Abstract
Metabolic bone disease of prematurity is characterised by disordered bone mineralisation and is therefore an increased fracture risk. Preterm infants are especially at risk due to incomplete in utero bone accretion during the last trimester. Currently, diagnosing metabolic bone disease mainly relies on biochemistry and radiographs. Dual-energy x-ray absorptiometry and quantitative ultrasound (US) are used less frequently. However, biochemical measurements correlate poorly with bone mineralisation and although scoring systems exist for metabolic bone disease, radiographs are subjective and do not detect early features of osteopenia. Dual energy x-ray absorptiometry is the reference standard for determining bone density in older children and adults. However, challenges with this method include movement artefact, difficulty scanning small and sick infants and a lack of normative data for young children. Quantitative US has a relatively low cost, is radiation-free and portable, and may hence be suitable for assessing bone status in preterm infants. This review aims to provide an overview of the use of quantitative US in detecting metabolic bone disease in preterm infants.
Electronic supplementary material
The online version of this article (10.1007/s00247-018-4161-5) contains supplementary material, which is available to authorized users.
Keywords: Bone mineral density, Children, Metabolic bone disease, Preterm infants, Quantitative ultrasonography, Review, Speed of sound, Ultrasound
Introduction
Metabolic bone disease and osteogenesis imperfecta are the two most common causes of fragile bones in infancy [1]. Metabolic bone disease is characterised by skeletal demineralisation and fractures that can occur during normal handling [2]. The in utero process of bone accretion increases exponentially during the last trimester of pregnancy [3]. Preterm infants are, therefore, deprived of this period of mineral accumulation, have low skeletal mineral stores and are predisposed to developing metabolic bone disease [4].
Other factors that increase their risk of metabolic bone disease include comorbidity, immobility and the use of drugs such as steroids and loop diuretics [3]. Concurrent use of total parenteral nutrition with an inadequate mineral content to match the infant’s higher metabolic demand leads to abnormal bone remodeling and metabolic bone disease [2, 4].
In a recent study, 30.9% of extremely low birth weight infants had radiologic evidence of metabolic bone disease [5]. In the short term, metabolic bone disease may impair the infant’s respiratory status and may be a factor in the development of myopia of prematurity associated with impaired growth of the skull [4]. These infants are also more at risk of fractures beyond the neonatal period, especially during the first 2 years of life [6]. In the same study, about a third of infants with metabolic bone disease developed spontaneous bone fractures [5].
In adolescence, former preterm infants tend to be shorter and lighter for their age and have been reported to have lower bone mass, bone mineral content, bone density and cortical cross-sectional area [4, 7, 8]. Despite the use of mineral-enriched preterm formulas, advances in intensive neonatal care and a reduction in the use of steroids and diuretics, metabolic bone disease remains a significant comorbidity. It has been reported that the incidence of metabolic bone disease in very low birth weight infants and extremely low birth weight infants is 32% and 54%, respectively, and that 10% of very low birth weight infants may be at risk for fractures [9, 10].
Considering these short- and long-term complications of poor neonatal bone health and the increasing survival rates for very low and extremely low birth weight preterm infants, an improved method of assessing bone health is necessary.
Current assessment of bone health
Currently, metabolic bone disease diagnosis relies on biochemical evaluation and radiologic investigation [3]. Biochemical measurements include serum or urinary phosphate, serum calcium and alkaline phosphatase [4]. A raised alkaline phosphatase and low serum phosphate may indicate metabolic bone disease. However, biochemical features correlate poorly with bone mineralisation and may not be consistent indicators of bone strength or mineralisation [6]. Conventional radiographs may be used to look for osteopenia or fractures and to grade metabolic bone disease [10]. However, radiographs are poor at diagnosing mild bone disease and radiologic features of osteopenia only become reproducibly apparent after 30–40% of mineral loss [2, 4].
Dual energy x-ray absorptiometry (DXA) is used to determine bone mineral density, which correlates with bone mineralisation and bone mineral content. DXA is the gold standard in adults and children. However, the lack of portable machines and the small size of (preterm) neonates and infants (who may be very ill) pose challenges for its use [4]. Furthermore, data from DXA scans are difficult to interpret in newborns due to movement artefact and variations in technique [4]. Overall, it is also relatively expensive [7]. Another important limitation of DXA is that it measures bone in just two dimensions, thus only providing an estimate of bone mineral density, which in children is highly variable because of changes in bone geometry with growth. Scientists have not agreed on a mathematical formula to fully account for differences in bone size [11].
The main advantages of DXA are its wide availability, short scanning times and low radiation dose [11].
Assessing bone health and/or diagnosing metabolic bone disease in the preterm infant remains difficult as there is no screening test that is both specific and sensitive. Biochemical indices are not diagnostic, radiographs have low sensitivity, and DXA is impractical for routine use and of questionable reliability [4].
Quantitative ultrasonography
Quantitative ultrasonography (US) was developed in 1984 as a non-ionising, portable and low-cost alternative to conventional methods of measuring bone health [4]. Quantitative US follows the principle that velocity of transmission and amplitude are influenced when a US wave is propagated through bone [11]. Many quantitative US devices are specific to only one skeletal site, such as the calcaneum or tibia. A US transducer and receiver are placed at opposite ends of the bone. The US wave passes through the area of interest and parameters such as speed of sound (speed of propagation of US wave through bone) and bone transmission time (time taken for ultrasonic wave to pass through bone) are recorded [4]. Speed of sound increases and bone transmission time decreases with an increase in bone density and strength. The parameters reflect bone density, architecture and elasticity, including qualitative bone properties such as bone mineralisation and quantitative properties such as cortical thickness, elasticity and microarchitecture, providing a more complete picture of bone health as compared to current assessment techniques [4, 11]. This is useful in preterm infants because qualitative bone properties may be affected in addition to bone mineral density, further predisposing them to metabolic bone disease [3].
Quantitative US techniques can be applied to peripheral sites, are safe, easy to use and cost effective; the devices are portable and only a few minutes are needed to perform the measurements at the bedside. These characteristics make it favourable for use in assessing bone status in children [11].
In vitro studies have shown that forearm quantitative US variables correlate significantly with bone strength, and these parameters have been found to correspond to bone mineral assessment by DXA in children [7]. Results have demonstrated that quantitative US devices adapted for children can be used as frequently as DXA to estimate bone mineral status and bone fragility, but current data are not sufficient to establish which of them is the best choice [11]. This review will evaluate the potential of quantitative US as an important tool in the diagnosis, management and follow-up of metabolic bone disease in preterm infants. In this review, we evaluate studies that have used a total of four commercially available quantitative US devices: Omnisense 7000P (Sunlight Medical Inc., Tel Aviv, Israel), DBM Sonic (IGEA, Capri, Italy), DBM Bone Profiler (IGEA, Capri, Italy) and Osteoson KIV (Minhorst, Medut, Germany).
Search strategy
For literature analysis we used the Critical Appraisal Skills Programme tool [12]. A systematic search (Fig. 1) was performed of Medline and Embase (Table 1). Reference lists from identified studies were hand-searched to identify further relevant studies. No time limits were applied. Unpublished data such as conference proceedings were not included. Articles not written in English were excluded. Twenty-nine papers were included and are summarised in Table 1. The Critical Appraisal Skills Programme tool [12] was also used to assess the quality of these papers and is shown in Table 2.
Fig. 1.
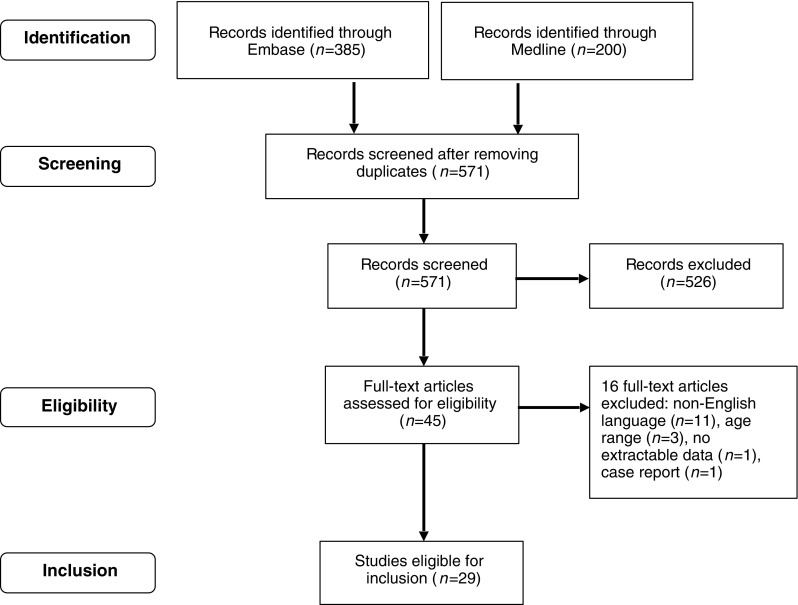
Identification and inclusion of articles for analysis
Table 1.
Summary of papers included in review
| Reference | Year | Quantitative ultrasound device | Site/parameter | Term/ preterm | Study design | n | Speed of sound (term) | Age at scan (term) | Preterm speed of sound values | Age at scan (preterm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mercy et al. [2] | 2007 | Omnisense | Tibia/ SOS | No/Yes | Longitudinal | 84 | 5 (2–9)b (days) | |||
| Ashmeade et al. [7] | 2007 | Omnisense | Tibia/ SOS | Yes/Yes | Cross-sectional/ longitudinal | 108 | 3,036 (2,843–3,333)b | ≤72 h of life | 2,924 (2,672–3,220)b | ≤1 week of life |
| McDevitt et al. [8] | 2007 | Omnisense | Tibia/ SOS | No/Yes | Cross-sectional/ longitudinal | 39 | 2,942 (2,609–3,064)b (corrected gestational age 0–6 months) 3,269 (3,009–3,413)b (corrected gestational age 6–12 months) 3,327 (3,110–3,495)b (corrected gestational age ≥ 12 months) |
32 (2–104)b (days) | ||
| Zuccotti et al. [13] | 2011 | Omnisense | Tibia/SOS | Yes/No | Cross-sectional/ Longitudinal | 116 | 2,964 (2,811–3,282)b (girls) 3,042 (2,656–3,349)b (boys) |
<9 days | ||
| Tansug et al. [14] | 2011 | Omnisense | Tibia/ SOS | Yes/Yes | Longitudinal | 126 | 3,114 (139)a | 10th day | 2,995 (143)a | 10th day |
| Gonnelli et al. [15] | 2004 | DBM Bone profiler | Humerus/ BTT, SOS | Yes/No | Cross-sectional | 140 | 1,724.8 (25.3)a | <3 days | ||
| Betto et al. [16] | 2014 | DBM Sonic | Metacarpal/ BTT, SOS | No/Yes | Cross-sectional/ Longitudinal | 154 | 1,642.17 (28.35)a | <24 h of birth | ||
| Ritschl et al. [17] | 2005 | DBM Sonic | Second metacarpus/BTT, SOS | Yes/Yes | Cross-sectional/ Longitudinal | 338 | 1,684 (27)a | <24 h | 1,636 (17)a | <24 h |
| Litmanovitz et al. [18] | 2007 | Omnisense | Tibia/ SOS | No/Yes | Interventional | 16 | ≤7 days | |||
| Liao et al. [19] | 2005 | Omnisense | Tibia/SOS | Yes/Yes | Cross-sectional | 542 | 2,984 (116)a | <3 months | 2,935 (96)a | <3 months |
| McDevitt et al. [20] | 2005 | Omnisense | Tibia, distal third of radius/ SOS | Yes/Yes | Cross-sectional | 110 | 3,079 (3,010–3,142)b | 3 (2–5)b (days) | 2,994 (2,917–3,043)b (gestational age 32–36 weeks) 2,911 (2,816–2,982)b (gestational age <32 weeks) |
3 (2–5)b (days) |
| Altuncu et al. [21] | 2007 | Omnisense | Tibia/SOS | Yes/Yes | Cross-sectional/ Longitudinal | 55 | z-score: 0.0 ([−0.8]-0.5)b | <1 week | z-score: 0.4 ([−0.2]-1.4)b | <1 week and term-corrected age |
| Chen et al. [22] | 2012 | Omnisense | Tibia/ SOS | Yes/Yes | Cross-sectional | 667 | 2,971.7 (1,06.3)a | ≤7 days | 2,932.9 (112.4)a | ≤7 days |
| Rack et al. [23] | 2012 | Osteoson KIV | 4 different sites/ SOS | Yes/Yes | Longitudinal | 172 | 1,785 (27)a | ≤7 days | 1,720 (24)a | ≤7 days |
| Littner et al. [24] | 2004 | Omnisense | Tibia/SOS | Yes/No | Cross-sectional | 25 | 3,082.4 (93.7)a | <96 h of life | ||
| Fewtrell et al. [25] | 2008 | Omnisense | Tibia/ SOS | No/Yes | Cross-sectional/ longitudinal | 99 | 2,950 (2,821–3,220)b | 2.6 (2.6)a (weeks) | ||
| Chen et al. [26] | 2010 | Omnisense | Tibia/ SOS | No/Yes | Interventional | 16 | 2,851.5 (89)a | At birth | ||
| Litmanovitz et al. [29] | 2003 | Omnisense | Tibia/SOS | No/Yes | Interventional | 24 | 2,892.3 (29.5)a (Control] 2,825.0 (32.2)a [Intervention] |
<1 week | ||
| Pereda et al. [30] | 2003 | Omnisense | Tibia/SOS | No/Yes | Cross-sectional | 95 | No numerical data | 2.7 (1.9)a [days] | ||
| Littner et al. [31] | 2003 | Omnisense | Tibia/SOS | Yes/Yes | Cross-sectional | 73 | No numerical data | <96 h of life | No numerical data | <96 h of life |
| Rubinacci et al. [32] | 2003 | DBM Sonic | Humerus/BTT, SOS | Yes/Yes | Cross-sectional | 94 | 1,734 (28)a | <1 week | 1,664 (42)a | At least 34 weeks post conceptual age |
| Littner et al. [33] | 2004 | Omnisense | Tibia/SOS | Yes/Yes | Cross-sectional | 50 | 3,010 (118)a (no specific data based on gestation) | <96 h of life | 3,010 (118)a (no specific data based on gestation) | <96 h of life |
| Littner et al. [34] | 2005 | Omnisense | Tibia/SOS | Yes/Yes | Cross-sectional | 22 | 3,063 (126)a (mean gestation: 34 weeks) | <96 h of life | 3,063 (126)a (mean gestation: 34 weeks) |
<96 h of life |
| Teitelbaum et al. [35] | 2006 | Omnisense | Tibia/SOS | Yes/Yes | Cross-sectional | 235 | 3,012 (98)a | <96 h of life | 2,963 (132)a | <96 hs of life |
| Chen et al. [36] | 2007 | Omnisense 7000P | Tibia/SOS | No/Yes | Cross-sectional | 144 | 3,098 (135)a (small for gestational age infants) 3,003 (122)a (appropriate for gestational age infants) |
<1 week of life | ||
| Ahmad et al. [37] | 2010 | Omnisense 7000P | Tibia/SOS | Yes/Yes | Cross-sectional | 102 | 3,168.4 (3,129.0–3,207.9)b | <3 months | 2,797.4 (2,720.4–2,874.4)b (23–28 weeks) 3,003.9 (2,949.8–3,058)b (29–32 weeks) 2,470 (2,267.2–2,673.4)b (33–36 weeks) |
<3 months |
| Liao et al. [38] | 2010 | Omnisense 7000P | Tibia/ SOS | Yes/Yes | Longitudinal | 267 | 2,979 (113)a | ≤6 days of delivery | 2,945 (89)a | ≤6 days of delivery |
| Savino et al. [39] | 2013 | DBM sonic | Metacarpal/ BTT, SOS | Yes/No | Cross-sectional | 103 | 1,640 (26)a | 127 (81)a (days) | ||
| Erdem et al. [40] | 2015 | Omnisense 7000P | Tibia/SOS | No/Yes | Interventional | 28 | 2,901.28 (120.08)a (control) 2,812.0 (149.69)a (Intervention) |
Unknown |
BTT bone transmission time, SOS speed of sound
amean (standard deviation), bmedian (range)
Table 2.
Application of the Critical Appraisal Skills Programme tool [12]
| Quantitative ultrasound device | Study | Year | Type of study | Are the results of the study valid? | What are the results? | Will the results help locally? |
|---|---|---|---|---|---|---|
| Omnisense 7000P | Mercy et al. [2] | 2007 | Cohort | + | + | ± |
| Ashmeade et al. [7] | 2007 | Case control | ± | ± | ± | |
| McDevitt et al. [8] | 2007 | Cohort | + | + | ± | |
| Zuccotti et al. [13] | 2011 | Cohort | ± | + | ± | |
| Tansug et al. [14] | 2011 | Case control | ± | + | ± | |
| Litmanovitz et al. [18] | 2007 | Randomised controlled trial | ± | + | ± | |
| Liao et al. [19] | 2005 | Case control | ± | + | – | |
| McDevitt et al. [20] | 2005 | Cohort | ± | + | ± | |
| Altuncu et al. [21] | 2007 | Diagnostic accuracy | ± | ± | ± | |
| Chen et al. [22] | 2012 | Case control | ± | + | ± | |
| Littner et al. [24] | 2004 | Case control | ± | ± | ± | |
| Fewtrell et al. [25] | 2008 | Cohort | ± | ± | ± | |
| Chen et al. [26] | 2010 | Randomised controlled trial | ± | + | ± | |
| Litmanovitz et al. [29] | 2003 | Randomised controlled trial | + | + | ± | |
| Pereda et al. [30] | 2003 | Cohort | ± | + | ± | |
| Littner et al. [31] | 2003 | Cohort | ± | ± | ± | |
| Littner et al. [33] | 2004 | Case control | ± | ± | ± | |
| Littner et al. [34] | 2005 | Case control | ± | ± | ± | |
| Teitelbaum et al. [35] | 2006 | Case control | ± | ± | ± | |
| Chen et al. [38] | 2007 | Case control | ± | + | ± | |
| Ahmad et al. [37] | 2010 | Case control | ± | ± | ± | |
| Liao et al. [38] | 2010 | Case control | – | ± | ± | |
| Erdem et al. [40] | 2015 | Randomised controlled trial | ± | + | ± | |
| DBM Sonic | Gonnelli et al. [15] | 2004 | Cohort | ± | + | ± |
| Betto et al. [16] | 2014 | Cohort | ± | + | ± | |
| Ritschl et al. [17] | 2005 | Cohort | ± | + | ± | |
| Rubinacci et al. [32] | 2003 | Case control | ± | + | ± | |
| Savino et al. [39] | 2013 | Cohort | ± | + | ± | |
| Osteon KIV | Rack et al. [23] | 2012 | Case control | – | + | ± |
+ Yes
- No
± Unable to tell
Analysis
Feasibility
Twenty-eight studies reported successful scanning of all study subjects including premature and very low birth weight infants, while one study reported a proportion of failed scans. Quantitative US appeared well-tolerated, had no adverse side effects, and was appropriate for use for both single and serial scans. Fewtrell et al. [25] reported failed scans, due to technical problems. In that study, 17 of 99 patients had at least one failed scan and 4 patients had no successful scans at all. There were no clinical features or patterns related to the failed scans, but it was suggested that oedema from illness or fat deposition from rapidly growing infants could be affecting scan success.
Reproducibility
Reproducibility of the technique (as mentioned in 11 studies) is summarised in Table 3. Intraobserver coefficient variant, interobserver coefficient variant and instrumental precision coefficient variant were all less than 2%. Instrumental precision reported for Omnisense 7000P is 0.25–0.5%.
Table 3.
Reproducibility of quantitative ultrasound technique
| Study | Year | Equipment name/model | Number of patients | Intraobserver coefficient variant (%) | Interobserver coefficient variant (%) | Instrumental precision coefficient variant (%) | Intersite variation coefficient variant (%) |
|---|---|---|---|---|---|---|---|
| Mercy et al. [2] | 2007 | Omnisense 7000P | 84 | 1.26 | |||
| McDevitt et al. [8] | 2007 | Omnisense 7000P | 39 | 1.1 | 1.2 | ||
| Zuccotti et al. [13] | 2011 | Omnisense 7000P | 116 | 0.34 | |||
| Gonnelli et al. [15] | 2004 | DBM Bone Profiler | 140 | 1.0 | |||
| McDevitt et al. [20] | 2005 | Omnisense 7000P | 110 | 1.2 | 2.4 | ||
| Rack et al. [23] | 2012 | Osteon KIV | 172 | 0.62 | |||
| Fewtrell et al. [25] | 2008 | 99 | 1–2 | ||||
| Littner et al. [31] | 2003 | Omnisense 7000P | 73 | <1.2 | |||
| Rubinacci et al. [32] | 2003 | DBM Sonic 1200 | 94 | 1.76 (standardised) | |||
| Littner et al. [34] | 2005 | Omnisense 7000P | 22 | <1.2 | |||
| Liao et al. [38] | 2010 | Omnisense 7000P | 267 | 1.23–1.84 |
No significant differences were found in readings taken from different anatomical sites [2]. The ability to take measurements from various sites has significant potential advantages and the absence of large differential measurement errors between sites is important.
Quantitative US values
Table 1 summarises the equipment used and speed of sound values in the 29 reviewed studies. Most studies (23) used Omnisense 7000P at the tibial site, and their values were comparable for the term and preterm populations.
Speed of sound and gestational age
Regardless of quantitative US equipment used, a positive correlation was found between speed of sound values and gestational age, with term infants having higher speed of sound values than preterm infants reflecting the increased maturity of their bones. It is to be noted that significant correlation does not mean diagnostic accuracy in any of the presented results.
Ashmeade et al. [7] found a positive correlation between speed of sound and gestational age in preterm but not in term infants. Similarly, Zuccotti et al. [13] found no correlation between gestational age and speed of sound values in term infants. Conversely, Tansug et al. [14] suggested that speed of sound and gestational age are positively correlated when reviewing values from preterm and term infants as a whole, but the correlation did not seem to apply to the preterm group alone. The small sample size (three infants with gestational age <28 weeks) could be the reason for this finding.
Postnatal trend of speed of sound values
Postnatal speed of sound values decrease in preterm infants. A similar decrease has been seen in term infants [15–17]. This is mentioned in 14 studies and summarised in Table 4. As postnatal age increases, speed of sound values decrease despite overall growth, as shown by limb length and biochemical markers [18]. The rate of decline in speed of sound values is related to the prematurity of the infant, with most preterm infants having the steepest decline in speed of sound values [7, 17, 19]. This trend seems counterintuitive as one would expect bone density and strength to increase as infants grow. This may be because the postnatal trend of speed of sound values in preterm infants differs from that of term infants, and quantitative US is able to reflect a decline in either quantitative or qualitative bone properties despite linear growth.
Table 4.
Postnatal trend in quantitative ultrasonography values
| Reference | Year | Quantitative ultrasound device | Site/parameter | Trend of speed of sound/bone transmission time values postnatally (preterm) | Trend of speed of sound/ bone transmission time values postnatally (term) | Comments |
|---|---|---|---|---|---|---|
| Mercy et al. [2] | 2007 | Omnisense 7000P | Tibia/SOS | Decreasing | The overall trend in tibial SOS showed a decrease with postnatal age. | |
| Ashmeade et al. [7] | 2007 | Omnisense 7000P | Tibia/SOS | Decreasing | There was a significant decrease over time for entire cohort of preterm infants. | |
| Tansug et al. [14] | 2011 | Omnisense 7000P | Tibia/ SOS | Decreasing | SOS values of preterm infants decreases until 2nd month of life. | |
| Gonnelli et al. [15] | 2004 | DBM Sonic | Humerus/BTT, SOS | Decreasing in SOS Increasing in BTT |
Decrease in SOS values for term infants at 12-months follow-up. Steady increases in BTT for term infants after birth at 12-months follow up. | |
| Betto et al. [16] | 2014 | DBM Sonic | Metacarpal/BTT, SOS | Decreasing | Decreasing | Deflection of metacarpal BTT from birth to 3rd week of life, followed by increase in this parameter during first few months of life. |
| Ritschl et al. [17] | 2005 | DBM Sonic | Second metacarpal/ BTT, SOS | Decreasing in SOS Increasing in metacarpal BTT |
Decreasing in SOS | Decline in SOS values for up to 6 months in term and preterm infants, then increasing trend up to 18 months of life. Steady increase in metacarpal BTT after birth in preterm infants. |
| Litmanovitz et al. [18] | 2007 | Omnisense 7000P | Tibia/ SOS | Decreasing | Bone SOS decreases during the first 4 postnatal weeks in very low birth weight premature infants. | |
| Liao et al. [19] | 2005 | Omnisense 7000P | Tibia/SOS | Decreasing | The SOS of infants showed an inverse correlation with postnatal age, and the decrease of bone SOS with age in premature infants was more marked than in full-term infants. | |
| Altuncu et al. [21] | 2007 | Omnisense 7000P | Tibia/SOS | Decreasing | Serial assessment of tibia SOS z-scores of preterm infants showed that tibia SOS z-scores of preterm infants at term-CA (corrected age) were significantly lower than the scores at first postnatal week of life. | |
| Rack et al. [23] | 2012 | Osteoson KIV | 4 different sites/SOS | Decreasing | Rapid decline in SOS values in first few weeks of life, plateauing after 40 weeks post-conceptual age. | |
| Fewtrell et al. [25] | 2008 | Omnisense 7000P | Tibia/SOS | Decreasing | Both absolute and z-scores relative to cross-sectional reference data fell during the postnatal period. | |
| Litmanovitz et al. [29] | 2003 | Omnisense 7000P | Tibia/SOS | Decreasing | ||
| Rubinacci et al. [32] | 2003 | DBM Sonic | Humerus/BTT, SOS | Decreasing | ||
| Savino et al. [39] | 2013 | DBM sonic | Metacarpal/BTT, SOS | Decreasing | Decreasing trend of SOS values lasted up to 240 days, followed by slow increases in next months. |
BTT bone transmission time, SOS speed of sound
Catch-up growth
Catch-up growth of preterm infants has been documented from longitudinal studies. This is shown by the postnatal equalising of speed of sound values between preterm and term infants. McDevitt et al. [8] reported that catch-up in speed of sound values is independent of postnatal growth and occurs in most infants by 6 months. The fastest rate of catch-up in speed of sound values was seen in infants who had the lowest initial speed of sound. This finding agrees with Tansug et al. [14], who demonstrated no significant difference in speed of sound values between term and preterm infants by month 12. A similar catch-up phenomenon was seen for metacarpal bone transition time in the preterm cohort in Ritschl et al. [17]. In this study, metacarpal bone transmission time values were stable for the term cohort, and the preterm cohort displayed increasing metacarpal bone transmission time values after birth, reaching the values of term infants at around 6 months of life [17].
Anthropometry
There are contradicting reports on whether speed of sound values are positively correlated, negatively correlated or not significantly correlated to birth weight. This is evaluated in 19 studies and summarised in Table 5. In Tansug et al. [14], Day 10 speed of sound values correlated with birth weight when considering both preterm and term infants as a whole, but when looking at preterm infants alone, there was no significant correlation. However, as previously alluded to, a limitation is the small number of preterm births included in this study. Zuccotti et al. [13] only looked at term infants and found no relation between weight and speed of sound values. In Ashmeade et al. [7], there was a significant positive correlation between speed of sound measurements and birth weight among preterm infants. In contrast, the correlation was negative in term infants. This suggests that lower rates of intrauterine growth are associated with high speed of sound values at birth.
Table 5.
Correlation between birth weight and quantitative ultrasonography (US) values
| Reference | Year | Quantitative US device | Site/parameter | Correlation between birth weight and quantitative US values | Comments | |
|---|---|---|---|---|---|---|
| Preterm infants | Term infants | |||||
| Mercy et al. [2] | 2007 | Omnisense 7000P | Tibia/SOS | Positive correlation | Significant positive correlation between birth weight and SOS values when using first measure cross-sectional data. | |
| Ashmeade et al. [7] | 2007 | Omnisense 7000P | Tibia/SOS | Positive correlation | Negative correlation | Significant positive correlation in birth weight and SOS measurements in preterm infants, but negative correlation in term infants. This might suggest that lower rates of interuterine growth are associated with high SOS values. |
| McDevitt et al. [8] | 2007 | Omnisense 7000P | Tibia/SOS | No significant correlation | No significant effect of weight or length gain on SOS values. | |
| Zuccotti et al. [13] | 2011 | Omnisense 7000P | Tibia/SOS | No significant correlation | No relation between birth weight and SOS values. | |
| Tansug et al. [14] | 2011 | Omnisense 7000P | Tibia/SOS | No significant correlation | There is positive correlation between birth weight when considering preterm and term infants as a whole, but no significant correlation when looking at preterm infants alone. There are only a small number of preterm births included in this study. | |
| Gonnelli et al. [15] | 2004 | DBM Bone profiler | Humerus/BTT, SOS | Positive correlation | BTT and humerus BTT of neonates showed significant relationship with birth weight. | |
| Betto et al. [16] | 2014 | DBM Sonic | Metacarpal/BTT, SOS | Positive correlation | Weight and length at 3rd week and 36th week of life correlated positively with metacarpal BTT. | |
| Ritschl et al. [17] | 2005 | DBM Sonic | Second metacarpus/ BTT, SOS | Positive correlation | Positive correlation | Quantitative US parameters were closely correlated with length and weight of infant. |
| Liao et al. [19] | 2005 | Omnisense 7000P | Tibia/SOS | No significant correlation | No significant correlation | SOS in infants with birth weights <1,500 g was lower than in infants with birth weights >2,500 g. However, there are no significant differences after accounting for gestational age and birth season. |
| McDevitt et al. [20] | 2005 | Omnisense 7000P | Tibia, distal third of radius/SOS | 32–36 weeks’ gestational age: no significant correlation <32 weeks’ gestational age: negative correlation |
No significant correlation | There was no significant difference in SOS for SGA and AGA infants in >37 weeks’ gestational age and 32–36 weeks’ gestational age groups. In the <32 weeks’ gestational age group, SGA infants had higher SOS values than AGA infants. However, there was no significant difference between LGA and AGA infants in all groups. |
| Chen et al. [22] | 2012 | Omnisense 7000P | Tibia/SOS | Negative correlation | Negative correlation | Birth weight had a negative effect on increasing SOS values. SOS values were higher in SGA infants than in AGA infants. |
| Rack et al. [23] | 2012 | Osteoson KIV | 4 different sites/SOS | Positive correlation | No significant correlation | Birth weight was the strongest predictor of quantitative US values in the most immature infants, but predictive value becomes insignificant in term infants. |
| Fewtrell et al. [25] | 2008 | Omnisense 7000P | Tibia/SOS | No significant correlation | There is no significant correlation between SOS and birth weight at time of scan. | |
| Littner et al. [31] | 2003 | Omnisense 7000P | Tibia/SOS | Positive correlation | Positive correlation | SOS values were more closely correlated to gestational age than with birth weight. |
| Rubinacci et al. [32] | 2003 | DBM Sonic | Humerus/BTT, SOS | Positive correlation | SOS values were found to be significantly correlated to birth weight and weight at measurement (postconceptual age of at least 34 weeks for preterm infants). | |
| Littner et al. [33] | 2004 | Omnisense 7000P | Tibia/SOS | Negative correlation | LGA infants had lower SOS values than normal AGA values predicted from standard curves. | |
| Teitelbaum et al. [35] | 2006 | Omnisense 7000P | Tibia/SOS | Positive correlation | Positive correlation | There was a significant positive correlation between SOS and birth weight, independent of gestational age. |
| Liao et al. [38] | 2010 | Omnisense 7000P | Tibia/ SOS | Positive correlation | Positive correlation | SOS values of infants with birth weight of <1,500 g was significantly lower than infants with birth weight of >2,500 g. |
| Savino et al. [39] | 2013 | DBM Sonic | Metacarpal/ BTT, SOS | No significant correlation | No significant correlation | Negative correlation was observed between SOS, length and weight. However with multiple regression modelling, no significant relationship was found. |
AGA appropriate for gestational age, BTT bone transmission time, LGA large for gestational age, SGA small for gestational age, SOS speed of sound
Perhaps more interesting is the new insight into appropriate, small and large for gestational age infants and how their speed of sound values differ. Ten studies in this review have made mention of the effects of size for gestational age on speed of sound values (Table 6).
Table 6.
Relationship between speed of sound values of appropriate for gestational age (AGA), small for gestational age (SGA) and large for gestational age (LGA) infants
| Study | Year | Quantitative ultrasonography device | Site/parameter | Relationship between speed of sound values of AGA and SGA infants | Relationship between speed of sound values of AGA and LGA infants |
|---|---|---|---|---|---|
| Mercy et al. [2] | 2007 | Omnisense 7000P | Tibia/ SOS | Rapid decline in SOS values in SGA infants postnatally as compared to AGA infants. | |
| Ashmeade et al. [7] | 2007 | Omnisense 7000P | Tibia/ SOS | SOS values were higher in SGA infants as compared to AGA infants. | |
| Liao et al. [19] | 2005 | Omnisense 7000P | Tibia/SOS | No difference in SOS values between SGA and AGA infants. | No difference in SOS values between AGA and LGA infants. |
| McDevitt et al. [20] | 2005 | Omnisense 7000P | Tibia, distal third of radius/ SOS | >32 weeks’ gestation: No significant difference in SOS values between AGA and SGA infants <32 weeks’ gestation: SGA infants had higher SOS values than AGA infants |
|
| Altuncu et al. [21] | 2007 | Omnisense 7000P | Tibia/SOS | No difference in SOS values between SGA and AGA infants. | |
| Chen et al. [22] | 2012 | Omnisense 7000P | Tibia/ SOS | SOS values were higher in SGA infants with higher gestational age as compared to AGA infants with similar birthweight. | |
| Rack et al. [23] | 2012 | Osteoson KIV | 4 different sites/ SOS | Lower SOS values in SGA infants than AGA infants. | |
| Littner et al. [24] | 2004 | Omnisense 7000P | Tibia/SOS | LGA infants were found to have lower SOS values than AGA infants. | |
| Littner et al. [34] | 2005 | Omnisense 7000P | Tibia/SOS | SGA infants have higher SOS values than AGA controls. | |
| Chen et al. [36] | 2007 | Omnisense 7000P | Tibia/SOS | Preterm SGA infants had higher tibial SOS values than their AGA counterparts; findings were similar regardless of the reference chart used to categorize infants as SGA or AGA. |
SOS speed of sound, US ultrasonography
McDevitt et al. [20] found no significant difference in speed of sound values between small for gestational age and appropriate for gestational age infants of more than 32 weeks’ gestation. Younger than 32 weeks’ gestation, small for gestational age infants had higher speed of sound values than appropriate for gestational age infants. Liao et al. [19] and Altuncu et al. [21] also found no difference in speed of sound values between small for gestational age and appropriate for gestational age infants. Chen et al. [22] suggested that the higher speed of sound may be attributable to the older gestational age in small for gestational age infants compared to appropriate for gestational age infants with similar birth weight. This may show that maturity of the fetus has a larger bearing on bone speed of sound than birth weight. However, Rack et al. [23] reported lower speed of sound values in small for gestational age infants than appropriate for gestational age infants. This could be explained by a deficiency in calcium and phosphate leading to reduced placental transfer and diminished bone mineralisation in small for gestational age infants or perhaps a soft-tissue effect causing higher speed of sound values in small for gestational age infants than appropriate for gestational age infants. Mercy et al. [2] found a rapid decline in speed of sound values postnatally in small for gestational age infants as compared to appropriate for gestational age infants, while there was an upward trend for large for gestational age infants. There were no explanations provided, but it was stated that this is the first time such a trend has been reported.
In Littner et al. [24], large for gestational age infants were found to have lower speed of sound values than appropriate for gestational age infants. This finding is not reproduced in Liao et al. [19], where it was concluded that no differences in speed of sound values were found between appropriate for gestational age, small for gestational age and large for gestational age infants. Littner et al. [24] speculate that the relative lack of motion of macrosomic infants as compared to appropriate for gestational age infants may lead to lower speed of sound, as physical activity is known to enhance mineral accretion.
Biochemical bone markers
Fewtrell et al. [25], Chen et al. [26] and Tansug et al. [14] did not find any relationship between speed of sound values and the bone turnover markers serum alkaline phosphatase and serum phosphate. In Chen et al. [26], there was only a slight upward trend in alkaline phosphatase, which did not correlate with any speed of sound trends. Serum alkaline phosphatase is the sum of three isoforms from the liver, intestines and bone, as such an increase in serum alkaline phosphatase might be due to a liver dysfunction. Tansug et al. [14] explained that their findings might be because there were no infants with very low serum phosphate or high serum alkaline phosphatase in their study. As a high serum alkaline phosphatase is known to develop relatively late in the pathological process of metabolic bone disease, Fewtrell et al. [25] aimed to assess the ability of early speed of sound measurements to predict a high serum alkaline phosphatase level later on. They found that speed of sound measurements did not predict a high alkaline phosphatase. Conversely, a high serum alkaline phosphatase was also not associated with a lower final speed of sound measurement. However, this study did not consider some confounding factors, such as factors related to the severity of illness or infant characteristics such as gestational age or birth weight. Conversely, Altuncu et al. [21] found that there was an inverse correlation between alkaline phosphatase levels and tibia z score at term corrected age in preterm infants. In their study, patients with alkaline phosphatase>900 international units per litre were found to have significantly lower tibia z score for speed of sound, indicating ongoing osteoblastic activity [21].
Other studies have found significant correlations between biochemical markers and speed of sound values. McDevitt et al. [8] found that serum phosphate and speed of sound were significantly positively correlated. This correlation is replicated in Betto et al. [16], with another quantitative US parameter. The study found that metacarpal bone transmission time was correlated to serum phosphate, phosphaturia and calciuria in the third week of life and suggested that these three biochemical tests could be used in the workup of metabolic bone disease. This observation was also made in Ashmeade et al. [7] and Rack et al. [23]. Additionally, in Ashmeade et al. [7], a significant negative correlation was found at various time points between serum alkaline phosphatase and speed of sound values. This shows that serum markers in combination with longitudinal speed of sound measurements may be useful for identifying infants at risk of developing metabolic bone disease. Rack et al. [23] also found a negative correlation between serum alkaline phosphatase and quantitative US parameters. The study also measured urine calcium and phosphate concentrations and serum calcium concentration and found that none of these variables correlated with quantitative US, contrary to Betto et al. [16].
Litmanovitz et al. [18] used bone specific alkaline phosphatase and carboxy terminal cross-links telopeptide of Type-I collagen as markers of bone formation and bone resorption, respectively. They found that although there was a significant increase in bone specific alkaline phosphatase and significant decrease in carboxy terminal cross-links telopeptide of Type-1 collagen, both parameters remained within the normal range and there were no significant correlations between bone turnover markers and speed of sound.
Summary of findings
In neonates, quantitative US can be measured with Omnisense 7000P, DBM sonic and Osteon KIV devices. The measurements are well tolerated by all infants, even those in intensive care. This review did not compare the reliability of different US devices; however, the trend of speed of sound values was similar for each device. Intraobserver, interobserver and intersite precision were high in all devices. The studies reviewed showed a difference between preterm and term infants at birth, and a decreasing trend in speed of sound values in preterm infants when longitudinal measurements were taken. This may reflect either that the postnatal trend of speed of sound values in preterm infants differs from term infants, or that quantitative US is able to assess both quantitative and qualitative bone properties, and gives a more holistic picture of bone health. Catch-up growth of preterm infants has been demonstrated in longitudinal studies.
Although quantitative US is now widely used in adults in the context of osteoporosis, its use in infants and children is limited to studies of small sample size [23]. Lack of reference data, use of different quantitative US devices and assessment of different sites makes it challenging to compare the outcome between studies [27]. The correlation of quantitative US parameters with various factors mentioned in this review, for example biochemical markers and anthropometry, has not provided consistent results. The correlation between quantitative US parameters and the current gold standard assessment DXA is also lacking consistent data [22]. US reference values are available for term and preterm infants, but they are specific to the manufacturer of the device used and standardised values have not been achieved [28]. Most importantly, values for predicting or monitoring metabolic bone disease have not been established [14].
Conclusion
The noninvasive, financially viable and convenient monitoring of bone health with US might hold potential as an initial screening tool to predict metabolic bone disease but also for follow-up to review treatment efficacy and assess subsequent trends in bone health. However, the results presented in the papers we evaluated were not always concordant. More studies focusing on the association of biochemical bone markers, DXA, radiographs and quantitative US parameters will be essential in assessing the accuracy and reproducibility of quantitative US variables before widespread clinical use on neonatal units.
Electronic supplementary material
(DOC 42 kb)
Acknowledgements
We thank Mrs. Sarah Massey for her help with the literature search.
Compliance with ethical standards
Conflicts of interest
None
References
- 1.Bishop N, Sprigg A, Dalton A. Unexplained fractures in infancy: looking for fragile bones. Arch Dis Child. 2007;92:251–256. doi: 10.1136/adc.2006.106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercy J, Dilon B, Morris J, et al. Relationship of tibial speed of sound and lower limb length to nutrient intake in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:381–385. doi: 10.1136/adc.2006.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemet D, Dolfin T, Wolach B, et al. Quantitative ultrasound measurements of bone speed of sound in premature infants. Eur J Pediatr. 2001;160:736–740. doi: 10.1007/s004310100849. [DOI] [PubMed] [Google Scholar]
- 4.McDevitt H, Ahmed SF. Quantitative ultrasound assessment of bone health in the neonate. Neonatology. 2007;91:2–11. doi: 10.1159/000096965. [DOI] [PubMed] [Google Scholar]
- 5.Visawanathan S, Khasawneh W, McNelis K, et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. J Parenter Enter Nutr. 2014;38:982–990. doi: 10.1177/0148607113499590. [DOI] [PubMed] [Google Scholar]
- 6.Dahlenburg SL, Bishop NJ, Lucas A. Are preterm infants at risk for subsequent fractures? Arch Dis Child. 1989;64:1384–1393. doi: 10.1136/adc.64.10_Spec_No.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashmeade T, Pereda L, Chen M, et al. Longitudinal measurements of bone status in preterm infants. J Pediatr Endocrinol Metab. 2007;20:415–424. doi: 10.1515/JPEM.2007.20.3.415. [DOI] [PubMed] [Google Scholar]
- 8.McDevitt H, Tomlinson C, White MP, et al. Changes in quantitative ultrasound in infants born at less than 32 weeks gestation over the first 2 years of life: influence of clinical and biochemical changes. Calcif Tissue Int. 2007;81:263–269. doi: 10.1007/s00223-007-9064-7. [DOI] [PubMed] [Google Scholar]
- 9.Vachharajani AJ, Mathur AM, Rao R. Metabolic bone disease of prematurity. NeoReviews. 2009;10:402–411. doi: 10.1542/neo.10-8-e423. [DOI] [Google Scholar]
- 10.Koo WKK, Gupta JM, Nayanar VV, et al. Skeletal changes in preterm infants. Arch Dis Child. 1982;57:447–452. doi: 10.1136/adc.57.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baroncelli GI. Quantitative ultrasound methods to assess bone mineral status in children: technical characteristics, performance and clinical application. Pediatr Res. 2008;63:220–228. doi: 10.1203/PDR.0b013e318163a286. [DOI] [PubMed] [Google Scholar]
- 12.CASP UK (1993) CASP Checklists. CASP International. https://casp-uk.net/casp-tools-checklists/. Accessed 27 March 2018
- 13.Zuccotti G, Vigano A, Cafarelli L, et al. Longitudinal changes of bone ultrasound measurements in healthy infants during the first year of life: influence of gender and type of feeding. Calcif Tissue Int. 2011;89:312–317. doi: 10.1007/s00223-011-9520-2. [DOI] [PubMed] [Google Scholar]
- 14.Tansug N, Yildirim SA, Canda E, et al. Changes in quantitative ultrasound in preterm and term infants during the first year of life. Eur J Radiol. 2011;79:428–431. doi: 10.1016/j.ejrad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Gonnelli S, Montagnani A, Gennari L, et al. Feasibility of quantitative ultrasound measurements on the humerus of newborn infants for the assessment of the skeletal status. Osteoporos Int. 2004;15:541–546. doi: 10.1007/s00198-003-1558-1. [DOI] [PubMed] [Google Scholar]
- 16.Betto M, Gaio P, Ferrini I, et al. Assessment of bone health in preterm infants through quantitative ultrasound and biochemical markers. J Matern Fetal Neonatal Med. 2014;27:1343–1347. doi: 10.3109/14767058.2013.858317. [DOI] [PubMed] [Google Scholar]
- 17.Ritschl E, Wehmeijer K, Terlizzi FD, et al. Assessment of skeletal development in preterm and term infants by quantitative ultrasound. Pediatr Res. 2005;58:341–346. doi: 10.1203/01.PDR.0000169996.25179.EC. [DOI] [PubMed] [Google Scholar]
- 18.Litmanovitz I, Dolfin T, Arnon S, et al. Assisted exercise and bone strength in preterm infants. Calcif Tissue Int. 2007;80:39–43. doi: 10.1007/s00223-006-0149-5. [DOI] [PubMed] [Google Scholar]
- 19.Liao XP, Zhang WL, He J, et al. Bone measurements of infants in the first 3 months of life by quantitative ultrasound: the influence of gestational age, season, and postnatal age. Pediatr Radiol. 2005;35:847–853. doi: 10.1007/s00247-005-1481-z. [DOI] [PubMed] [Google Scholar]
- 20.McDevitt H, Tomlinson C, White MP, et al. Quantitative ultrasound assessment of bone in preterm and term neonates. Arch Dis Child Fetal Neonatal Ed. 2005;90:341–342. doi: 10.1136/adc.2004.065276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altuncu E, Akman I, Yurdakul Z, et al. Quantitative ultrasound and biochemical parameters for the assessment of osteopenia in preterm infants. J Matern Fetal Neonatal Med. 2007;20:401–405. doi: 10.1080/14767050701287418. [DOI] [PubMed] [Google Scholar]
- 22.Chen HL, Tseng HI, Yang SN, et al. Bone status and associated factors measured by quantitative ultrasound in preterm and full-term newborn infants. Early Hum Dev. 2012;88:617–622. doi: 10.1016/j.earlhumdev.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Rack B, Lochmuller EM, Janni W, et al. Ultrasound for the assessment of bone quality in preterm and term infants. J Perinatol. 2012;32:218–226. doi: 10.1038/jp.2011.82. [DOI] [PubMed] [Google Scholar]
- 24.Littner Y, Mandel D, Mimouni FB, et al. Decreased bone ultrasound velocity in large for gestational age infants. J Perinatol. 2004;24:21–23. doi: 10.1038/sj.jp.7211013. [DOI] [PubMed] [Google Scholar]
- 25.Fewtrell MS, Loh KL, Chomtho S, et al. Quantitative ultrasound (QUS): a useful tool for monitoring bone health in preterm infants? Acta Paediatr. 2008;97(12):1625–1630. doi: 10.1111/j.1651-2227.2008.00992.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen HL, Lee CL, Tseng HI, et al. Assisted exercise improves bone strength in very low birth weight infants by bone quantitative ultrasound. J Paediatr Child Health. 2010;46:653–659. doi: 10.1111/j.1440-1754.2010.01822.x. [DOI] [PubMed] [Google Scholar]
- 27.Visser F, Sprij AJ, Brus F. The validity of biochemical markers in metabolic bone disease in preterm infants: a systematic review. Acta Paediatr. 2012;101:562–568. doi: 10.1111/j.1651-2227.2012.02626.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki K. Is QUS available for clinical use? Clin Calcium. 2012;22:120–123. [PubMed] [Google Scholar]
- 29.Litmanovitz I, Dolfin T, Friedland O, et al. Early physical activity intervention prevents decrease of bone strength in very low birth weight infants. Pediatrics. 2003;112:15–19. doi: 10.1542/peds.112.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Pereda L, Ashmeade T, Zaritt J, et al. The use of quantitative ultrasound in assessing bone status in newborn preterm infants. J Perinatol. 2003;23:655–659. doi: 10.1038/sj.jp.7211006. [DOI] [PubMed] [Google Scholar]
- 31.Littner Y, Mandel D, Mimouni FB, et al. Bone ultrasound velocity curves of newly born term and preterm infants. J Pediatr Endocrinol Metab. 2003;16:43–47. doi: 10.1515/JPEM.2003.16.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Rubinacci A, Moro GE, Boehm G, et al. Quantitative ultrasound for the assessment of osteopenia in preterm infants. Eur J Endocrinol. 2003;149:307–315. doi: 10.1530/eje.0.1490307. [DOI] [PubMed] [Google Scholar]
- 33.Littner Y, Mandel D, Cohen S, et al. Bone ultrasound velocity of appropriately grown for gestational age concordant twins. Am J Perinatol. 2004;21:269–273. doi: 10.1055/s-2004-829873. [DOI] [PubMed] [Google Scholar]
- 34.Littner Y, Mandel D, Mimouni FB, et al. Bone ultrasound velocity of infants born small for gestational age. J Pediatr Endocrinol Metab. 2005;18:793–797. doi: 10.1515/JPEM.2005.18.8.793. [DOI] [PubMed] [Google Scholar]
- 35.Teitelbaum JE, Rodriguez RJ, Ashmeade TL, et al. Quantitative ultrasound in the evaluation of bone status in premature and full-term infants. J Clin Denistrom. 2006;9:358–362. doi: 10.1016/j.jocd.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Ashmeade R, Carver JD. Bone ultrasound velocity in small versus appropriate for gestational age preterm infants. J Perinatol. 2007;27:485–489. doi: 10.1038/sj.jp.7211769. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad I, Nemet D, Eliakim A, et al. Body composition and its components in preterm and term newborns: a cross-sectional, multimodal investigation. Am J Hum Biol. 2010;22:69–75. doi: 10.1002/ajhb.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao XP, Zhang WL, Yan CH, et al. Reduced tibial speed of sound in Chinese infants at birth compared with Caucasian peers: the effects of race, gender, and vitamin D on fetal bone development. Osteoporos Int. 2010;21:2003–2011. doi: 10.1007/s00198-009-1158-9. [DOI] [PubMed] [Google Scholar]
- 39.Savino F, Viola S, Benetti S, et al. Quantitative ultrasound applied to metacarpal bone in infants. PeerJ. 2013;1:e141. doi: 10.7717/peerj.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erdem E, Tosun O, Bayat M, et al. Daily physical activity in low-risk extremely low birth weight preterm infants: positive impact on bone mineral density and anthropometric measurements. J Bone Miner Metab. 2015;33:329–334. doi: 10.1007/s00774-014-0594-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 42 kb)


