Abstract
Botulinum toxin type A (BoNT) is considered an effective therapeutic option in cervical dystonia (CD). The pathophysiology of CD and other focal dystonias has not yet been fully explained. Results from neurophysiological and imaging studies suggest a significant involvement of the basal ganglia and thalamus, and functional abnormalities in premotor and primary sensorimotor cortical areas are considered a crucial factor in the development of focal dystonias. Twelve BoNT-naïve patients with CD were examined with functional MRI during a skilled hand motor task; the examination was repeated 4 weeks after the first BoNT injection to the dystonic neck muscles. Twelve age- and gender-matched healthy controls were examined using the same functional MRI paradigm without BoNT injection. In BoNT-naïve patients with CD, BoNT treatment was associated with a significant increase of activation in finger movement-induced fMRI activation of several brain areas, especially in the bilateral primary and secondary somatosensory cortex, bilateral superior and inferior parietal lobule, bilateral SMA and premotor cortex, predominantly contralateral primary motor cortex, bilateral anterior cingulate cortex, ipsilateral thalamus, insula, putamen, and in the central part of cerebellum, close to the vermis. The results of the study support observations that the BoNT effect may have a correlate in the central nervous system level, and this effect may not be limited to cortical and subcortical representations of the treated muscles. The results show that abnormalities in sensorimotor activation extend beyond circuits controlling the affected body parts in CD even the first BoNT injection is associated with changes in sensorimotor activation. The differences in activation between patients with CD after treatment and healthy controls at baseline were no longer present.
Keywords: Functional MRI, Cervical dystonia, Botulinum toxin, Brain plasticity
Background
Cervical dystonia (CD) is the most common form of focal dystonia, characterized by involuntary sustained contractions of neck muscles resulting in an abnormal rotation or tilt of the head in specific directions (Stacy 2000). The pathophysiology of CD and other focal dystonias has not yet been fully elucidated. Results from neurophysiological and imaging studies suggest a significant contribution of the basal ganglia and thalamus in the development of focal dystonias (Peterson et al. 2010). Recently, it has become clear that the role of the basal ganglia extends beyond motor control into cognitive and sensory functions as well as into sensorimotor integration (Tinazzi et al. 2009a, b). In the last few years, an increasing number of studies have also presented the cerebellum as another important subcortical brain structure in patients with dystonia (Filip et al. 2013a, b, 2017). Finally, other functional imaging and electrophysiological experiments suggest functional abnormalities in the premotor and primary sensorimotor cortical areas together with aberrant sensorimotor integration, which is considered to be a crucial factor in the development of focal dystonia (Tinazzi et al. 2009a, b; Hinkley et al. 2009; Opavský et al. 2011, 2012). However, the published studies differ in terms of observed hypo- and hyperactivation in these cortical areas. Differences among task conditions, including testing of dystonia-affected and unaffected body parts, can partly explain this variance.
Further important insights into the pathophysiology of focal dystonias have come from studies investigating the effects of botulinum toxin (BoNT) treatment. BoNT is currently considered to be one of the most effective therapeutic options in the management of focal dystonias (Jankovic 2004). Undoubtedly, the introduction of the first-generation BoNT products not only led to a breakthrough in dystonia treatment but also to advances in dystonia research. We now know that the dystonic hyperactive and cholinergically sensitive extrafusal fibers as well as the intrafusal muscle fibers are the prime targets of BoNT therapy (Rosales and Dressler 2010). It is the effect of BoNT in muscle spindles that would eventually modify proprioceptive spindle afferents, as these are partly dependent on the intrafusal muscle fiber tensions. A modification of the central programs with BoNT may eventually occur at the spinal and supraspinal levels (Rosales and Dressler 2010). The clinical effect of BoNT on dystonia is, therefore, assumed to be mediated by dynamic changes at multiple levels of the sensorimotor system, from the neuromuscular junction up to the cerebral cortex, as documented by the previous behavioral and electrophysiological studies (Kaňovský et al. 1998; Abbruzzese and Berardelli 2006). The previous functional magnetic resonance imaging (fMRI) studies from our center showed significant treatment-related changes in the sensorimotor network in patients receiving long-term treatment with BoNT type A (Opavský et al. 2011, 2012).
Nevertheless, specialists in movement disorders clinics soon realized that dystonia may behave differently over the course of BoNT treatment. The first reports described the changes of the muscular pattern (Gelb et al. 1991; Deuschl et al. 1992; Marin et al. 1992, 1995; Kaňovský et al. 1997) that may have also implied a central mechanism of dystonia.
We assume that the changing clinical behavior and evolving clinical response to BoNT treatment will also be reflected in changes of task-related functional MRI activation after therapy. The aim of the presented work is to study changes in the sensorimotor network in patients after the very first BoNT injection, using the same task as in our previous work (Opavský et al. 2011).
Subject and methods
Patients enrolled in the project underwent a comprehensive neurological examination by a movement disorders specialist. All subjects had typical clinical symptoms for at least 12 months and underwent polyelectromyographic examination of neck muscles. To be eligible for the study each patient had to have magnetic resonance (MR) imaging of the brain with no structure abnormality. Each patient was informed in detail about the goal and the course of investigation, and signed an informed consent form. The study protocol was approved by the local ethics committee, in accordance with the principles and recommendations of the Declaration of Helsinki, 1975 and later revisions.
Twelve BoNT-naïve patients (1 male and 11 females; aged 48.8 ± 11.7 years, range 31–70 years) with CD were examined with fMRI during a skilled hand movement with their eyes closed. The examination was repeated 4 weeks after the first BoNT injection to the dystonic neck muscles. Twelve age- and gender-matched healthy controls (2 males and 10 females; aged 49.7 ± 13.9 years, range 25–64 years) were examined using the same functional MRI paradigm without BoNT injection.
The severity of CD was evaluated using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) (Consky and Lang 1994) at two sessions: at week 0, on the day of screening, of the first fMRI examination before the BoNT injection, and at week 4, on the day of the second fMRI examination. In all patients, the injected muscles were determined on the basis of a polyelectromyographic examination, provided by 4-channel Keypoint workstation, Medtronic®, Minneapolis, MN, USA. The details of the electromyographic examination and BoNT injection were described in our previous work (Kaňovský et al. 1998). All patients were treated with onabotulinum toxin type A (Botox®; Allergan, Inc, Irvine, CA, USA) in concentrations of 25 IU/ml. The demographic and clinical data of the patients are presented in Table 1.
Table 1.
Demographic data of the patients (both CD and control group) and results of TWSTRS before and after BoNT-A injection
| Control group | Study group | |||||
|---|---|---|---|---|---|---|
| Sex | Age (years) | Sex | Age (years) | Total BoNT-A dose (Botox U) | TWSTRS at week 0 | TWSTRS at week 4 |
| F | 52 | F | 45 | 200 | 19 | 10 |
| M | 59 | F | 45 | 200 | 15 | 8 |
| F | 34 | M | 60 | 150 | 10 | 4 |
| F | 25 | F | 42 | 150 | 24 | 19 |
| F | 26 | F | 56 | 150 | 18 | 9 |
| F | 55 | F | 40 | 200 | 17 | 7 |
| F | 64 | F | 64 | 100 | 16 | 6 |
| F | 61 | F | 33 | 200 | 19 | 12 |
| F | 62 | F | 55 | 100 | 13 | 7 |
| M | 57 | F | 44 | 200 | 15 | 8 |
| F | 46 | F | 70 | 100 | 13 | 7 |
| F | 55 | F | 31 | 200 | 12 | 7 |
| Mean | 49.7 | Mean | 48.8 | 162.5 | 15.9 | 8.7 |
Prior to the imaging session, participants were trained in the laboratory in the active task to be performed in the scanner. The task was a complex sequential opposition of individual fingers to thumb with the following order of movements: index finger 1×, ring finger 2×, middle finger 3×, and little finger 4×. During fMRI scanning, patients had their eyes closed, and instructions to start and stop movement were given verbally in MR-compatible headphones. In a block paradigm, movement (7.5 s) was alternated with rest (7.5 s). Each experimental run consisted of 16 movement-rest block pairs, for a total of 4 min. Experimental conditions were repeated twice with the same hand. Performance was visually monitored, recording the number of finger sequences completed per block.
MR imaging data were acquired on 1.5 T scanners (Avanto and Symphony, Siemens, Erlangen, Germany) with a standard head coil. The MR imaging protocol covered the whole brain and included anatomical T1-weighted images to provide an immediate overlay with functional data, fluid-attenuated inversion recovery (FLAIR) images to visualize brain lesions, functional T2*-weighted (BOLD) images during task performance and rest, and a high-resolution 3D anatomical scan (MPRAGE). BOLD images were acquired using gradient-echo echo-planar imaging (EPI) sequence, with repetition/echo time (TR/TE) = 2500/40 ms, field of view (FOV) 220 mm, and 30 axial slices, to provide 3.4 × 3.4 × 5 mm resolution. A total of 96 images were acquired per each 4-min functional run. The subject’s head was immobilized with cushions to assure maximum comfort and minimize head motion.
Prior to fMRI analysis, the imaging data of patients with the left-sided dystonia leading muscle were flipped in the left–right direction (Johansen-Berg et al. 2002). FMRI data processing was carried out using the FSL version 5.0 (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl) (Smith et al. 2004; Jenkinson et al. 2012). The following pre-statistics processing was applied: motion correction; slice-timing correction using Fourier-space time-series phase-shifting; non-brain removal; spatial smoothing using a Gaussian kernel of full-width at half-maximum (FWHM) 8 mm; grand-mean intensity normalization; high-pass temporal filtering with sigma 7.5 s; and spatial normalization/registration to the standard-space MNI template. Time-series statistical analysis was carried out using a generalized linear model, implemented in FMRIB’s improved linear model (FILM) with local autocorrelation correction. Group analysis was performed using FMRIB’s Local Analysis of Mixed Effects (FLAME) stages 1 and stage 2 with automatic outlier detection. Statistical maps were thresholded using clusters corrected P = 0.05. The voxelwise Z (Gaussianized T) threshold was adjusted to reflect the expected effect size. We evaluated (1) mean activation thresholded at Z > 3.5; (2) within-group differences in patients at Z > 2; and (3) between-group differences at each timepoint and within-group changes over time at Z > 1.7. The differences were evaluated within the respective significant clusters of mean activation.
Results
All patients were injected into the muscles identified by polyelectromyography. The dose for each cervical muscle was 50 IU, and the mean total dose for a patient was 162.5 ± 43.3 IU. The significant clinical effect of BoNT injections was evaluated using the TWSTRS at week 4. The mean value of TWSTRS at week 0 was 15.9 ± 3.8, and at week 4, it was 8.7 ± 3.8 (P = 0.00002, one-sided paired t test). Details are provided in Table 1. All patients and controls were right-handed, and conventional brain MRI was completely normal in all subjects. For the hand motor task, patients used the hand ipsilateral to the dystonic leading muscle. In 7/12 patients, it was the dominant hand, and in the remaining 5/12 cases, the non-dominant one. Subjects in the control group used hand randomly. The proportion of dominant vs. non-dominant hands was the same in both groups.
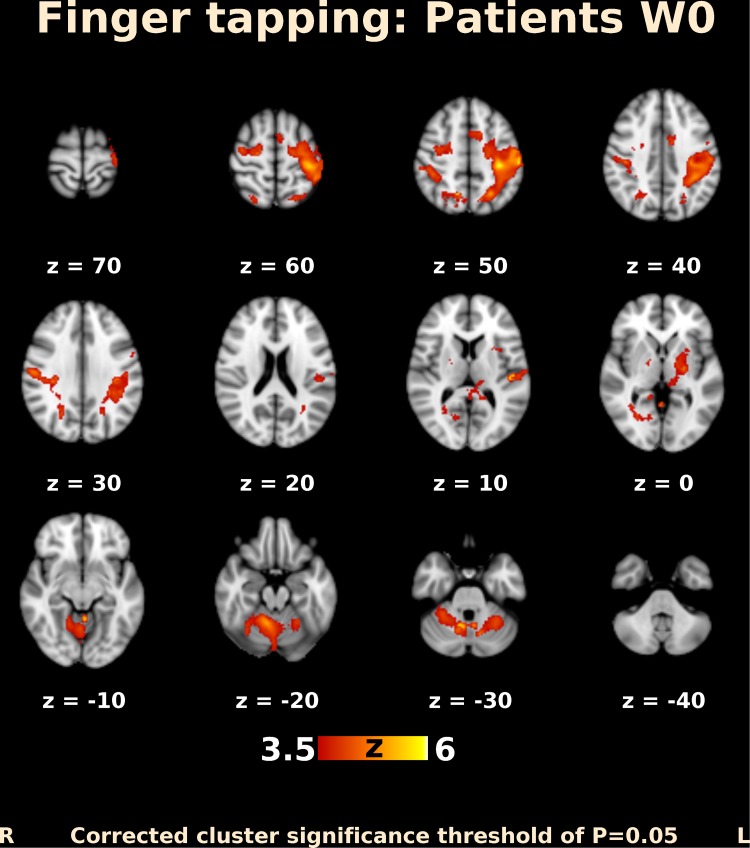
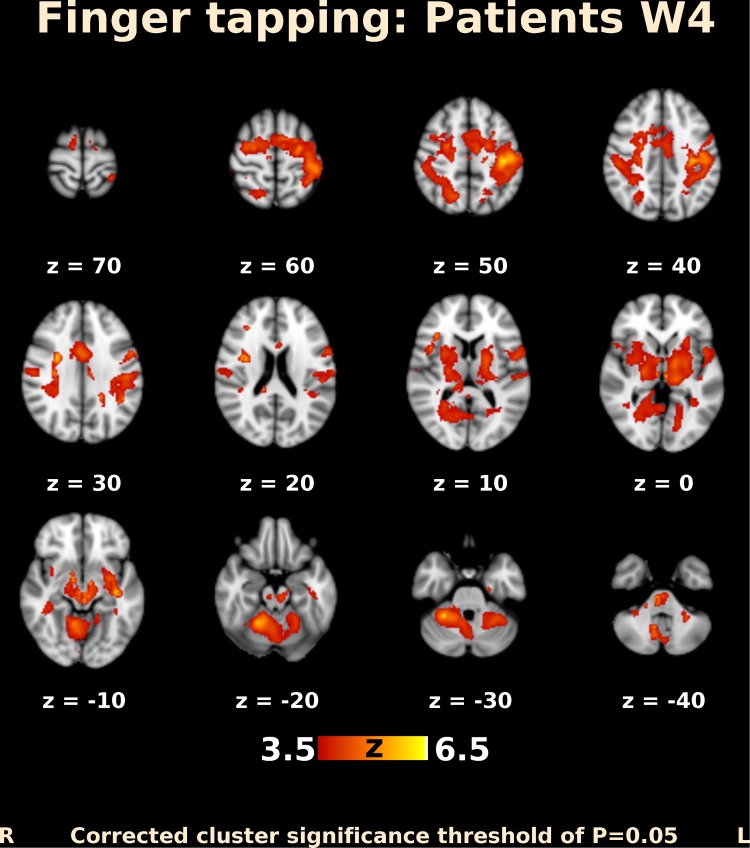
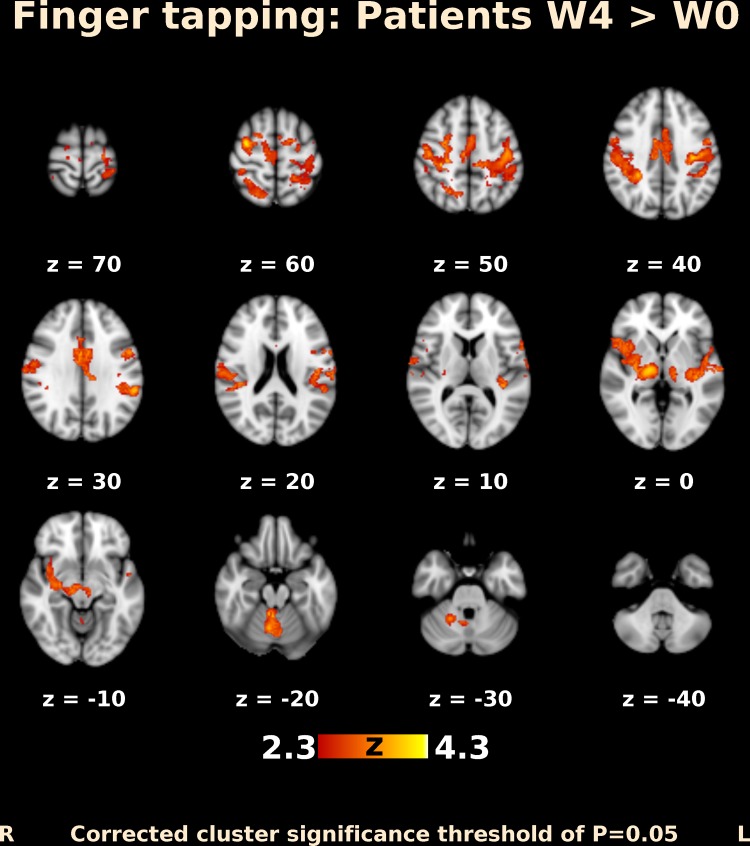
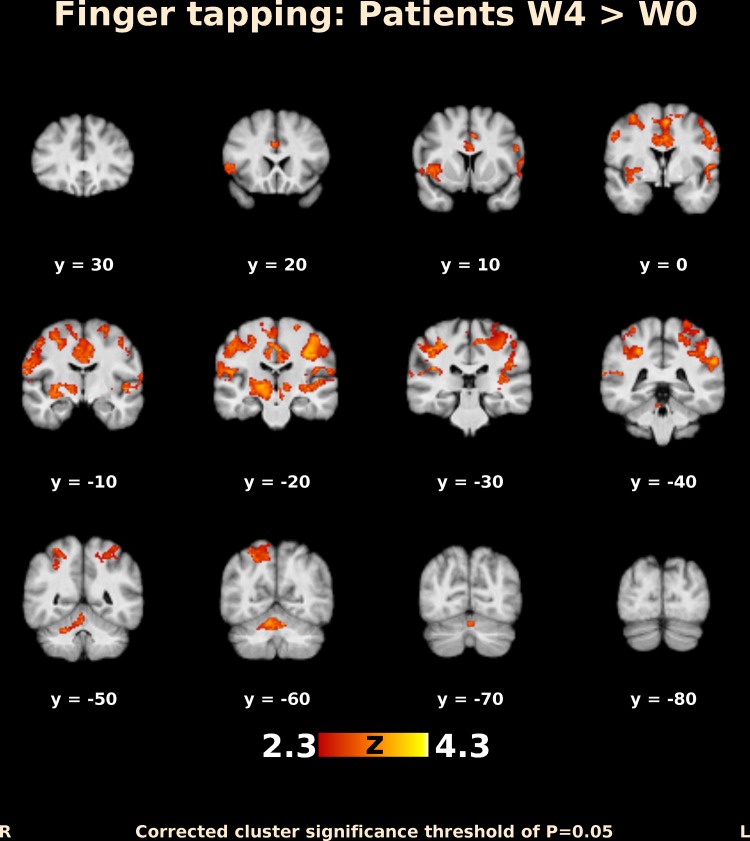
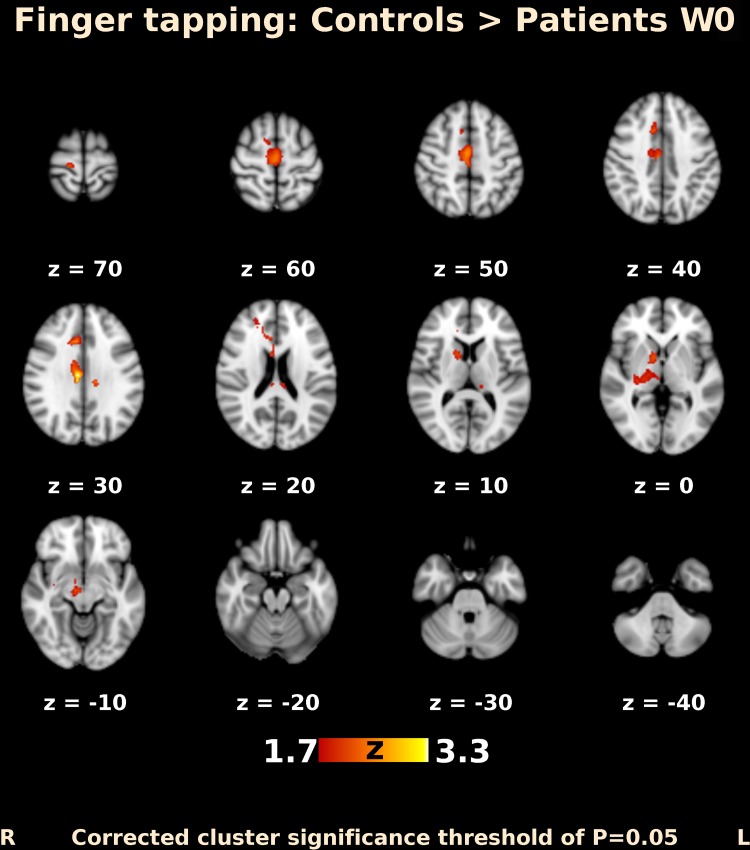
Before BoNT injection, patients performing finger movements activated multiple brain areas, predominantly within the sensorimotor system, including the contralateral primary motor and somatosensory cortex, contralateral secondary somatosensory cortex, bilateral premotor cortex, contralateral supplementary motor area (SMA), bilateral superior and inferior parietal lobule, bilateral cerebellum, contralateral thalamus, bilateral pallidum, and putamen (Fig. 1). The activation map at week 4 after BoNT injection showed a more extended but similar distributed brain network (Fig. 2). A direct comparison of both timepoints (paired contrast) revealed that the activation increased after treatment in most of the brain areas activated before BoNT injection, especially in the bilateral primary and secondary somatosensory cortex, bilateral superior and inferior parietal lobule, bilateral SMA and premotor cortex, predominantly contralateral primary motor cortex, bilateral anterior cingulate cortex, as well as in the predominantly ipsilateral thalamus, insula, and putamen. A significant increase in activation was also apparent in the central part of cerebellum, close to the vermis (Figs. 3, 4); however, there was no significant activation decrease after BoNT injection. When compared to the control group, patients before treatment showed significantly lower activation mainly in the bilateral SMA, ipsilateral cingulate and paracingulate cortex, as well as in the ipsilateral caudate, pallidum, and thalamus (Fig. 5), whereas there was no significant difference between the control group and the patient group after BoNT injection. No significant movement artifacts (maximal framewise movement head displacement was 2.28 mm in one subject; in the rest, it was smaller than 2 mm) were found in any of the MRI images.
Fig. 1.
Functional MRI activation map in patients with CD before BoNT-A injection. Slices are labeled with Z/Y coordinate in standard MNI152 space
Fig. 2.
Functional MRI activation map in patients with CD 4 weeks after BoNT-A injection. Slices are labeled with Z/Y coordinate in standard MNI152 space
Fig. 3.
Functional MRI activation map (transversal slices) in patients with CD. Differences in activation after and before BoNT-A injection. Slices are labeled with Z/Y coordinate in standard MNI152 space
Fig. 4.
Functional MRI activation map (coronar slices) in patients with CD. Differences in activation after and before BoNT-A injection. Slices are labeled with Z/Y coordinate in standard MNI152 space
Fig. 5.
Functional MRI activation map (transversal slices). Differences in activation between CD patients group before BoNT-A injection and control group. Slices are labeled with Z/Y coordinate in standard MNI152 space
The possible influence of faster movements was correlated with the expected task-related hemodynamic response function, which could potentially negatively affect presented results. Evaluating the amount and influencing of task-correlated motion were extracted six original motion parameters (three rotations and three translations) and two derived motion parameters (the absolute voxel displacement from a reference volume and the relative voxel displacement between two consecutive volumes), which were estimated in each subject during the preprocessing. Next, Spearman correlation coefficient was used to correlate each motion vector with the task vector convolved with the hemodynamic response function. The absolute values of the correlation coefficients were compared between the sessions using pairwise sign rank Wilcoxon test. As a result, none of the tested coefficients differed significantly between the sessions (p = 0.2 or greater). The overall correlation coefficients were rather low (ranging from ρ = 0.08 to ρ = 0.19). Thus, we consider any potential effect of task-correlated motion to be negligible.
Discussion
In this work, we have studied changes in fMRI activation after the first BoNT injection. We consider this trait of the study population as one of the significant contributions of our study, since most of the previous papers reported either changes in long-term-treated patients with CD (Carbon et al. 2008; Obermann et al. 2008, 2010; Opavský et al. 2011, 2012; Burciu et al. 2017) or focused on differences between treated patients and controls, rather than on effects of therapy (de Vries et al. 2008). In BoNT-naïve patients with CD, BoNT treatment was associated with a significant increase of activation in finger movement-induced fMRI activation of several brain areas, especially in the bilateral primary and secondary somatosensory cortex, bilateral superior and inferior parietal lobule, bilateral SMA and premotor cortex, predominantly contralateral primary motor cortex, bilateral anterior cingulate cortex, ipsilateral thalamus, insula, and putamen, and the central part of cerebellum, close to the vermis. These results support the previous observations that the BoNT effect has a correlate at the central nervous system level (e.g., Kaňovský et al. 1998; Šenkárová et al. 2010; Palomar and Mir 2012). The abnormal cortical activation detected during skilled motor tasks performed with a non-dystonic body part also confirms the previous electrophysiological and functional imaging observations that sensorimotor abnormalities in the dystonic brain extend beyond the directly clinically affected sensorimotor representations (Kaňovský et al. 2003; Thickbroom et al. 2003; Opavský et al. 2011, 2012).
In cervical dystonia, earlier fMRI studies by Opavský et al. (2011, 2012) showed significant changes in the sensorimotor network in patients receiving long-term treatment with BoNT. The results of the present study in patients after the first BoNT injection show certain similarities, especially with respect to the localization of the activation changes. However, in contrast to the decrease of activation in long-term-treated patients reported by Opavský et al. (2011, 2012), the present results demonstrate an increase of activation in patients with CD after the very first BoNT injection. Although the limitation of a small patient cohort has to be acknowledged, the opposite direction of activation changes occurring in almost the same brain areas in a single type of focal dystonia in response to either initial or long-term BoNT therapy could provide evidence for long-term brain plasticity and more complex changes induced by BoNT, which involve not only the neuromuscular junction, but also the central nervous system.
The significant treatment-induced changes in our study were detected in areas involved in sensorimotor control and motor learning. In the following section, we will review the function of these regions and discuss their role in the pathophysiology of CD and in the response to BoNT treatment.
The SMA, which was hypoactivated in CD and showed activation increase after BoNT treatment, is considered to be involved in many processes such as posture regulation, internal generation of movement, bimanual coordination, and movement sequencing (Tanji 1996; Chouinard and Paus 2010). In primates, dystonia models demonstrated SMA hyperexcitability, an abnormal increase of proprioceptive inputs to the SMA, and wider sensory receptive fields and a mismatch between sensory inputs and motor outputs (Cuny et al. 2008). These observations may suggest that abnormal sensory inputs coming to SMA neurons participate in the development of dystonia. Hyperexcitability may then decrease the demand for recruitment of SMA neurons to control voluntary movement, which would manifest as reduced task-related activation in functional MRI. BoNT treatment supposedly reduces the abnormal afferent input, thereby reducing the baseline hyperexcitability of the SMA. After treatment, voluntary movement may, therefore, require increased engagement of SMA neurons. Such adaptive increase in activation of the medial premotor cortex has been demonstrated in response to many pathological processes, such as stroke, injury, etc. (e.g., Kantak et al. 2012).
The cingulate cortex is another structure that showed significant hypoactivation in patients with CD and treatment-related activation increase. It is a structurally heterogeneous brain region involved in emotional, cognitive, and motor tasks (Torta and Cauda 2011). The dorsal cingulate sulcus has several motor regions that are active during movement. The cingulate cortex has rich anatomical connections with SMA and both structures are implicated in integration of emotional and motor processing (Oliveri et al. 2003). Therefore, the presented changes in the cingulate cortex probably reflect a similar mechanism as the changes in SMA.
Further intriguing treatment-related activation changes were detected in the central and pericentral parts of the cerebellum. Although the cerebellum is not traditionally noted among the major substrates for development of dystonia, interest in this neuronal structure has increased recently (Avanzino and Abbruzzese 2017,2012; Sadnicka et al. 2012; Filip et al. 2013a, b) as its role in the pathophysiology of dystonia has been suggested by animal models (Jinnah et al. 2005; Raike et al. 2005; Vidailhet et al. 2009), imaging studies (Carbon et al. 2008; Obermann et al. 2010; Opavský et al. 2011, 2012; Prudente et al. 2016; Burciu et al. 2017), neurophysiological studies (Liepert et al. 2004), and even analyses of secondary CD (LeDoux and Brand 2003; Extremera et al. 2008). Neurological disorders originating from the cerebellum (e.g., ataxia) are usually associated with a loss of function. However, different syndromes can arise from the same pathway as different defects alter the output in different ways. In dystonia, it is still disputable whether the cerebellum is the source of the disease or just a node in a complex network trying to compensate for dysfunction of other parts of the brain.
The activation increases post-treatment and was also observed in the secondary somatosensory cortex, which is located in the parietal operculum. It is implicated in higher order functions in somatosensory processing, but it is also believed to integrate information from the two sides of the body, and to participate in visuospatial attention, learning, and memory. According to the previous electrophysiological and imaging evidence, CD seems to be associated with disorders of not only motor but also sensory cortical processing, perhaps at the level of sensorimotor integration (Siggelkow et al. 2002; Abbruzzese et al. 2001; Frasson et al. 2001; Rosales and Dressler 2010). Abbruzzese and Berardelli (2003) consider the aberrant sensorimotor processing to be a key factor for the development of focal dystonias. In a broader sense, the sensorimotor integration involves all parts of the motor and sensory system, including the motor circuits, in which the basal ganglia and the premotor and motor cortex are the principal components.
Finally, the hypoactivations and treatment-related activation increases were also detected in the ipsilateral striatum, pallidum, and thalamus. The involvement of subcortical structures is not unexpected as some previous studies in CD-reported abnormal bilateral activation of the basal ganglia and thalamus during non-dystonia-associated tasks (Obermann et al. 2008; Opavský et al. 2011). Moreover, the internal pallidum serves as a target for effective modulation of CD and other forms of primary dystonias using deep brain stimulation, with an imprecisely characterized mode of action. Whereas higher field MR scanners have certainly provided better spatial and temporal resolution, our spatial resolution of 3.5 × 3.5 × 5 mm appears sufficient to reliably detect basal ganglia activation in normal subjects and neurological patients (Obermann et al. 2008; Hok et al. 2017; Marchal-Crespo et al. 2017).
With respect to overall pattern and direction of activation changes, the previous studies in long-term-treated patients with CD reported that CD was associated with hyperactivations before the BoNT injection (Obermann et al. 2008; Opavský et al. 2011), whereas our study in untreated patients demonstrates widespread hypoactivations. Similar activation decrease was documented previously in a heterogeneous group of eight patients with CD, where five of them were BoNT-naïve (de Vries et al. 2008) and also in another focal dystonia, writer´s cramp (Castrop et al. 2012). However, the global picture seems to be even more complex, since a recent study utilizing fMRI in CD during a force production task reported both distributed activation increases and decreases in comparison with healthy controls (Burciu et al. 2017). Although the provided evidence is difficult to reconcile, the differing direction of functional changes (increased vs. decreased activity compared to healthy controls) may be explained by differences in patient populations, especially the differences in treatment [e.g., naïve vs. long-term-treated patients (Obermann et al. 2008; Opavský et al. 2011)], and functional MRI activation tasks [sequential finger opposition (Opavský et al. 2011) vs. forearm contraction (Obermann et al. 2008) vs. wrist flexion/extension and fist clenching (de Vries et al. 2008)].
Both in neurophysiology and functional imaging, cortical differences between baseline HC and patients with dystonia diminish following a successful treatment with BoNT. The implication is that a peripheral blockade of effectors may influence the central motor programs in dystonia. As we await more data on the probable ‘direct’ retrograde effects of BoNT (e.g., Antonucci et al. 2008), the ‘indirect’ effects remain tenable to date, the latter being hinged upon the normalization of abnormal muscle-spindle functioning in dystonia (Rosales and Dressler 2010). The consequent and apparent normalization of the cortical disorder following BoNT injections in dystonia as observed in neurophysiological studies may indicate that the manipulation of proprioceptive afferent input has a substantial impact on the disorder directly at the central level (Kaňovský et al. 1998; Gilio et al. 2000; Kaňovský and Rosales 2011). It is important to emphasize that treatment with BoNT leads to changes in the central nervous system not only in dystonia, but also in spasticity, as shown in the previous fMRI (Šenkárová et al. 2010; Veverka et al. 2012; Tomášová et al. 2013) and transcranial magnetic stimulation (Huynh et al. 2013) studies. We are aware that there are similarities as well as differences in the two BoNT indications. Dystonia reflects maladaptive plasticity, whereas patients with stroke manifest both adaptive changes related to recovery of function and maladaptive changes likely underlying spasticity. BoNT aims to specifically targets the maladaptive process in both conditions. The results of these studies showed a much more complex effect of the long term and regular BoNT injections, although the pathophysiology of spasticity differs from pathophysiological processes in dystonia (Veverka et al. 2016).
We acknowledge that the mechanism how BoNT could affect the central activity is not fully elucidated. Marchand-Pauvert et al. (2013) summarize the recent evidence of blockade of the gamma motor endings, of plastic changes following blockade of the neuromuscular transmission and of retrograde transport and transcytosis. Presently, it is not clear, which of these mechanisms contributes to the changes observed in functional imaging studies.
We acknowledge several limitations of the study which temper our conclusions and should be addressed in future research: recording the number of finger sequences completed per block does not capture all aspects of motor performance. The results should be replicated in a larger patient cohort, possibly using several different motor tasks, so that effects of a specific, carefully controlled and/or monitored motor task and a specific patient cohort might be separated. More timepoints post-treatment from baseline would permit better insight as to whether the changes in fMRI occurred before, after or at the same time as the improvements in clinical parameters—this may help explain whether the central changes are in fact primary driving the improvement or secondary effects. We acknowledge that the controls were scanned only once, whereas a balanced design would be more powerful to rule out effects of repeated motor testing. Nevertheless, single repetition of a motor task typically leads to a decrease, rather than increase, in sensorimotor activation (Hok et al. 2017 + další mimoOl citace). MRI-compatible electromyographic recording from the cervical musculature would permit modeling the influences of possible changes in dystonic activity after BoNT treatment. Finally, combining multiple examination methods in the same protocol, e.g., functional MRI and TMS, would provide richer data to help describe the complex pathophysiological processes (de Vries et al. 2012).
In conclusion, the results of the present study demonstrate that in treatment of CD, the first BoNT injection is associated with changes in widespread sensorimotor networks, which diminish the observed baseline differences between the patients and healthy controls. This study also confirms that abnormalities in sensorimotor activation extend beyond circuits controlling the affected body parts in CD.
Acknowledgements
Research supported by Grant AZV MH CR 16-30210A from the Czech Health Research Council and by the Institutional support of the Research Organisation—Ministry of Health, Czech Republic, RVO—FNOL 2017. Thanks to Anne Johnson for grammatical assistance.
References
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;3:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Abbruzzese G, Berardelli A. Neurophysiological effects of botulinum toxin type A. Neurotox Res. 2006;9:109–114. doi: 10.1007/BF03033927. [DOI] [PubMed] [Google Scholar]
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124(Pt 3):537–545. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzino L, Abbruzzese G. How does the cerebellum contribute to the pathophysiology of dystonia? Basal Ganglia. 2012;2(4):231–235. doi: 10.1016/j.baga.2012.05.003. [DOI] [Google Scholar]
- Burciu RG, Hess CW, Coombes SA, Ofori E, Shukla P, Chung JW, McFarland NR, Wagle Shukla A, Okun MS, Vaillancourt DE. Functional activity of the sensorimotor cortex and cerebellum relates to cervical dystonia symptoms. Hum Brain Mapp. 2017;38(9):4563–4573. doi: 10.1002/hbm.23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131(1):146–154. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrop F, Dresel C, Hennenlotter A, Zimmer C, Haslinger B. Basal ganglia-premotor dysfunction during movement imagination in writer’s cramp. Mov Disord. 2012;27(11):1432–1439. doi: 10.1002/mds.24944. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation? Front Hum Neurosci. 2010;19(4):173. doi: 10.3389/fnhum.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consky E, Lang A. Clinical assessments of patients with cervical dystonia. In: Jankovic JHM, editor. Therapy with Botulinum Toxin. New York: Marcel Dekker Inc.; 1994. [Google Scholar]
- Cuny E, Ghorayeb I, Guehl D, Escola L, Bioulac B, Burbaud P. Sensory motor mismatch within the supplementary motor area in the dystonic monkey. Neurobiol Dis. 2008;2:151–161. doi: 10.1016/j.nbd.2007.12.011. [DOI] [PubMed] [Google Scholar]
- de Vries PM, Johnson KA, de Jong BM, Gieteling EW, Bohning DE, George MS, Leenders KL. Changed patterns of cerebral activation related to clinically normal hand movement in cervical dystonia. Clin Neurol Neurosurg. 2008;110(2):120–128. doi: 10.1016/j.clineuro.2007.09.020. [DOI] [PubMed] [Google Scholar]
- de Vries PM, de Jong BM, Bohning DE, Hinson VK, George MS, Leenders KL. Reduced parietal activation in cervical dystonia after parietal TMS interleaved with fMRI. Clin Neurol Neurosurg. 2012;114(7):914–921. doi: 10.1016/j.clineuro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Heinen F, Kleedorfer B, Wagner M, Lücking CH, Poewe W. Clinical and polymyographic investigation of spasmodic torticollis. J Neurol. 1992;239(1):9–15. doi: 10.1007/BF00839204. [DOI] [PubMed] [Google Scholar]
- Extremera VC, Alvarez-Coca J, Rodrıguez GA, Perez JM, de Villanueva JLR, Dıaz CP. Torticollis is a usual symptom in posterior fossa tumors. Eur J Pediatr. 2008;167(2):249–250. doi: 10.1007/s00431-007-0453-8. [DOI] [PubMed] [Google Scholar]
- Filip P, Lungu OV, Bares M. Dystonia and the cerebellum: a new field of interest in movement disorders? Clin Neurophysiol. 2013;124(7):1269–1276. doi: 10.1016/j.clinph.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Filip P, Lungu OV, Shaw DJ, Kasparek T, Bareš M. The mechanisms of movement control and time estimation in cervical dystonia patients. Neural Plast. 2013;2013:908741. doi: 10.1155/2013/908741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip P, Gallea C, Lehéricy S, Bertasi E, Popa T, Mareček R, Lungu OV, Kašpárek T, Vaníček J, Bareš M. Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord. 2017;32(5):757–768. doi: 10.1002/mds.26930. [DOI] [PubMed] [Google Scholar]
- Frasson E, Priori A, Bertolasi L, Mauguière F, Fiaschi A, Tinazzi M. Somatosensory disinhibition in dystonia. Mov Disord. 2001;16(4):674–682. doi: 10.1002/mds.1142. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Yoshimura DM, Olney RK, Lowenstein DH, Aminoff MJ. Change in pattern of muscle activity following botulinum toxin injections for torticollis. Ann Neurol. 1991;29(4):370–376. doi: 10.1002/ana.410290407. [DOI] [PubMed] [Google Scholar]
- Gilio F, Currà A, Lorenzano C, Modugno N, Manfredi M, Berardelli A. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann Neurol. 2000;48(1):20–26. doi: 10.1002/1531-8249(200007)48:1<20::AID-ANA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Hinkley LB, Webster RL, Byl NN, Nagarajan SS. Neuroimaging characteristics of patients with focal hand dystonia. J Hand Ther. 2009;2:125–134. doi: 10.1016/j.jht.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok P, Opavský J, Kutín M, Tüdös Z, Kaňovský P, Hluštík P. Modulation of the sensorimotor system by sustained manual pressure stimulation. Neuroscience. 2017;21:11–22. doi: 10.1016/j.neuroscience.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Huynh W, Krishnan AV, Lin CS, Vucic S, Katrak P, Hornberger M, Kiernan MC. Botulinum toxin modulates cortical maladaptation in poststroke spasticity. Muscle Nerve. 2013;48(1):93–99. doi: 10.1002/mus.23719. [DOI] [PubMed] [Google Scholar]
- Jankovic Joseph. Treatment of cervical dystonia with botulinum toxin. Movement Disorders. 2004;19(S8):S109–S115. doi: 10.1002/mds.20024. [DOI] [PubMed] [Google Scholar]
- Jenkinson Mark, Beckmann Christian F., Behrens Timothy E.J., Woolrich Mark W., Smith Stephen M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Hess EJ, LeDoux MS, Sharma N, Baxter MG, DeLong MR. Rodent models for dystonia research: characteristics, evaluation, and utility. Mov Disord. 2005;20(3):283–292. doi: 10.1002/mds.20364. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Kaňovský P, Rosales RL. Debunking the pathophysiological puzzle of dystonia with special reference to botulinum toxin therapy. Parkinsonism Relat Disord. 2011;17(Suppl 1):S11–S14. doi: 10.1016/j.parkreldis.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Kaňovský P, Dufek J, Halačková H, Rektor I. Change in the pattern of cervical dystonia might be the cause of benefit loss during botulinum toxin treatment. Eur J Neurol. 1997;4(1):79–84. doi: 10.1111/j.1468-1331.1997.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Kaňovský P, Streitová H, Dufek J, Znojil V, Daniel P, Rektor I. Change in lateralization of the P22/N30 cortical component of median nerve somatosensory evoked potentials in patients with cervical dystonia after successful treatment with botulinum toxin A. Mov Disord. 1998;1:108–117. doi: 10.1002/mds.870130122. [DOI] [PubMed] [Google Scholar]
- Kaňovský P, Bareš M, Streitová H, Klajblová H, Daniel P, Rektor I. Abnormalities of cortical excitability and cortical inhibition in cervical dystonia. Evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J Neurol. 2003;250:42–50. doi: 10.1007/s00415-003-0942-2. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Stinear JW, Buch ER, Cohen LG. Rewiring the brain: potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil Neural Repair. 2012;26(3):282–292. doi: 10.1177/1545968311420845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux MS, Brand KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18(1):60–69. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- Liepert J, Kucinski T, Tuscher O, Pawlas F, Baumer T, Weiller C. Motor cortex excitability after cerebellar infarction. Stroke. 2004;35(11):2484–2488. doi: 10.1161/01.STR.0000143152.45801.ca. [DOI] [PubMed] [Google Scholar]
- Marchal-Crespo L, Michels L, Jaeger L, López-Olóriz J, Riener R. Effect of error augmentation on brain activation and motor learning of a complex locomotor task. Front Neurosci. 2017;11:526. doi: 10.3389/fnins.2017.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Aymard C, Giboin LS, Dominici F, Rossi A, Mazzocchio R. Beyond muscular effects: depression of spinal recurrent inhibition after botulinum neurotoxin A. J Physiol. 2013;15(4):1017–1029. doi: 10.1113/jphysiol.2012.239178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin C, Martí MJ, Tolosa E, Alvarez R, Montserrat LL, Santamaria J. Modification of muscle activity after BOTOX injections in spasmodic torticollis. Ann Neurol. 1992;32(3):411–412. doi: 10.1002/ana.410320323. [DOI] [PubMed] [Google Scholar]
- Marin C, Martí MJ, Tolosa E, Alvarez R, Montserrat L, Santamaria J. Muscle activity changes in spasmodic torticollis after botulinum toxin treatment. Eur J Neurol. 1995;1(3):243–247. doi: 10.1111/j.1468-1331.1995.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Obermann M, Yaldizli O, de Greiff A, Konczak J, Lachenmayer ML, Tumczak F, et al. Increased basal-ganglia activation performing a non-dystonia-related task in focal dystonia. Eur J Neurol. 2008;8:831–838. doi: 10.1111/j.1468-1331.2008.02196.x. [DOI] [PubMed] [Google Scholar]
- Obermann M, Vollrath C, de Greiff A, Gizewski ER, Diener HC, Hallett M, Maschke M. Sensory disinhibition on passive movement in cervical dystonia. Mov Disord. 2010;25(15):2627–2633. doi: 10.1002/mds.23321. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Babiloni C, Filippi MM, Caltagirone C, Babiloni F, Cicinelli P, Traversa R, Palmieri MG, Rossini PM. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp Brain Res. 2003;149(2):214–221. doi: 10.1007/s00221-002-1346-8. [DOI] [PubMed] [Google Scholar]
- Opavský R, Hluštík P, Otruba P, Kaňovský P. Sensorimotor network in cervical dystonia and the effect of botulinum toxin treatment: a functional MRI study. J Neur Sci. 2011;306(1–2):71–75. doi: 10.1016/j.jns.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Opavský R, Hluštík P, Otruba P, Kaňovský P. Somatosensory cortical activation in cervical dystonia and its modulation with botulinum toxin: an FMRI study. Int J Neurosci. 2012;122(1):45–52. doi: 10.3109/00207454.2011.623807. [DOI] [PubMed] [Google Scholar]
- Palomar FJ, Mir P. Neurophysiological changes after intramuscular injection of botulinum toxin. Clin Neurophysiol. 2012;123(1):54–60. doi: 10.1016/j.clinph.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 2010;3:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, Stilla R, Singh S, Buetefisch C, Evatt M, Factor SA, Freeman A, Hu XP, Hess EJ, Sathian K, Jinnah HA. A functional magnetic resonance imaging study of head movements in cervical dystonia. Front Neurol. 2016;7:201. doi: 10.3389/fneur.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raike RS, Jinnah HA, Hess EJ. Animal models of generalized dystonia. NeuroRx. 2005;2(3):504–512. doi: 10.1602/neurorx.2.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales RL, Dressler D. On muscle spindles, dystonia and botulinum toxin. Eur J Neurol. 2010;17(Suppl 1):71–80. doi: 10.1111/j.1468-1331.2010.03056.x. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia help or hindrance? Clin Neurophysiol. 2012;123(1):65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Šenkárová Z, Hlustík P, Otruba P, Herzig R, Kanovský P. Modulation of cortical activity in patients suffering from upper arm spasticity following stroke and treated with botulinum toxin A: an fMRI study. J Neuroimaging. 2010;20(1):9–15. doi: 10.1111/j.1552-6569.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- Siggelkow S, Kossev A, Moll C, Däuper J, Dengler R, Rollnik JD. Impaired sensorimotor integration in cervical dystonia: a study using transcranial magnetic stimulation and muscle vibration. J Clin Neurophysiol. 2002;3:232–239. doi: 10.1097/00004691-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stacy M. Idiopathic cervical dystonia: an overview. Neurology. 2000;12(Suppl 5):2–8. [PubMed] [Google Scholar]
- Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol. 1996;6:782–787. doi: 10.1016/S0959-4388(96)80028-6. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Stell R, Mastaglia FL. Reversible reorganisation of the motor cortical representation of the hand in cervical dystonia. Mov Disord. 2003;18(4):395–402. doi: 10.1002/mds.10383. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Fiorio M, Fiaschi A, Rothwell JC, Bhatia KP. Sensory functions indystonia: insights from behavioral studies. Mov Disord. 2009;10:1427–1436. doi: 10.1002/mds.22490. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Squintani G, Berardelli A. Does neurophysiological testing provide the information we need to improve the clinical management of primary dystonia? Clin Neurophysiol. 2009;8:1424–1432. doi: 10.1016/j.clinph.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Tomášová Z, Hluštík P, Král M, Otruba P, Herzig R, Krobot A, Kaňovský P. Cortical activation changes in patients suffering from post-stroke arm spasticity and treated with botulinum toxin a. J Neuroimaging. 2013;23(3):337–344. doi: 10.1111/j.1552-6569.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56(4):2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Veverka T, Hluštík P, Tomášová Z, Hok P, Otruba P, Král M, Tüdös Z, Zapletalová J, Herzig R, Krobot A, Kaňovský P. BoNT-A related changes of cortical activity in patients suffering from severe hand paralysis with arm spasticity following ischemic stroke. J Neurol Sci. 2012;319(1–2):89–95. doi: 10.1016/j.jns.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Veverka Tomáš, Nevrly Martin, Otruba Pavel, Hluštík Petr, Kanovsky Petr. Botulinum Toxin Therapy Manual for Dystonia and Spasticity. 2016. How Much Evidence do we have on the Central Effects of Botulinum Toxin in Spasticity and Dystonia? [Google Scholar]
- Vidailhet M, Grabli D, Roze E. Pathophysiology of dystonia. Curr Opin Neurol. 2009;22(4):406–413. doi: 10.1097/WCO.0b013e32832d9ef3. [DOI] [PubMed] [Google Scholar]