | 1.421 (1.298–1.548) | 2.843 (2.419–3.325) |
CI, confidence interval.
Activity of ebselen in combination with clinically used antifungal drugs.
A very likely scenario in the clinics is that repurposed drugs, such as ebselen, may be used in conjunction with clinically used and currently approved antifungals (52). Thus, we performed experiments to determine the activity of ebselen in combination with conventional antifungal agents. We tested for synergistic, indifferent, or antagonistic effects of the combinations of ebselen with fluconazole, amphotericin B, and caspofungin against C. auris and C. albicans under two different conditions, including the inhibition of planktonic growth and the inhibition of biofilm formation. As seen in Tables S3 and S4, we observed indifference in the overwhelming majority of paired combinations, with the only exception being synergy in the inhibition of C. albicans by the combination of ebselen with fluconazole. Overall, from these experiments, we interpret the lack of antagonism as a positive finding, as this may open up new opportunities for combination treatment.
Ebselen displays a broad spectrum of antifungal actions against pathogenic yeasts and molds.
We tested the activity of ebselen against a panel of medically important fungi in a preliminary assessment of the antifungal actions of this repositionable candidate. These assays were performed by the Fungus Testing Laboratory according to CLSI methodology. Regarding yeasts, the results confirmed its activity against Candida spp. and also demonstrated similar levels of activity against Cryptococcus neoformans (Table 3). One of the most pressing needs in the field of medical mycology is to develop agents with activity against difficult-to-treat mold infections. As also seen in Table 3, ebselen also demonstrated in vitro activity against Aspergillus fumigatus, Fusarium spp., Scedosporium apiospermum, Lomentospora prolificans, and Rhizopus oryzae, with MIC values generally comparable to or even lower than those observed for the current antifungal agents used in these assays for comparison purposes.
TABLE 3.
MIC values of ebselen and current antifungals against multiple clinical isolates belonging to different fungal species for determination of antifungal action
| Species | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| 50% |
100% |
|||||
| FLC | CSP | Ebselen | Ebselen | VOR | POS | |
| C. parapsilosisa | 0.5 | 0.5 | 4 | |||
| C. kruseia | 16 | 1 | 4 | |||
| Paecilomyces variotiia | 4 | 8 | 0.125 | |||
| C. albicans | <0.125 | 2 | 4 | |||
| <0.125 | 2 | 4 | ||||
| >64 | 2 | 2 | ||||
| C. glabrata | 1 | 1 | 4 | |||
| 0.25 | 0.5 | 1 | ||||
| 0.25 | 1 | 2 | ||||
| Cryptococcus neoformans | 8 | 4 | 4 | |||
| 32 | 2 | 2 | ||||
| 32 | 1 | 2 | ||||
| Rhizopus arrhizus | 8 | 16 | 0.5 | |||
| 8 | 16 | 0.25 | ||||
| Aspergillus fumigatus | 4 | 4 | 0.5 | |||
| 4 | 4 | 2 | ||||
| 4 | 4 | 0.25 | ||||
| Fusarium oxysporum | 4 | 4 | 4 | |||
| 4 | 4 | 4 | ||||
| Fusarium solani | 4 | 8 | >16 | |||
| Scedosporium apiospermum | 8 | 8 | 2 | |||
| 2 | 8 | 1 | ||||
| Lomentospora prolificans | 4 | >32 | >16 | |||
| 4 | >32 | >16 | ||||
| 2 | 8 | >16 | ||||
Quality control strains.
In summary, the emergence and rapid spread of C. auris as a nosocomial pathogen, frequently associated with multidrug resistance to different classes of antifungals, demands the rapid deployment of novel effective antifungal drugs against this formidable pathogen of increasing concern. Unlike the tortuous path of “de novo” drug discovery, estimated to take more than 15 years and cost more than $2 billion with high attrition rates, drug repurposing, or finding new uses for existing drugs, significantly reduces the time and cost associated with the development of novel drugs and can rapidly bring benefits to patients (26). Here, by screening a repurposing library, we identified the potent activity of ebselen against C. auris. Besides this emergent pathogen, ebselen also displayed antifungal activity against all other Candida spp. tested, expanding on its antifungal properties from previous reports (41, 53). Furthermore, ebselen displayed a broad spectrum of antifungal actions, including difficult-to-treat molds. Although further investigations are required, altogether, these data point to the promise of ebselen as a repositionable clinical agent for the treatment of C. auris infections as well as for the treatment of candidiasis and possibly other fungal infections in patients refractory to therapy with conventional antifungal agents. As with any repositionable candidate, a caveat is that nonantifungal off-target effects may result in a lack of specificity and lead to adverse effects that may limit the clinical utility of ebselen for the treatment of fungal infections.
MATERIALS AND METHODS
Chemical library.
The Prestwick Chemical library (Prestwick Chemical, France) contains approximately 1,280 chemically and pharmacologically diverse compounds, mostly off-patent approved drugs used for the treatment of a variety of diseases and approximately 5% of other drugs at different stages of development, all with known bioavailability and safety in humans. The compounds in the library are provided in 96-well microtiter plates as 10 mM solutions in dimethyl sulfoxide (DMSO). An initial 1:100 dilution for each compound was prepared by pipetting 2 μl of this concentrated solution into 198 μl of phosphate-buffered saline (PBS) using the wells of presterilized, polystyrene, round-bottomed 96-well microtiter plates (Corning Incorporated, Corning, NY) and was stored as working stock solutions at −20°C. For follow-up experiments, pharmaceutical grade ebselen was commercially purchased from AdipoGen (San Diego, CA, USA).
Strains and culture conditions.
The C. auris panel was obtained from the U.S. Centers for Disease Control and Prevention (30). From this panel, the multidrug-resistant C. auris 0390 clinical isolate was used for initial experiments, including primary screening. This isolate is resistant to azoles and amphotericin B and shows decreased susceptibility against echinocandins. Nine other C. auris isolates, one C. lusitaniae, and one C. krusei isolate from the CDC panel were used in follow-up experiments, as well as the C. albicans SC5314 type strain and clinical isolates representative of different Candida species, including C. dubliniensis, C. parapsilosis, C. tropicalis, and C. glabrata, which were obtained from the Fungus Testing Laboratory at the University of Texas Health Sciences Center at San Antonio.
Overnight cultures of the strains were grown by inoculating cells in 20 ml of yeast extract-peptone-dextrose (YPD) (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose) liquid medium in 150-ml flasks and incubating in an orbital shaker (150 to 180 rpm) at 30°C. After 18 h, the cells were washed with PBS and counted with a hemocytometer. The cells were then adjusted to the desired final density (typically 0.5 × 103 cells/ml for planktonic testing and 1 × 106 for biofilm testing) in RPMI medium supplemented with l-glutamine (Cellgro, Manassas, VA) and buffered with 165 mM morpholinepropanesulfonic acid ([MOPS] Thermo-Fisher Scientific, Waltham, MA) at pH 6.9.
Primary screening for inhibitors of C. auris.
The initial screening for compounds with antifungal activity against C. auris was performed according to the CLSI document M27-A3 for antifungal susceptibility testing of yeasts with minor modifications (54). Briefly, the inoculum of C. auris strain 0390 was prepared and added to wells in the 96-well microtiter plates, each containing an individual compound from the Prestwick library at a final concentration of 20 μM. The plates were then incubated at 35°C and the MICs were read visually (for >50% inhibition) at 24 and 48 h. At the end of the 48-h incubation period, the cells in the wells were homogenized and the absorbance determined spectrophotometrically with a microtiter plate reader to provide a more quantitative measure of inhibition. The first column of the plate contained only RPMI medium without drugs and the last column contained no cells, which served as negative and background controls, respectively. The screening was performed in duplicates. Compounds found to inhibit greater than 70% of planktonic growth (based on absorbance readings at 48 h) were initially selected as “hits.”
Dose-response assays for confirmation of initial hits.
The confirmation of the antifungal activity of drugs that were identified as hits from the initial (i.e., primary) screening was performed by dose-response assays using broth microdilution techniques according to CLSI methodology (54). The starting concentration of the hits was 40 μM, and serial 2-fold dilutions were performed across the rows of the 96-well microtiter plates to 0.078 μM. Positive and negative controls were also included, and experiments were performed in duplicates at each dose in two different plates. The plates were incubated for 48 h, after which the cells in the wells were homogenized and the absorbance determined with a microtiter plate reader. To prepare the dose-response curve, the spectrophotometric readings were converted into normalized responses, for which values from positive control (untreated) wells and negative control (uninoculated) wells were arbitrarily set as 100% and 0% growth, respectively. After this, the IC50s, defined as the concentration of drug required to reduce planktonic growth by half, were determined by fitting the normalized values to the variable slope Hill equation (an equation determining the nonlinear drug dose-response relationship) using Prism (GraphPad Software Inc., San Diego, CA).
Determination of the antifungal activity of ebselen against different Candida spp.
The in vitro antifungal activity of ebselen against C. auris strains in the CDC panel, C. albicans SC5314 type strain, and different clinical isolates representing different Candida spp. was determined by using the same assay, based on CLSI document M27-A3 for yeast susceptibility testing, described above for dose-response experiments, and using spectrophotometric readings. For these experiments, the range of ebselen concentrations tested was from 32 to 0.0625 μg/ml. IC50s were determined as described above.
Activity of ebselen against biofilms of C. auris isolates and other Candida spp.
We used the 96-well microtiter plate model of Candida biofilm formation previously developed by our group to test the activity of ebselen for the prevention of biofilm formation of C. auris and other species (50, 55). Briefly, to evaluate the effect of the drugs in preventing biofilm formation, 50 μl of ebselen diluted in RPMI medium to appropriate concentrations (ranging from 32 μg/ml to 0.0625 μg/ml) was added to 96-well plates containing 50 μl of 2 × 106/ml of yeast cells, and the plates were incubated at 37°C for an additional 24 h. The plates were then washed once with PBS to remove nonadherent cells. The extent of biofilm inhibition was estimated with a colorimetric assay on the basis of the reduction of XTT (Sigma, St. Louis, MO) by metabolically active cells as previously described by us. From these, the IC50s were determined as described above, but this time using the XTT colorimetric readings. For comparison purposes, the same assays were used in parallel to determine the activity of current antifungals (fluconazole, amphotericin B, and caspofungin) against C. auris biofilms. For these studies, a stock solution of fluconazole (Hospira, Lake Forest, IL) was prepared in sodium chloride for injection at 2 mg/ml and stored at 4°C. Amphotericin B was obtained in solution at 250 μg/ml (Gibco Life Technologies, Grand Island, NY) and stored at −20°C. Caspofungin (Merck & Co., Inc., Whitehouse Station, NJ) was obtained as a powder and stored at 4°C; a stock solution was prepared in PBS at 2 mg/ml the day it was added to the plates.
An alternative method was used to assess the biofilm biomass of the 10 C. auris isolates, in which the biofilms were stained with crystal violet as previously described (56). Briefly, after biofilm formation, the plates were washed once with PBS, and each well was treated with 100 μl of methanol for 20 min for fixation. The methanol was removed, and the plates were allowed to dry. Adherent biofilms were stained for 10 min with 150 μl of 3% (wt/vol) crystal violet. After crystal violet was removed and the plates allowed to dry, they were washed 3 times with 200 μl of distilled water. For microscopy, the stained samples were directly observed on the 96-well plate by using a 40× objective on an inverted microscope system (Westover Scientific, Mill Creek, WA) equipped for photography. The images were processed for display using Micron software (Westover Scientific). To measure biomass, 100 μl of 33% glacial acetic acid was added to each well to dissolve the dye after microscopy. Glacial acetic acid was left in the wells for 5 min while the plates were slowly shaken. The solution was then transferred to a new microtiter plate for optical density at 550 nm (OD550) measurements to calculate the extent of biofilm inhibition relative to untreated controls.
Drug combination studies with ebselen and clinically used antifungals.
We assessed the efficacy of combinations of ebselen together with fluconazole, caspofungin, and amphotericin B by checkerboard assays. Briefly, 2-fold serial dilutions of the clinically used antifungal were placed from rows A to G of a 96-well microtiter plate, whereas 2-fold serial dilutions of ebselen were placed from columns 1 to 9 of the same plate. In this scheme, column 10 contains the antifungal by itself, while row H contains ebselen alone. Appropriate positive (no drug) and negative (no organism) controls were in columns 11 and 12. Combination studies were performed for both C. auris and C. albicans under planktonic conditions and for the inhibition of biofilm formation (using the methods described above). To assess whether each combination of drugs resulted in synergistic, indifferent, or antagonistic effects, the fractional inhibitory concentration index (FICI) was used, defined as MICAB/MICA + MICBA/MICB. This calculation takes the MIC of each drug when mixed with the other and divides it by the MIC of the drug by itself. FICI values of ≤0.5 indicate synergy, indifference is defined as >0.5 to ≤4.0, and antagonism is defined as >4.0 (57).
Determination of the spectrum of antifungal action of ebselen.
Antifungal susceptibility testing against a variety of medically important fungi was performed by the Fungus Testing Laboratory in accordance with the CLSI M27-A3 and M38-A2 reference standards for antifungal susceptibility testing for yeast and molds, respectively (54, 58). All clinical fungal isolates tested form part of the collection available in the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention (CDC), Clinical and Environmental Microbiology Branch for providing the Candida auris panel (recipient J.L.L.-R.).
This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health (grant UL1 TR001120). Additional support was provided by the Margaret Batts Tobin Foundation, San Antonio, TX, and by a Clusters in Research Excellence grant from the San Antonio Life Science Institute (SALSI). G.W. is supported by the UTSA RISE-PhD trainee program (NIH/NIGMS RISE GM60655).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, and the content is solely the responsibility of the authors.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01084-18.
REFERENCES
- 1.Banerjee SN, Emori TG, Culver DH, Gaynes RP, Jarvis WR, Horan T, Edwards JR, Tolson J, Henderson T, Martone WJ. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med 91:86S–89S. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Sagué C, Jarvis WR. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J Infect Dis 167:1247–1251. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 5.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Siyabi T, Al Busaidi I, Balkhair A, Al-Muharrmi Z, Al-Salti M, Al'Adawi B. 2017. First report of Candida auris in Oman: clinical and microbiological description of five candidemia cases. J Infect 75:373–376. doi: 10.1016/j.jinf.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, MSD, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus - United States, May 2013-August 2016. MMWR Morb Mortal Wkly Rep 65:1234–1237. doi: 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- 10.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang HC. 2011. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peidrahita CT, Cadnum JL, Jencson AL, Shaikh AA, Ghannoum MA, Donskey CJ. 2017. Environmental surfaces in healthcare facilities are a potential source for transmission of Candida auris and other Candida species. Infect Control Hosp Epidemiol 38:1107–1109. doi: 10.1017/ice.2017.127. [DOI] [PubMed] [Google Scholar]
- 12.Abdolrasouli A, Armstrong-James D, Ryan L, Schelenz S. 2017. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses 60:758–763. doi: 10.1111/myc.12699. [DOI] [PubMed] [Google Scholar]
- 13.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. 2016. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Candida auris. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/fungal/diseases/candidiasis/candida-auris.html. Accessed 6 April 2018. [Google Scholar]
- 15.Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. 2016. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect 73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Tsay S, Kallen A, Jackson BR, Chiller TM, Vallabhaneni S. 2018. Approach to the investigation and management of patients with Candida auris, an emerging multidrug-resistant yeast. Clin Infect Dis 66:306–311. doi: 10.1093/cid/cix744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, Tarai B, Singh A, Upadhyaya G, Upadhyay S, Yadav P, Singh PK, Khillan V, Sachdeva N, Perlin DS, Meis JF. 2018. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother 73:891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 18.Sharma C, Kumar N, Pandey R, Meis JF, Chowdhary A. 2016. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect 13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R. 2017. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis 23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee S, Alampalli SV, Nageshan RK, Chettiar ST, Joshi S, Tatu US. 2015. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics 16:686. doi: 10.1186/s12864-015-1863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarma S, Upadhyay S. 2017. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect Drug Resist 10:155–165. doi: 10.2147/IDR.S116229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butts A, Krysan DJ. 2012. Antifungal drug discovery: something old and something new. PLoS Pathog 8:e1002870. doi: 10.1371/journal.ppat.1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clancy CJ, Nguyen MH. 2017. Emergence of Candida auris: an international call to arms. Clin Infect Dis 64:141–143. doi: 10.1093/cid/ciw696. [DOI] [PubMed] [Google Scholar]
- 24.Sears D, Schwartz BS. 2017. Candida auris: an emerging multidrug-resistant pathogen. Int J Infect Dis 63:95–98. doi: 10.1016/j.ijid.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Nosengo N. 2016. New tricks for old drugs. Nature 534:314–316. doi: 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- 26.Ashburn TT, Thor KB. 2004. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 27.Siles SA, Srinivasan A, Pierce CG, Lopez-Ribot JL, Ramasubramanian AK. 2013. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 57:3681–3687. doi: 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh N, Sharpley AL, Emir UE, Masaki C, Herzallah MM, Gluck MA, Sharp T, Harmer CJ, Vasudevan SR, Cowen PJ, Churchill GC. 2016. Effect of the putative lithium mimetic ebselen on brain myo-inositol, sleep, and emotional processing in humans. Neuropsychopharmacology 41:1768–1778. doi: 10.1038/npp.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi N. 2016. Ebselen, a useful tool for understanding cellular redox biology and a promising drug candidate for use in human diseases. Arch Biochem Biophys 595:109–112. doi: 10.1016/j.abb.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Antibiotic resistance (AR) isolate bank. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/resistance-bank/ Accessed 27 March 2018. [Google Scholar]
- 31.Kean R, Sherry L, Townsend E, McKloud E, Short B, Akinbobola A, Mackay WG, Williams C, Jones BL, Ramage G. 2018. Surface disinfection challenges for Candida auris: an in vitro study. J Hosp Infect 98:433–436. doi: 10.1016/j.jhin.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, Litvintseva AP. 2017. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol 55:2996–3005. doi: 10.1128/JCM.00921-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta AK, Skinner AR. 2003. Ciclopirox for the treatment of superficial fungal infections: a review. Int J Dermatol 42(Suppl 1):S3–S9. doi: 10.1046/j.1365-4362.42.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 34.Masumoto H, Hashimoto K, Hakusui H, Takaichi M, Yokota T, Honda T, Esumi Y. 1997. Studies on the pharmacokinetics of ebselen in rats (1): absorption, distribution, metabolism and excretion after single oral administration. Drug Metab Pharmacokinet 12:596–609. doi: 10.2133/dmpk.12.596. [DOI] [Google Scholar]
- 35.Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. 2001. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke 32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 36.Martini F, Pesarico AP, Bruning CA, Zeni G, Nogueira CW. 2018. Ebselen inhibits the activity of acetylcholinesterase globular isoform G4 in vitro and attenuates scopolamine-induced amnesia in mice. J Cell Biochem 119:5598–5608. doi: 10.1002/jcb.26731. [DOI] [PubMed] [Google Scholar]
- 37.Jin Y, Li D, Lu S, Zhao H, Chen Z, Hou W, Ruan BH. 2018. Ebselen reversibly inhibits human glutamate dehydrogenase at the catalytic site. Assay Drug Dev Technol 16:115–122. doi: 10.1089/adt.2017.822. [DOI] [PubMed] [Google Scholar]
- 38.Thabet NM, Moustafa EM. 2017. Synergistic effect of ebselen and gamma radiation on breast cancer cells. Int J Radiat Biol 93:784–792. doi: 10.1080/09553002.2017.1325024. [DOI] [PubMed] [Google Scholar]
- 39.Santofimia-Castaño P, Izquierdo-Alvarez A, de la Casa-Resino I, Martinez-Ruiz A, Perez-Lopez M, Portilla JC, Salido GM, Gonzalez A. 2016. Ebselen alters cellular oxidative status and induces endoplasmic reticulum stress in rat hippocampal astrocytes. Toxicology 357-358:74–84. [DOI] [PubMed] [Google Scholar]
- 40.Baek JM, Kim JY, Yoon KH, Oh J, Lee MS. 2016. Ebselen is a potential anti-osteoporosis agent by suppressing receptor activator of nuclear factor kappa-B ligand-induced osteoclast differentiation in vitro and lipopolysaccharide-induced inflammatory bone destruction in vivo. Int J Biol Sci 12:478–488. doi: 10.7150/ijbs.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thangamani S, Eldesouky HE, Mohammad H, Pascuzzi PE, Avramova L, Hazbun TR, Seleem MN. 2017. Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells. Biochim Biophys Acta 1861:3002–3010. doi: 10.1016/j.bbagen.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schewe T. 1995. Molecular actions of ebselen–an antiinflammatory antioxidant. Gen Pharmacol 26:1153–1169. doi: 10.1016/0306-3623(95)00003-J. [DOI] [PubMed] [Google Scholar]
- 43.Parnham MJ, Kindt S. 1984. A novel biologically active seleno-organic compound–III. Effects of PZ 51 (ebselen) on glutathione peroxidase and secretory activities of mouse macrophages. Biochem Pharmacol 33:3247–3250. [DOI] [PubMed] [Google Scholar]
- 44.Kil J, Lobarinas E, Spankovich C, Griffiths SK, Antonelli PJ, Lynch ED, Le Prell CG. 2017. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 390:969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson TN, Osman H, Werngren J, Hoffner S, Engman L, Holmgren A. 2016. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species, Staphylococcus aureus and Mycobacterium tuberculosis. Biochim Biophys Acta 1860:1265–1271. doi: 10.1016/j.bbagen.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Thangamani S, Younis W, Seleem MN. 2015. Repurposing clinical molecule ebselen to combat drug resistant pathogens. PLoS One 10:e0133877. doi: 10.1371/journal.pone.0133877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thangamani S, Younis W, Seleem MN. 2015. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep 5:11596. doi: 10.1038/srep11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azad GK, Singh V, Mandal P, Singh P, Golla U, Baranwal S, Chauhan S, Tomar RS. 2014. Ebselen induces reactive oxygen species (ROS)-mediated cytotoxicity in Saccharomyces cerevisiae with inhibition of glutamate dehydrogenase being a target. FEBS Open Bio 4:77–89. doi: 10.1016/j.fob.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierce CG, Srinivasan A, Uppuluri P, Ramasubramanian AK, Lopez-Ribot JL. 2013. Antifungal therapy with an emphasis on biofilms. Curr Opin Pharmacol 13:726–730. doi: 10.1016/j.coph.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, Long L, Isham N, Kovanda L, Borroto-Esoda K, Wring S, Angulo D, Ghannoum M. 2017. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 61:e02396-16. doi: 10.1128/AAC.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng W, Sun W, Simeonov A. 2018. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br J Pharmacol 175:181–191. doi: 10.1111/bph.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chassot F, Pozzebon Venturini T, Baldissera Piasentin F, Morais Santurio J, Estivalet Svidzinski TI, Hartz Alves S. 2016. Antifungal activities of diphenyl diselenide and ebselen against echinocandin-susceptible and -resistant strains of Candida parapsilosis. New Microbiol 39:301–303. [PubMed] [Google Scholar]
- 54.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard–3rd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 55.Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Toole GA. 2011. Microtiter dish biofilm formation assay. J Vis Exp 2011:2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bassolé IH, Juliani HR. 2012. Essential oils in combination and their antimicrobial properties. Molecules 17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard– 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.