Abstract
Purpose
The purpose of our work was to collate information from studies published to date focusing on switching in anti-VEGF therapy and describe the currently available data on anti-VEGF switching in nAMD.
Methods
A PubMed search of published articles from January 2010 to January 2017 was conducted. Published studies were compared in parameters of sample size, reason for switch, duration of follow-up, and switch outcome (functional and anatomical).
Results
Our search revealed 31 relevant publications. Switching from bevacizumab to ranibizumab mostly resulted in improvement in visual acuity (VA) and anatomical outcomes (CMT, CRT; 7/8 and 6/8 studies, respectively), whereas switching from ranibizumab to bevacizumab was less effective (no VA or anatomical improvement in 2/4 studies). Switching from either agent to aflibercept resulted mostly in improvement of anatomical outcomes (19/21 studies), but rarely in VA improvement (6/21 studies). Not all results were statistically significant, likely due to small sample sizes.
Conclusion
Switching anti-VEGF therapy from bevacizumab to ranibizumab might be of benefit (functionally and anatomically) for patients who failed to improve with intravitreal bevacizumab injections, whereas switching from either agent to aflibercept resulted mostly in reduced macular thickness only.
Keywords: Anti-VEGF, Intravitreal injections, Bevacizumab, Ranibizumab, Aflibercept
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible loss of vision in people older than 50 years in the developed world [1]. Neovascular AMD (nAMD) is responsible for almost 90% of severe vision loss in these patients [2, 3].
Anti-VEGF (vascular endothelial growth factor) intravitreal injections are the current standard of care for nAMD: ranibizumab (Lucentis; Genentech, San Francisco, CA) has been established as an effective treatment for nAMD in large-scale, prospective, randomized, controlled multicenter studies. Another anti-VEGF alternative is aflibercept (Eylea; Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) which was approved for the treatment of nAMD by the US Food and Drug Administration in 2011. In several countries, the off-label use of bevacizumab (Avastin; Genentech) is the initial anti-VEGF choice due to economic considerations. Several large-scale, prospective, comparative studies demonstrated that bevacizumab is non-inferior to ranibizumab in terms of effect on visual acuity when administered according to the same regimen [4–8].
Bevacizumab and ranibizumab are considered by many physicians as equally safe and effective treatments for nAMD; however, some patients fail to respond even after multiple injections.
The failure of a drug to be effective might be due to either tachyphylaxis, lack of reaction when drugs are used repeatedly over a short period of time, tolerance, or a slow loss of efficacy over time [9]. Tachyphylaxis develops quickly/suddenly and does not improve with dose increase, but the drug’s efficacy might be restored if the drug is stopped and then restarted. On the contrary, with tolerance, improvement might occur with dose increase or shortening the interval between doses, but efficacy is not restored if the drug is stopped and then restarted. Deciding which mechanism is relevant to a specific case can prove to be challenging and oftentimes irrelevant to routine clinical care.
Possible mechanisms for decreased drug response in nAMD might be due to change in the neovascular membrane (more fibrosis), change in lesion type (classic vs. occult), irreversible change in the vessel walls or in neighboring structures (photoreceptors, RPE), or development of chronic inflammatory changes [9–11].
In routine clinical care setting, when confronted with cases of poor initial response or loss of efficacy in a patient who initially responded well to treatment, the retina specialist faces a challenging dilemma. In most cases, clinical decision making is based on personal experience since high-quality publications guiding these treatment decisions are scarce. Some questions which arise in these cases include the following: What benefit would the patient gain from switching to another agent versus continuing care with the current agent? What is the appropriate timing for switching? Which agent to switch to?
Since there are currently no treatment algorithms to guide us through these decisions, we tried to collect data from existing publications describing cohorts of “switch patients” hoping to at least outline what could be the expected outcome of switch from one anti-VEGF agent to another. This work is descriptive in nature and does not attempt to provide recommendations, but rather to trigger a discussion about the clinical usefulness and expected outcomes of switching between anti-VEGF agents in patients with nAMD.
Methods
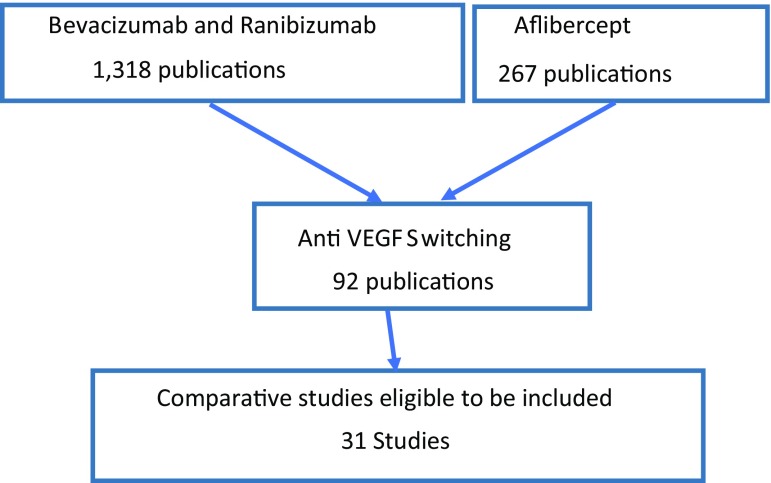
A PubMed search of published articles up to January 2017 was conducted. (There were no limitations on past publications.) Key words used were bevacizumab and ranibizumab which returned 1318 publications. When the key word aflibercept was added, an additional 267 publications were identified. Filtering these 1585 publications by using the key words anti-VEGF switching, this number was narrowed to 92 (first publications that used these key words appeared in 2012 in PubMed).
Upon further examination the following publications were excluded: non-peer-review publications, publications published prior to 2010, and case reports and reports where no switching had been reported. Finally, 31 publications were identified as comparative studies eligible to be included in our analysis (Fig. 1).
Fig. 1.
Process of including publications in the analyses
The following data were collected from these publications:
Sample size (the number of eyes in the study).
Reason for switching the anti-VEGF agent.
Duration of post-switch follow-up.
Functional outcome (visual acuity improved, no change, or decreased).
Anatomical outcomes (OCT outcomes improved, no change, or decreased).
The studies were divided into four groups:
Studies dealing with the switch from bevacizumab to ranibizumab injections (a total of 8 studies).
Studies dealing with the switch from bevacizumab and/or ranibizumab to aflibercept injections (a total of 16 studies).
Studies dealing with the switch from ranibizumab to bevacizumab injections (a total of 4 studies).
Studies dealing with the switch from ranibizumab to aflibercept injections (a total of 6 studies).
Results
We present our findings in 4 tables according to the four groups of switching. Abbreviations used in all tables are as follows:
VA—visual acuity, CMT—central macular thickness, CFT—central foveal thickness, CME—cystoid macular edema, PED—pigment epithelial detachment, CME—central macular edema, SRF—subretinal fluid, CSF—central subretinal fluid. (We deliberately choose to use the same terms that were used by the authors of the various studies.)
Eight studies reported the outcome of switching from bevacizumab intravitreal injections to ranibizumab intravitreal injections [10, 12–16].
Results of these studies are summarized in Table 1.
Table 1.
Results of switching from bevacizumab to ranibizumab
| Study | Sample size (eyes) | Reason for switch | FU duration | Outcome (mean change) | |
|---|---|---|---|---|---|
| Aslankurt et al. [12] | 20 | Cost and general health insurance applications | 21.8 ± 13.1 months | VA | Improved* (−0.2 logMAR) |
| CMT | No change (−2 μm) | ||||
| Ehlken et al. [13] | 114 | Unresponsiveness to treatment (no improvement or deterioration in VA and morphology) | 3 months | VA | Improved* (actual value not reported) |
| CFT | Decreased* (−66 μm) | ||||
| Gasperini et al. [10] | 10 | Poor response to treatment (lack of definite reduction or an increase in exudation in any compartment) | 13 months | VA | Improved (actual value not reported) |
| SRF CME PED | Decreased (actual value not reported) | ||||
| Kaiser et al. [14] | 19 (previous treatment: pegaptanib n = 1, BCZ n = 13, both n = 5) | Inadequate clinical response: gain of less than 1 line of VA or a persistence of 300 μm or greater CRT | 12 months | VA | Improved (1.17 ± 0.62 ETDRS lines) |
| CRT | Decreased (−62.16 μm) | ||||
| Kent et al. [15] | 87 | Uniform switch due to pharmacoeconomic governmental decision | 39 weeks | VA | Improved* (−0.07 logMAR) |
| CRT | Decreased* (−63.6 μm) | ||||
| Moisseiev et al. [16] | 114 | Persistent intra- or subretinal fluid and/or the absence of visual improvement | 14.2 ± 8.6 months | VA | No change (0.04 logMAR) |
| CRT | No change (−28 μm) | ||||
| Martin Df et al. [17] | 57 | Persistent intra- or subretinal fluid and/or the absence of visual improvement | 12 months | VA | Improved (0.08 logMAR) |
| Shachat AP [18] | 23 | Inadequate clinical response: gain of less than 1 line of VA or a persistence of 300 μm or greater CRT | 18 months | CRT | Improved (−55 ± µm) |
| VA | Improved (0.03 logMAR) | ||||
| CRT | Improved (−110 + 56 µm) | ||||
* p value ≤ 0.05
An additional 4 studies reported the outcome of switching therapy from ranibizumab to bevacizumab intravitreal injections [10, 12, 13, 17].
Results of these studies are summarized in Table 2.
Table 2.
Results of switching from ranibizumab to bevacizumab
| Study | Sample size (eyes) | Reason for switch | FU duration | Outcome (mean change) | |
|---|---|---|---|---|---|
| Aslankurt et al. [12] | 20 | Cost and general health insurance applications | 19.7 ± 9.4 months | VA | Improved (−0.03 logMAR) |
| CMT | No change (−1 μm) | ||||
| Ehlken et al. [13] | 24 | Unresponsiveness to treatment (no improvement or deterioration in VA and morphology) | 3 months | VA | No change* (actual value not reported) |
| CFT | Decreased (−28 μm) | ||||
| Gasperini et al. [10] | 16 | Poor response to treatment (lack of definite reduction or an increase in exudation in any compartment) | 13 months | VA | Improved (actual value not reported) |
| SRF | Decreased (actual value not reported) | ||||
| CME | |||||
| PED | |||||
| Pinheiro-Costa et al. [19] | 110 | Pharmacoeconomic nonmedical board decision | 12.2 ± 2.6 months | VA | Decreased* (−2.4 letters) |
| CRT | Increased (+19.1 μm) | ||||
* p value ≤ 0.05
Fifteen articles described switching either from bevacizumab and/or ranibizumab to aflibercept intravitreal injections [20–34]. Previous treatment in this group might have been either bevacizumab or ranibizumab. Some reports included patients for whom treatment was switched to the other drug and then to aflibercept (as third line), while other cases were switched directly to aflibercept. Results of these studies are summarized in Table 3, where previous treatments are documented as well as the final outcome. Changing the treatment strategy was different in terms of timing and cause in the various studies; the reasons for switching and previous agent or agents are detailed in Table 3.
Table 3.
Results of switching from ranibizumab and/or bevacizumab to aflibercept
| Study | Sample size (eyes) | Reason for switch | FU duration | Outcome (mean change) | |
|---|---|---|---|---|---|
| Fassnacht et al. [20] | 96 | Insufficient anatomical response to RBZ/BCZ, defined as any persisting or increasing sub- or intraretinal fluid | 16 weeks | VA | Improved (1.9 letters) |
| CRT | Decreased* (−39 μm) | ||||
| Singh et al. [21] | 26 (previous treatment: BCZ n = 7, RBZ n = 17, both = 2) | Responder population to previous anti-VEGF treatment | 6 months | VA | Improved* (5.9 letters) |
| CRT | Decreased* (−38.6 μm) | ||||
| Clement et al. [22] | 189 (previous treatment: BCZ n = 95, RBZ n = 84, both = 10) | Non-responders: persistent or recurrent macular edema, SRF, hemorrhage, exudates, and/or PED (82%) | 6 months | VA | Improved* (actual value not reported) |
| Responders: continued decrease in SRF, cystoid macular edema (CME), macular thickness and/or PED (18%) | SRF | Decreased* (actual value not reported) | |||
| CME | |||||
| PED | |||||
| Bakall et al. [23] | 36 | Recurrent, increase, or persistent subretinal fluid or edema for a minimum of 3 months RBZ/BCZ treatment prior to switching | 6 months | VA | No change (0.05 logMAR) |
| CMT | Decreased* (−65 μm) | ||||
| Cho et al. [24] | 28 | Persistent intra- or subretinal fluid 28–35 days after a minimum of 6 RBZ and/or BCZ injections prior to switching | 6 months | VA | No change (0.03 logMAR) |
| CSF | Decreased* (−21 μm) | ||||
| Ferrone et al. [25] | 221 (previous treatment: BCZ n = 76, RBZ n = 145) | Physician’s perception of limited degree or duration of effect, from previous therapy (RBZ/BCZ) | 21 weeks | VA | Not change* (actual value not reported) |
| CFT | No change* (actual value not reported) | ||||
| Grewal et al. [26] | 21 (previous treatment: BCZ n = 4, RBZ n = 5, both n = 12) | Persistent exudation: intraretinal fluid/cysts, or subretinal fluid (SRF), or both | 12 months | VA | No change (−0.02 logMAR) |
| CFT | Decreased* (−36.67 μm) | ||||
| Hall et al. [27] | 30 (previous treatment: BCZ n = 18, RBZ n = 2, both n = 10) | Patients who responded well to previous anti-VEGF therapy as well as refractory patients were switched | 12 months | VA | No change† (0.015 logMAR) |
| CMT | Decreased* (−24 μm) | ||||
| Messenger et al. [28] | 109 (previous treatment: BCZ n = 51, RBZ n = 40, both n = 18) | VA at conversion was ≥20/400 | 12 months | VA | No change (0 letters) |
| CMT | Decreased* (−26 μm) | ||||
| Oh et al. [29] | 96 (previous treatment: BCZ n = 30, RBZ n = 43, both n = 23) | Persistent, recurrent, or worsening exudative fluid or hemorrhage. Patients also were transitioned if they had intolerance to previous anti-VEGF treatments | 4 months | VA | No change (0.02 logMAR) |
| CFT | Decreased (−18 μm) | ||||
| Yonekawa et al. [30] | 102 (previous treatment: BCZ n = 26, RBZ n = 48, both n = 28) | Refractory: persistent exudation despite monthly injection (n = 68) | 18 weeks | VA | No change (0.04 logMAR) |
| Recurrent: exudation suppressed but requiring frequent injection (n = 34) | CMT | Decreased* (−29 μm) | |||
| Thorell et al. [31] | 73 | Persistent or recurrent intraretinal or subretinal macular fluid | 6 months | VA | No change (0.5 letters) |
| CRT | Decreased* (−19 μm) | ||||
| Arcinue et al. [32] | 63 | Multiple recurrences or persistence of exudation following monthly RBZ/BCZ treatments | 12 months | VA | No change (−2 letters) |
| Maximum retinal thickness | Decreased* (−107 μm) | ||||
| Homer et al. [33] | 21 | Patients who required treatment on a 4–8-week interval to remain exudation-free (on an OCT-guided T&E protocol) | 24 months | VA | No change (0.0 logMAR) |
| CST | No change (−8.5 μm) | ||||
| Pinherio-Costa et al. [34] | 85 | Refractory and recurrent AMD | 14.7 months | VA | Decreased (−2.1 letters) |
| CRT | Decreased* (−79.2 μm) | ||||
| Pfau et al. [35] | 96 | Injection interval of less than 6 weeks or permanently persisting intra- and/or subretinal fluid or persistent pigment epithelial detachments | 12 months | VA | No change |
| CRT | Decreased (− 31.36 µm; SD ± 70.64 µm) | ||||
BCZ previous treatment with bevacizumab, RBZ previous treatment with ranibizumab
* p value ≤ 0.05
†BCVA improved significantly at 6 months (from 20/64 to 20/52, p = 0.036), but showed no improvement at 12 months of follow-up (from 20/64 to 20/66)
Six studies were identified which described switching a homogenous cohort from ranibizumab as first-line treatment to aflibercept as second-line treatment (Table 4) [33–38].
Table 4.
Results of switching from ranibizumab to aflibercept
| Study | Sample size (eyes) | Reason for switch | FU duration | Outcome (mean change) | |
|---|---|---|---|---|---|
| Gharbiya et al. [36] | 31 | Persistent intraretinal or subretinal fluid with or without PED following RBZ treatments | 6 months | VA | No change (0.3 letters) |
| CSF | Decreased* (−180 μm) | ||||
| Heussen et al. [37] | 12 | Diminishing effect over time or persistent intra- or subretinal fluid following RBZ treatments | 4 injections after the switch | VA | Improved (−0.22 logMAR) |
| CSF | Decreased* (−67 μm) | ||||
| Kumar et al. [38] | 34 | Persistent subretinal and/or intraretinal fluid despite previous RBZ treatments | 6 months | VA | Improved* (−0.1 logMAR) |
| CFT | Decreased* (−168 μm) | ||||
| Kawashima et al. [39] | 15 | Recurrent or residual exudative changes after the last three RBZ injections | 6 months | VA | No change (0.01 logMAR) |
| CRT | Decreased* (−71 μm) | ||||
| Batioglu et al. [40] | 29 | Persistent intraretinal or subretinal fluid and PED following RBZ treatments | 4.55 ± 2.14 months | VA | No change (−0.06 logMAR) |
| CMT | Decreased* (−126 μm) | ||||
| Gerding et al. [41] | 40 | Persistent or recurrent intra- and/or subretinal fluid | 6 months | VA | Improved* (0.65 ETDRS lines) |
| CFT | Decreased* (−96 μm) | ||||
* p value ≤ 0.05
Overall, switching from bevacizumab to ranibizumab resulted in VA and anatomical improvement in the majority of studies (7/8 and 6/8 studies, respectively), whereas switching from ranibizumab to bevacizumab was less effective (no VA or anatomical improvement in 2/4 studies).
Switching from either agent (bevacizumab and/or ranibizumab) to aflibercept resulted in improvement of retina anatomy in most cases (20/22 studies), but rarely in VA improvement (6/22 studies).
Discussion
Despite the well-proven efficacy of anti-VEGF agents in treating nAMD, not all patients experience the desired extent of functional and anatomical improvement. This could prove to be a very frustrating situation for both treating retinal specialist and patient, as the treatment and follow-up can be cumbersome, and failing to achieve the desired result may lead to loss of confidence and reduced compliance to the follow-up and treatment regimen.
With several available anti-VEGFs on the market, patients with unsatisfactory responses to one anti-VEGF can readily be switched to another.
Our literature review focused on analyzing the response of switching resistant nAMD patients from the initially chosen anti-VEGF (by the judgment of the treating retina specialist) to another anti-VEGF agent, but we also concluded results of switching anti-VEGF agents due to other reasons such as economic considerations or regulatory/insurance decisions. It is important to note that this is not a head-to-head comparison and caution should be taken when comparing results from different studies. Another important disclaimer is the fact that all of these studies are retrospective in nature, with an inherent patient selection bias.
Our analyses revealed 31 relevant publications. In most studies, switching from bevacizumab to ranibizumab showed improvement in VA and reduction in anatomical features (7/8 and 6/8 studies, respectively), whereas switching from ranibizumab to bevacizumab was less effective (no VA or anatomical improvement in 2/4 studies). Switching from either agent to aflibercept generally resulted in anatomical improvement (19/21 studies), but rarely in functional improvement (6/22 studies). To date, there are no large data available on direct switch from bevacizumab to aflibercept.
Similarly, a meta-analysis of seven retrospective and prospective studies indicated that following treatment switch from ranibizumab or bevacizumab to aflibercept, resistant nAMD patients may have a significant improvement in CRT, while the VA was mostly stabilized after 6-month follow-up [42].
Since the desired outcome would be of sustained change rather than a temporary improvement, the available duration of post-switch follow-up is a key consideration when analyzing results.
This was illustrated by Hall and colleagues who reported significant improvement in BCVA at 6 months (from 20/64 to 20/52, p = 0.036), but no such improvement was recorded at 12 months of follow-up (from 20/64 to 20/66) [27].
There are now emerging data on “switchback” cohorts, demonstrating that while switching may have an effect it may be limited in nature, and there are situations where switching back to an agent which was deemed ineffective in the past may actually produce clinically significant results. This was recently reported by Despreaux and colleagues in a study where 47 eyes with nAMD were switched back from aflibercept to ranibizumab demonstrating a short-term benefit of this switchback in patients who had shown no benefit from the initial switch from ranibizumab to aflibercept [43].
One major limitation to these studies is the lack of uniform guidelines for switching treatment and also the pooling of data from heterogeneous patient cohorts that were treated by different retina specialists. In most cases, these publications provide little or no information regarding the treatment regimen prior to or after the switch. This of course may impact both functional and anatomical outcomes and be significant in the definition of treatment failure. Also, the timing of switch is not provided or is not uniform, making it difficult to draw conclusions on the optimal time for treatment switch, which could potentially be after 3 injections, 6 injections, or perhaps more. All of these are crucial questions in the current clinical environment, and additional well-designed larger studies would be needed to answer them.
In conclusion, switching anti-VEGF agents from bevacizumab to ranibizumab may be of benefit for patients who fail to improve with intravitreal bevacizumab injections. Ranibizumab was shown in various the publications included in this analysis as a good alternative treatment in nAMD after bevacizumab failure. When switching from either bevacizumab or ranibizumab to aflibercept, anatomical improvement was seen in most cases, but only a minority of publications described improvement in functional outcomes. To date, there are no data available on direct switch from bevacizumab to aflibercept.
While we do not aim to provide a definitive treatment guideline as to “what to inject next,” this publication review may be useful to manage our expectations as retina specialists, as well as inform our patients of what to expect when treatment switch is recommended.
References
- 1.Bressler NM. Age related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 2.Kahn HA, et al. The Framingham eye study: I. Outline and major prevalence findings. Am J Epidemiol. 1977;106:17–32. doi: 10.1093/oxfordjournals.aje.a112428. [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL, et al. Age-related macular degeneration and blindness due to Neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 4.Maguire et al (2016) Five-year outcomes with anti-vascular endothelial growth factor treatment of Neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016 Apr 20. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 5.Chakravarthy U, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 6.Berg K, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the lucentis compared to avastin study treat-and-extend protocol: two-year results. Ophthalmology. 2016;123(1):51–59. doi: 10.1016/j.ophtha.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Kodjikian L, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL non-inferiority randomized trial. Ophthalmology. 2013;120(11):2300–2309. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Krebs I, et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97(3):266–271. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 9.Binder S, et al. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012;96(1):1–2. doi: 10.1136/bjophthalmol-2011-301236. [DOI] [PubMed] [Google Scholar]
- 10.Gasperini JL, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012;96(1):14–20. doi: 10.1136/bjo.2011.204685. [DOI] [PubMed] [Google Scholar]
- 11.Eghoj MS, et al. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2012;96(1):21–23. doi: 10.1136/bjo.2011.203893. [DOI] [PubMed] [Google Scholar]
- 12.Aslankurt M, et al. The results of switching between 2 anti-VEGF drugs, bevacizumab and ranibizumab, in the treatment of neovascular age-related macular degeneration. Eur J Ophthalmol. 2013;23(4):553–557. doi: 10.5301/ejo.5000268. [DOI] [PubMed] [Google Scholar]
- 13.Ehlken C, et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye. 2014;28:538–545. doi: 10.1038/eye.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser RS, et al. Ranibizumab for Eyes Previously Treated With Pegaptanib or Bevacizumab Without Clinical Response. Ophthalmic Surg Lasers Imaging. 2011;43(1):13–19. doi: 10.3928/15428877-20111006-01. [DOI] [PubMed] [Google Scholar]
- 15.Kent JS, et al. Comparison of outcomes after switching treatment from intravitreal bevacizumab to ranibizumab in Neovascular age-related macular degeneration. Can J Ophthalmol. 2012;47:159–164. doi: 10.1016/j.jcjo.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Moisseiev E, et al. Switching treatment for neovascular age-related macular degeneration from bevacizumab to ranibizumab. Retina. 2015;35(7):1323–1330. doi: 10.1097/IAE.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 17.Martin DF, et al. Ranibizumab and bevacizumab for treatment of neovascular AMD. Int Ophthalmol. 2012;119(7):112–115. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schachat AP. Switching anti-vascular endothelial growth factors therapy for neovascular AMD. Am J Ophthalmol. 2013;156(1):12–16. doi: 10.1016/j.ajo.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Pinherio-Costa J, et al. Switch from intravitreal ranibizumab to bevacizumab for the treatment of neovascular age-related macular degeneration: clinical comparison. Ophthalmologica. 2014;232(3):149–155. doi: 10.1159/000363422. [DOI] [PubMed] [Google Scholar]
- 20.Fassnacht H, et al. Effect of aflibercept in insufficient responders to prior anti-VEGF therapy in neovascular AMD. Grafes Arch Clin Exp Ophthalmol. 2014;252(11):1705–1709. doi: 10.1007/s00417-014-2589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh RP, et al. A single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration, previously treated with ranibizumab or bevacizumab: 6-month interim analysis. Br J Ophthalmol. 2014;98:i22–i27. doi: 10.1136/bjophthalmol-2013-304798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clement K, et al. Optical coherence tomographic and visual results at six months after transitioning to aflibracept for patients on prior ranibizumab or bevacizumab treatment for exudative age related macular degeneration. Trans Am Ophthalmol Soc. 2014;112:160–198. [PMC free article] [PubMed] [Google Scholar]
- 23.Bakall B, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156:15–22. doi: 10.1016/j.ajo.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Cho H, et al. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97:1032–1035. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 25.Ferrone PJ, et al. Early initial clinical experience with intravitreal aflibercept for wet age-related macular degeneration. Br J Ophthalmol. 2014;98:i17–i21. doi: 10.1136/bjophthalmol-2013-304474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grewal DS, et al. Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye. 2014;28:895–899. doi: 10.1038/eye.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall LB, et al. Aflibercept in the treatment of neovascular age-related macular degeneration in previously treated patients. J Ocul Pharmacol Ther. 2014;30(4):346–352. doi: 10.1089/jop.2013.0188. [DOI] [PubMed] [Google Scholar]
- 28.Messenger WB, et al. Injection frequency and anatomic outcomes 1 year following conversion to aflibercept in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2014;98:1205–1207. doi: 10.1136/bjophthalmol-2013-304829. [DOI] [PubMed] [Google Scholar]
- 29.Oh VY, et al. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factors inhibitors. Am J Ophthalmol. 2013;156:23–28. doi: 10.1016/j.ajo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonekawa Y, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156:29–35. doi: 10.1016/j.ajo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Thorell, et al. Response to aflibercept after frequent retreatment with bevacizumab or ranibizumab in eyes with neovascular AMD. OSLI Retina. 2014;45(6):526–533. doi: 10.3928/23258160-20141118-07. [DOI] [PubMed] [Google Scholar]
- 32.Arcinue, et al. One-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(3):426–436. doi: 10.1016/j.ajo.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homer N, et al. Transitioning to intravitreal aflibercept following a previous treat-and-extend dosing regimen in neovascular age-related macular degeneration: 24-month results. Eye. 2015;29:1–4. doi: 10.1038/eye.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinheiro-Costa J, et al. Switch to aflibercept in the treatment of neovascular AMD: one-year results in clinical practice. Ophthalmologica. 2015;233(3–4):155–161. doi: 10.1159/000381221. [DOI] [PubMed] [Google Scholar]
- 35.Pfau M, et al. Switching therapy from ranibizumab and/or bevacizumab to aflibercept in neovascular age-related macular degeneration: one-year results. Klin Monbl Augenhelikd. 2016;233(8):945–950. doi: 10.1055/s-0042-101348. [DOI] [PubMed] [Google Scholar]
- 36.Gharbiya M et al (2014) Visual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. BioMed Res Int. Article ID 273754 [DOI] [PMC free article] [PubMed]
- 37.Heussen FM, et al. Clinical outcomes after switching treatment from intravitreal ranibizumab to aflibercept in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2014;252:909–915. doi: 10.1007/s00417-013-2553-7. [DOI] [PubMed] [Google Scholar]
- 38.Kumar N, et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age related macular degeneration. Retina. 2013;33:1605–1612. doi: 10.1097/IAE.0b013e31828e8551. [DOI] [PubMed] [Google Scholar]
- 39.Kawashima, et al. Effects of aflibercept for ranibizumab-resistant Neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(9):1471–1477. doi: 10.1007/s00417-014-2838-5. [DOI] [PubMed] [Google Scholar]
- 40.Batioglu, et al. Short-term outcomes of switching anti-VEGF agents in eyes with treatment-resistant wet AMD. BMC Ophthalmol. 2015;15:40. doi: 10.1186/s12886-015-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerding H. Functional and anatomic efficacy of a conversion to aflibercept in eyes with age-related macular degeneration after long-term ranibizumab treatment. Klin Monatsbl Augenheilkd. 2015;232:560–563. doi: 10.1055/s-0035-1545775. [DOI] [PubMed] [Google Scholar]
- 42.Seguin-Greenstein S et al (2016) A meta-analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant Neovascular age-related macular degeneration following switching to treatment with aflibercept. J Ophthalmol. Article ID 4095852 [DOI] [PMC free article] [PubMed]
- 43.Despreaux, et al. Short-term results of switchback from aflibercept to ranibizumab in neovascular age-related macular degeneration in clinical practice. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):639–644. doi: 10.1007/s00417-015-3084-1. [DOI] [PubMed] [Google Scholar]