Abstract
A new sesquiterpene, named neo-acorane A (1), and two known ones, acoric acid (2) and calamusin D (3), were isolated from a 95% ethanol extract of the rhizome parts of Acorus calamus L. Their structures were elucidated by spectroscopic methods, and the absolute configurations were determined by single-crystal X-ray diffraction analysis. Compounds 1 and 2 are nonisoprenoid sesquiterpenoids, likely biosynthesized from an acorane-type sesquiterpene by oxidative fission of the six- or five-membered ring. Moreover, compounds 1 (10 μM), 2 (5 μM and 10 μM) and 3 (10 μM) showed cell proliferation activity on the SK-N-BE (2) cell line.
Keywords: Acorus calamus L., Araceae, acorane-type sesquiterpene, biosynthetic pathway, cell proliferation
1. Introduction
Acorus calamus L. is a member of the monocot family Araceae and is widely distributed among the northern temperate and subtropical regions of Asia, North America, and Europe. In China, the rhizomes of A. calamus have long been used as a folk medicine for treatments of phlegm syncope, stroke, epilepsy, palpitation, amnesia, tinnitus, deafness, dyspepsia-inducing abdominal pain, dysentery, rheumatic pain, eczema and scabies [1]. Previous phytochemical studies on A. calamus discovered the presence of sesquiterpenes, alkaloids, flavones, and fatty acids [2,3,4,5,6]. Bioactivity studies showed that extracts and compounds from the plant had anti-dementia, anti-microbial, anti-epileptic, insecticidal, and anti-diabetic activities [2,3,4,5,6]. These facts encouraged us to reinvestigate and find new and bioactive secondary metabolites, which can treat neurodegenerative diseases such as Alzheimer’s disease, epilepsy, and stroke. In our continuous search, we had isolated and elucidated a new sesquiterpene, neo-acorane A (1), and two known ones from the ethanol extract of the rhizomes of A. calamus. Details of the structural elucidation and plausible biogenesis, and the cell proliferation activity of compounds 1–3 are reported herein.
2. Results and Discussion
2.1. Phytochemical Study
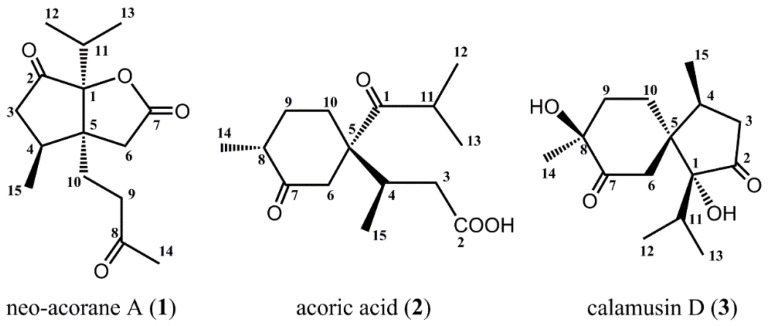
As part of our search for novel medicinal compounds from plants, we investigated the rhizome parts of A. calamus. Chromatographic separation and purification resulted in the isolation and full identification of a new sesquiterpene, neo-acorane A (1), and two known ones, acoric acid (2) and calamusin D (3) (Figure 1).
Figure 1.
Structures of isolated compounds from A. calamus.
Neo-acorane A (1) was obtained as colorless needle crystals (MeOH) and was determined to have a molecular formula of C15H22O4 on the basis of the HRESIMS data at m/z 267.1643 [M + H]+ (calcd. 267.1596), 289.1455 [M + Na]+ (calcd. 289.1416). The IR spectrum indicated the characteristic absorption bands of carbonyl groups (1778, 1749, 1715 cm−1). From the analysis of the 1H- and 13C-NMR spectra (Table 1) of compound 1, together with the examination of the HSQC and HMBC experiments, 15 carbon signals of the 13C-NMR data were identified as four methyls, four methylenes, two methines, and five quaternary carbons (assigned as three carbonyl groups, and two sp3 hybridize). It can be speculated that compound 1 is a sesquiterpene lactone with the characteristic signals, such as one downfield-shifted signal of the carbonyl group of lactone (δC 173.8, C-7), and one oxygenated quaternary carbon (δC 94.8, C-1).
Table 1.
NMR spectroscopic data (500 MHz, CDCl3) of neo-acorane A (1).
| Position | δC, Type | δH (J in Hz) | HMBC (H→C) |
|---|---|---|---|
| 1 | 94.8, C | ||
| 2 | 213.2, C | ||
| 3 | 43.4,CH2 | a 2.63, m b 2.07, m overlap |
2, 4, 5 |
| 4 | 34.1, CH | 2.37, m | 3, 5, 6, 10, 15 |
| 5 | 52.8, C | ||
| 6 | 35.4, CH2 | a 2.43, d (17.9) b 2.24, d (17.9) |
1, 4, 5, 7, 10 |
| 7 | 173.8, C | ||
| 8 | 206.9, C | ||
| 9 | 38.1, CH2 | a 2.59, m b 2.50, m |
5, 8, 10 |
| 10 | 26.1, CH2 | a 2.11, m overlap | 1, 4, 5, 6, 8, 9 |
| b 1.92, m | |||
| 11 | 30.4, CH | 2.15, m overlap | 1, 2, 5, 12, 13 |
| 12 | 18.1, CH3 | 1.02, d (7.0) | 1, 11, 13 |
| 13 | 17.8, CH3 | 1.15, d (7.0) | 1, 11, 12 |
| 14 | 30.4, CH3 | 2.18, s | 8, 9 |
| 15 | 16.5, CH3 | 1.09, d (6.7) | 3, 4, 5 |
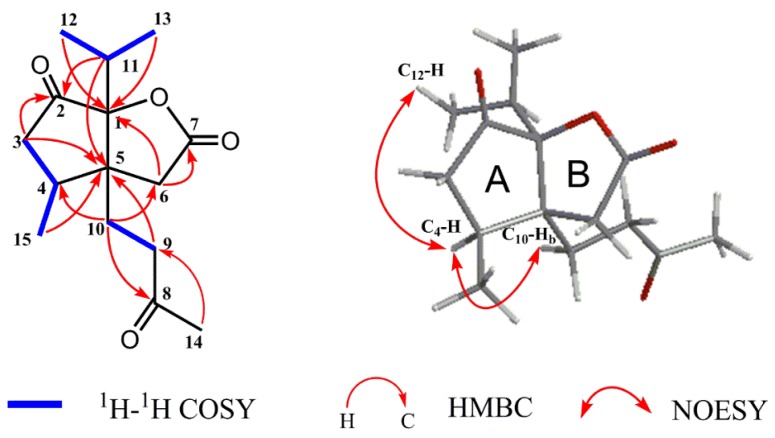
The planar structure of compound 1 was constructed by detailed analyses of 1H-1H COSY, HSQC, and HMBC spectra. Three proton-bearing structural fragments, corresponding to H2-3/H-4/H3-15, H2-9/H2-10, and H3-12/H-11/H3-13, were observed by analysis of the 1H-1H COSY spectra (Figure 2). The HMBC spectrum of compound 1 exhibited the correlations of H2-3/C-2 and C-5, of H3-15/C-5, of H2-6/C-1 and C-7, of H2-9/C-5, of H2-10/ C-4, C-6, and C-8, of H-11/ C-2, and C-5, of H3-12/C-1, of H3-13/C-1, of H3-14/C-8, and C-9. Through analysis of one ketone carbon (δC 213.2, C-2) and the key HMBC correlations, as depicted with arrows from H to C (Figure 2, Table 1), it was confirmed that unit A was a cyclopentanone moiety and linked with unit B via the bridge carbons C-1 and C-5.
Figure 2.
Key 1H-1H COSY, HMBC, and NOESY correlations of compound 1.
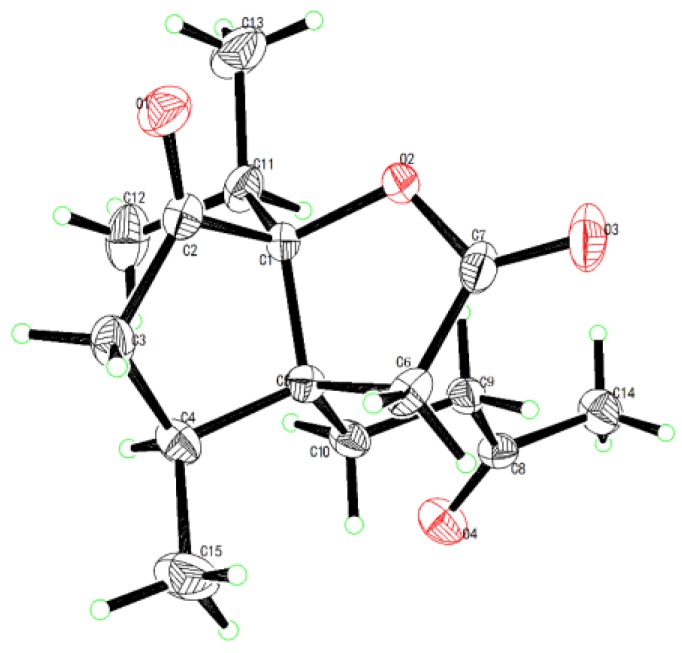
The relative configuration of compound 1 was determined by NOESY correlations (Figure 2). The key NOE correlations of H-4/H-10b and H-4/H3-12 indicated they are on the same face and were arbitrarily assigned as α-oriented. The structure of compound 1 was further confirmed by X-ray crystallographic analysis using Cu Kα radiation, and its absolute configuration was unambiguously determined as 1S, 4S, 5S (Figure 3). The chemical name of compound 1 is (3aS, 4S, 6aS)-3a-(3-oxobutyl)-4-methyl-6a-isopropyl-tetrahydro-2H-cyclopenta [b] furan-2,6(3H)-dione [7,8], and we named it neo-acorane A. NMR, HRESIMS, IR and UV spectra of compound 1 are available in Supplementary Materials.
Figure 3.
X-ray crystallographic structure of compound 1.
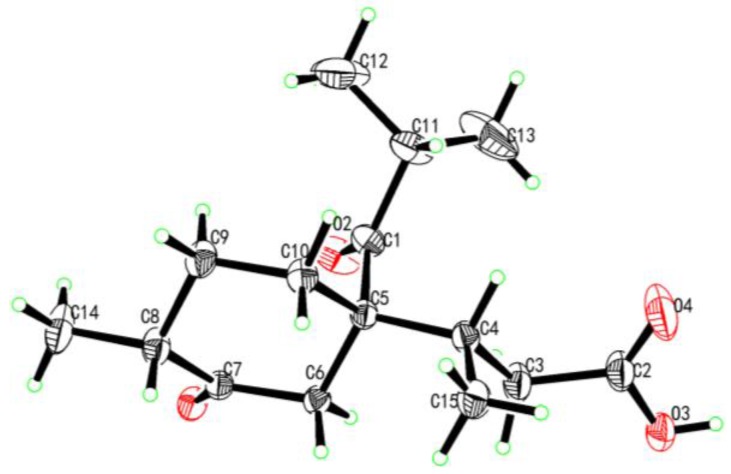
The structures of compounds 2 and 3 were identified by comparison of their physiochemical properties and spectrometric data with those reported in the literature [2,3]. The absolute configuration of compound 2 was previously unknown, and it was determined as 4S, 5S, 8R by X-ray crystallographic analysis using Cu Kα radiation (Figure 4).
Figure 4.
X-ray crystallographic structure of compound 2.
2.2. Possible Biosynthetic Pathway
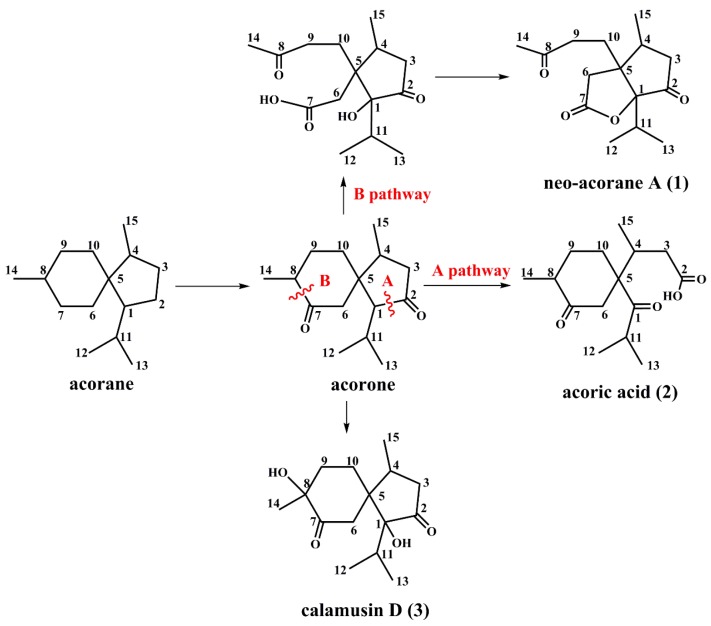
So far, many sesquiterpenes from A. calamus mainly consist of acorane, guaiane, cadinane, and elemane-type sesquiterpenes [9,10,11]. Compounds 1 and 2 are nonisoprenoid sesquiterpenoids, and may derive from the acorane skeleton with new biogenetic pathways. We speculated that compound 2 should be formed via the oxidative fission of the single bond between C-1 and C-2 (the A pathway) [3], and compound 1 might be biosynthesized by the oxidative fission of the single bond between C-7 and C-8 firstly, and then forms a five-membered lactone ring through dehydration and cyclization (the B pathway) (Scheme 1). Thus compounds 1 and 2 represent two new skeleton types deriving from acorane-type sesquiterpene.
Scheme 1.
Possible biosynthetic pathways of compounds 1–3.
2.3. Cell Proliferation Assay
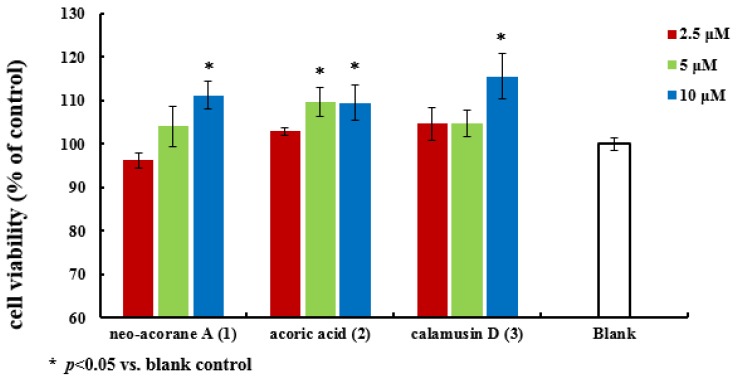
All of the isolated compounds (1–3) were evaluated for cell proliferation activity on the SK-N-BE (2) cell line with 1‰ dimethylsulfoxide (DMSO) medium as a blank control. The results (Figure 5) showed that compounds 1 (10 μM), 2 (5 μM and 10 μM) and 3 (10 μM) significantly promoted cell proliferation activity comparing with the blank control in vitro.
Figure 5.
Cell proliferation activity of compounds 1–3.
3. Materials and Methods
3.1. General Experimental Procedures
Melting points were measured with a X-4 melting point apparatus (Beijing KWF Sci-Tech Development Co., Ltd., Bejing, China). Specific rotations were measured with a Rudolph Research AutoPol IV polarimeter (Rudolph Research Flangers, Hackettstown, NJ, USA) at room temperature. UV spectra were collected by a Hewlett-Packard 8452A UV-vis spectrometer (Hewlett Packard, Palo Alto, CA, USA). IR spectra were measured with a Bruker Tensor 27 and MIRacle ATR FT-IR spectrometers (Bruker Optics, Fremont, CA, USA). NMR spectra were recorded on an Agilent DD2-500 NMR spectrometer ( Agilent, Santa Clara, CA, USA) with a OneNMR probe at 500 MHz for 1H and 125 MHz for 13C using the pulse programs provided in the Agilent Vnmrj 3.2 software. All chemical shifts were quoted on the δ scale in ppm using residual solvent as the internal standard (CDCl3: 7.26 ppm for 1H-NMR, 77.2 ppm for 13C-NMR). Coupling constants (J) are reported in Hz. HRESIMS were measured on HR-ESI-TOF-MS spectrometer (Agilent Technologies, Palo Alto, CA, USA) with the AnalystTM QS software (Agilent Series 1100 SL) for data acquisition and processing. X-ray single-crystal diffraction experiment was carried out on a Bruker APEX-II CCD detector (Bruker, Fremont, CA, USA ) employing graphite monochromated Cu Kα radiation. Column chromatograph (CC) was performed on silica gel (100–200 and 200–300 mesh; Qingdao Marine Chemical Factory, Qingdao, China) and ODS (YMC-gel, 60–80 µm, YMC Co. Ltd, Kyoto, Japan). Precoated silica gel GF254 plates (Qingdao Marine Chemical Factory, Qingdao, China) were used for TLC. Spots were visualized using UV light (254 and/or 365 nm) and by spraying with 5% (v/v) H2SO4 ethanol solution followed by heating at 105 °C.
3.2. Plant Material
A. calamus was collected in December of 2011 at Dawei Mountain, Liuyang City, Hunan Province, China, and authenticated by Prof. Ta-Si Liu (School of Pharmacy, Hunan University of Chinese Medicine, Changsha, China). A voucher specimen (No. 20111211) was deposited in Hunan Province Engineering Research Center of Bioactive Substance Discovery of TCM at Hunan University of Chinese Medicine, Changsha, China.
3.3. Extraction and Isolation
The air-dried rhizomes of A. calamus (10 kg) were powdered and extracted with ethanol (95% v/v) three times at room temperature for 24 h each time. The combined extraction was filtered and concentrated under reduced pressure to give a residue (1.1 kg, oily residue), which was then suspended in water and partitioned with petroleum ether (PE, 60–90 °C), trichloromethane (CHCl3), ethyl acetate (EtOAc) and n-butanol (n-BuOH) in succession, to give PE (618.6 g), CHCl3 (58.9 g, oily residue), EtOAc (5.2 g) and n-BuOH (16.0 g) extracts, respectively. The CHCl3 extract was subjected to dry column on silica gel (200–300 mesh, 6.0 cm× 100 cm, 1.0 kg; PE-acetone, 50:1 to 0:50) to afford six main fractions (Frs.1–6). Fr. 2 (10.5 g) was chromatographed on a silica gel column (200–300 mesh, 4.0 × 60 cm, 300 g, PE-acetone, 30:1 to 0:30) to obtain eight subfractions (Frs. 2.1–2.8). Fr. 2.4 (1.0 g) was further purified by preparative HPLC using MeOH‑H2O (70:30, 2 mL/min) as the eluent to afford compounds 3 (5.0 mg, tR = 25 min) and 1 (6.0 mg, tR = 38 min). Fr. 2.6 (1.2 g) was purified by recrystallization to obtain compound 2 (300 mg).
3.4. Spectroscopic Data of New Compound
Neo-acorane A (1): Colorless crystals (MeOH) in needle-type; m.p. 91–92 °C; +21.3° (c 1.87 × 10−3, MeOH); UV (MeOH) λmax (log ε): 203 (2.69) nm; IR (KBr) νmax 2968, 2936, 1778, 1749, 1715, 1227, 1186, 1167, 1012, 958, 895 cm−1; NMR data see Table 1; (+)-HRESIMS m/z 267.1643 [M + H]+ (calcd. for C15H23O4, 267.1596), 289.1455 [M + Na]+ (calcd. for C15H22O4Na, 289.1416).
3.5. Crystallographic Data of Compounds
Data collection was performed with a Bruker APEX-II CCD detector employing graphite monochromated Cu Kα radiation with Bruker SAINT software. The programs used to solve and refine structures were SHELXS-97 and SHELXL-97, respectively.
Crystal data for neo-acorane A (1): C15H22O4 (M = 266.33 g/mol), orthorhombic, space group P 21 21 21, a = 7.88520(10) Å, α = 90°, b = 12.4542(2) Å, β = 90°, c = 15.1058(2) Å, γ = 90°, V = 1483.45(4) Å3, Z = 4, T = 296(2)K, λ(CuKα) = 1.54178 Å, Dcalc = 1.130 g/cm3, F(000) = 544, independent reflections 2677 [R(int) = 0.1239], 9.203° < 2θ < 130.2°, the final R1 was 0.0429 (I > 2σ(I)) and wR2 was 0.1173 (all data).
Crystal data for acoric acid (2): C15H24O4 (M = 268.34 g/mol), orthorhombic, space group P 21 21 21, a = 9.4392(4) Å, α = 90°, b = 11.4671(4) Å, β = 90°, c = 14.3042(5) Å, γ = 90°, V = 1548.29(10) Å3, Z = 4, T = 296(2) K, λ(CuKα) = 1.54178 Å, Dcalc = 1.143 g/cm3, F(000) = 576, independent reflections 3026 [R(int) = 0.0357], 9.886° < 2θ < 145.6°, the final R1 was 0.0465 (I > 2σ(I)) and wR2 was 0.1401 (all data).
CCDC 1 446 115 and CCDC 1 446 116 contains the supplementary crystallographic data for compounds 1 and 2. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk.
3.6. Cell Proliferation Assay
The cell proliferation of the compounds was tested using the MTT method, as reported previously [12], with 1‰ DMSO medium as the blank control. Human neuroblastoma cell line SK-N-BE (2) was maintained from American type culture collection (ATCC) and prepared in our lab. Compounds 1–3 were dissolved in DMSO as reserve liquid (0.1 M), which were diluted into different concentrations (2.5, 5, and 10 μM) with cell culture Medium (Eagle’s Minimum Essential Medium). SK-N-BE (2) cells (1.0 × 104 cells/mL, 90 μL/well) were seeded on the 96-well plates and incubated overnight, then treated with each test compound at various concentrations (10 μL/well) at 37 °C in a humidified atmosphere containing 5% CO2 for 72 h. After the incubation, MTT was added to each well as described [12]. Optical density at 595 nm was measured in a 96-well microtiter plate reader (BioTek, Winooski, Vermont, USA). The optical density of formazan formed in control (untreated) cells was taken as 100% of viability. Three replicate wells were used for each control and test concentrations per microplate, and the experiment was repeated three times.
4. Conclusions
We isolated and characterized a new sesquiterpene, neo-acorane A (1), and two known ones, acoric acid (2) and calamusin D (3), from the CHCl3 extract of the ethanol extract of the rhizome parts of A. calamus. Compounds 1 and 2 possess two kinds of new chemical skeletons deriving from acorane-type sesquiterpene. The postulated biosynthetic pathways for compounds 1 and 2 deserve further confirmation by a biomimetic synthesis. Meanwhile, considering their cell proliferation activity, compounds 1−3 will also be potential nerve cell protection leads.
Acknowledgments
This work was financially supported by the Key Laboratory of Neurodegenerative Diseases of the Ministry of Education, Capital Medical University (2016), Administration of Traditional Chinese Medicine of Hunan Province, China (201577), and the Scientific Research Fund of the Hunan Provincial Education Department, China (15C0114).
Supplementary Materials
The followings are available online: NMR, HRESIMS, IR, and UV spectra of compound 1 are available online at www.mdpi.com/1420-3049/22/4/529/s1, Figure S1–S9.
Author Contributions
Juan Li and Shun-Xiang Li designed and supervised the study, identified the structures of the isolated compounds and wrote/revised the manuscript. Lin Li, Lan Zhang and Fang Liu carried out the bioactivity assays. Jianping Zhao, Xiao-Fang Zhao and Qing-Ru Liu interpreted and assigned the NMR data and helped in preparing/revising the manuscript. Min Yang measured and analyzed the X-ray single-crystal diffraction of compounds. Wei Wang and Ikhlas A. Khan revised the manuscript.
Conflicts of Interest
All authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Chinese Herbalism Editorial Board, State Administration of Traditional Chinese Medicine of the People’s Republic of China . Chinese Materia Medica. Shanghai Science and Technology Press; Shanghai, China: 1999. pp. 468–478. [Google Scholar]
- 2.Hao Z.Y., Liang D., Luo H., Liu Y.F., Ni G., Zhang Q.J., Li L., Si Y.K., Sun H., Chen R.Y., et al. Bioactive sesquiterpenoids from the rhizomes of Acorus calamus. J. Nat. Prod. 2012;75:1083–1089. doi: 10.1021/np300095c. [DOI] [PubMed] [Google Scholar]
- 3.Birch A.J., Hochstein F.A., Quartey J.A.K., Turnbull J.P. Structure and some reactions of acoric acid. J. Chem. Soc. 1964:2923–2931. doi: 10.1039/jr9640002923. [DOI] [Google Scholar]
- 4.Yao Y.J., Cai W.L., Yang C.J., Zhang H.Y., Hua H.X. Isolation and characterization of the insecticidal constituent acorone from Acorus calamus (Araceae) Acta Entomol. Sin. 2010;53:985–992. [Google Scholar]
- 5.Zhou C.X., Qiao D., Yan Y.Y., Wu H.S., Mo J.X., Gan L.S. A new anti-diabetic sesquiterpenoid from Acorus calamus. Chinese Chem. Lett. 2012;23:1165–1168. doi: 10.1016/j.cclet.2012.08.004. [DOI] [Google Scholar]
- 6.Hao Z.Y. Ph.D. Thesis. Chinese Academy of Medical Sciences & Peking Union Medical College; Dongcheng District, Beijing, China: 2012. Studies on chemical constituents of the rhizomes of Acorus calamus Linn. and their bioactivities. [Google Scholar]
- 7.Khushrav C., Valentin R., Arnold U. A new synthesis of methyl 3-oxo-2-pentyl-1-cyclopentene-1-acetate. Synlett. 2001;7:1127–1128. [Google Scholar]
- 8.Ramey K.C., Lini D.C., Moriarty R.M., Gopal H., Welsh H.G. Analysis of the nuclear magnetic resonance spectra of some 2,6-bridged bicyclo [2.2.1] heptane derivatives. J. Am. Chem. Soc. 1967;89:2401–2408. doi: 10.1021/ja00986a028. [DOI] [Google Scholar]
- 9.Dong W.W., Yang D.J., Lu R.H. Chemical constituents from the rhizome of Acorus calamus L. Planta Med. 2010;76:454–457. doi: 10.1055/s-0029-1186217. [DOI] [PubMed] [Google Scholar]
- 10.Dong W.W., Li M.J., Yang D.J., Lu R.H. Two new sesquiterpenes from Acorus calamus. Planta Med. 2010;76:1742–1745. doi: 10.1055/s-0030-1249833. [DOI] [PubMed] [Google Scholar]
- 11.Nawamaki K., Kuroyanagi M. Sesquiterpenoids from Acorus calamus as germination inhibitors. Phytochemistry. 1996;43:1175–1182. doi: 10.1016/S0031-9422(96)00401-3. [DOI] [Google Scholar]
- 12.Zhou M., Du G., Yang H.Y., Xia C.F., Yang J.X., Li X.N., Ye Y.Q., Gao X.M., Du G., Hu Q.F. Antiviral butyrolactones from the endophytic fungus Aspergillus versicolor. Planta Med. 2015;81:235–240. doi: 10.1055/s-0034-1396153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.