Abstract
Muscular dystrophies represent a group of diseases which may develop in several forms, and severity of the disease is usually associated with gene mutations. In skeletal muscle regeneration and in muscular dystrophies, both innate and adaptive immune responses are involved. The regenerative potential of mesenchymal stem/stromal cells (MSCs) of bone marrow origin was confirmed by the ability to differentiate into diverse tissues and by their immunomodulatory and anti-inflammatory properties by secretion of a variety of growth factors and anti-inflammatory cytokines. Skeletal muscle comprises different types of stem/progenitor cells such as satellite cells and non-satellite stem cells including MSCs, interstitial stem cells positive for stress mediator PW1 expression and negative for PAX7 called PICs (PW1+/PAX7− interstitial cells), fibro/adipogenic progenitors/mesenchymal stem cells, muscle side population cells and muscle resident pericytes, and all of them actively participate in the muscle regeneration process. In this review, we present biological properties of MSCs of bone marrow origin and a heterogeneous population of muscle-resident stem/progenitor cells, their interaction with the inflammatory environment of dystrophic muscle and potential implications for cellular therapies for muscle regeneration. Subsequently, we propose—based on current research results, conclusions, and our own experience—hypothetical mechanisms for modulation of the complete muscle regeneration process to treat muscular dystrophies.
Keywords: Muscle stem/progenitor cells, Mesenchymal stem cells, Skeletal muscle regeneration, Muscular dystrophies
Introduction
Skeletal muscle is post-mitotic tissue capable of repair and regeneration after injury. Stimulus or damage of skeletal muscle arising from physiological conditions (exercise, aging) or diseases (cachexia, sarcopenia, muscular dystrophies) triggers the regenerative process. In the regenerative process of skeletal muscle, different types of cells are involved, including muscle-resident progenitors and cells involved in innate and adaptive immune responses (Judson et al. 2013; Madaro and Bouche 2014). The regenerative potential of skeletal muscle is maintained by the heterogeneous population of muscle-resident stem/progenitor cells including satellite cells (SCs) capable of regenerating muscle fibers and maintaining a functional satellite pool, and by non-satellite stem cells: mesenchymal stem/stromal cells (MSCs), PW1+/PAX7− interstitial cells (PICs), fibro/adipogenic progenitors/mesenchymal stem cells (FAPs/MSCs), muscle side population (SP) cells and muscle resident pericytes. In response to an injury signal, myogenic SCs become activated, release chemotactic factors and a panel of pro-inflammatory cytokines, and attract monocytes and macrophages to the injury site. Activated SCs proliferate, migrate from their niche to the injury site, and generate myoblasts, which either fuse to damaged myofibers or fuse together to form myotubes that mature and form new muscle fibers. However, myogenic activity of SCs is supported by a heterogeneous population of muscle-resident MSCs which contribute to skeletal muscle regeneration (Joe et al. 2010; Judson et al. 2013; Uezumi et al. 2011). Direct contact of SCs with immune cells, especially those responsible for innate immunity, permits proper muscle regeneration. In adult human muscle macrophages orchestrate myogenesis and muscle regeneration by the interactions of differentially activated macrophages with SCs. Studies performed in vitro on human SCs and macrophages documented that pro-inflammatory macrophages (M1) inhibited myogenic precursor cell (MPC) fusion while anti-inflammatory macrophages (M2) strongly promoted MPC differentiation by increasing their commitment to differentiated myoblasts and the formation of mature myotubes (Saclier et al. 2013). Dysregulation in cooperation between muscle progenitors and cells responsible for adaptive and innate immune responses leads to impaired muscle regeneration and deposition of non-functional adipose and fibrotic tissue as occurs in muscular dystrophies (Alexakis et al. 2007; Madaro and Bouche 2014).
Researchers worldwide are working on diverse strategies to create innovative therapies for injured and/or degenerated skeletal muscle of dystrophic or traumatic patients. But first, to make cellular therapies effective, we need to clearly understand the links between MSCs and other muscle regeneration progenitor cells and inflammatory cell systems in the process of muscle regeneration in vivo and in vitro. There is still a need to investigate and gain more information about this process, especially in the area of paracrine communications between cells. In this review, we propose—based on current research results, conclusions and own experience—to introduce biological properties of MSCs of bone marrow (BM) origin and the heterogeneous population of muscle-resident stem/progenitor cells, and subsequently hypothetical mechanisms modulating the complete muscle regeneration process to treat muscular dystrophies.
Influence of Innate and Adaptive Immune Response in Muscular Dystrophies
Muscular dystrophies are a group of diseases which may develop in several forms, and severity of disease is associated with mutations in genes coding proteins associated with the muscle membrane, such as the dystrophin–glycoprotein complex (Hoffman et al. 1988; Matsumura and Campbell 1993), or with the extracellular matrix (ECM), such as laminin and collagen VI (Qiao et al. 2005; Zou et al. 2008). The absence of dystrophin (a protein linking cytoskeletal component) leads to increased fragility of the sarcolemma. Damage of muscle fibers leads to activation of SCs, responsible for muscle growth and regeneration, and induces an inflammatory response. The inflammatory response in damaged muscle is initiated due to the ability of SCs to secrete chemotactic factors such as monocyte chemotactic protein 1, macrophage-derived chemokine, fractalkine, urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor (uPA/uPAR) and the vascular endothelial growth factor (VEGF) (Chazaud et al. 2003; Tidball and Villalta 2010).
Both innate and adaptive immune responses are involved in skeletal muscle regeneration in normal conditions and in muscular dystrophies. Acute injury of skeletal muscle triggers an innate immune response characterized by release of pro-inflammatory cytokines interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α at the site of injury. Myogenic precursor cells receive signals from injured muscle and attract monocytes from muscle-supplying vessels. Pro-inflammatory cytokines, especially interferon (IFN)-γ and TNF-α, transform monocytes into phagocytic M1 phenotype. M1 macrophages are important for pathogen prevention and for the phagocytosis of cellular debris, but they are not helpful in the muscle regeneration process because their ability to secrete a cytotoxic level of nitric oxide (NO) accelerates muscle injury (Villalta et al. 2009). A high concentration of M1 is associated with pro-inflammatory cytokine activity during the first step of muscle damage. This initial inflammatory response is diminished by anti-inflammatory Th2 cytokines, IL-4, IL-10 and IL-13, due to the switch of macrophage phenotype from M1 to M2. There are two pathways shifting macrophages from M1 to M2. The first is paracrine information delivered from Th2 lymphocytes by secretion of IL-4 and IL-13 facilitating transformation of M1 macrophage into M2a (CD206+) phenotype, and secretion of IL-10 leading to transformation of M1 into M2c (CD206+, CD163+) macrophages (Deng et al. 2012; Villalta et al. 2009). Both of them make an important contribution to the muscle regeneration process. M2a macrophages are known from supporting muscle regeneration, while M2c type suppresses activity of cytotoxic M1 macrophages and persists in damaged muscle until the termination of inflammation (reviewed by Tidball and Villalta 2010; Yin et al. 2013). There was also an assumption that phagocytic ability of M1 macrophages may contribute to shifting M1 phenotype into M2 phenotype. In vitro studies documented that after phagocytosis of dead muscle fiber debris M1 macrophages stopped secreting the inflammatory cytokine TNF-α and started to secrete transforming growth factor (TGF)-β, supportive for muscle regeneration, and this reflected the shift into M2 phenotype—that is the second mechanism in which this phenomenon occurs (Arnold et al. 2007). The switch of macrophage subsets is critical to muscle regeneration, as confirmed by depletion of monocytes/macrophages at different stages before and after muscle injury induced by a cardiotoxin in the mouse model (Wang et al. 2014). Moreover, M2 macrophages are able to suppress the inflammatory response and secrete paracrine factors fibroblast growth factor (FGF)-2, insulin growth factor (IGF)-1, IGF-2, hepatocyte growth factor (HGF), and IL-6 that support SCs activation, proliferation and differentiation, and additionally support neovascularization of new myofibers by platelet-derived growth factor (PDGF) secretion (Boonen and Post 2008; Tonkin et al. 2015). Macrophages also secrete the TGF-β family member, growth differentiation factor 3, which contributes to myoblast fusion (Varga et al. 2016). In situ transition of infiltrating macrophages from an inflammatory to a repair phenotype is dependent on the microenvironment and interaction with muscle progenitor cells as introduced in an acute sterile skeletal muscle injury mouse model (Patsalos et al. 2017).
Therefore, M2 macrophages and anti-inflammatory cytokines IL-4 and IL-10 reduce inflammation and contribute to satellite cell differentiation, thus promoting myogenic differentiation (Deng et al. 2012).
In the mdx mouse model, muscles are characterized by continuous cycles of myofiber necrosis and repair. Repetitive series of myofiber deterioration lead to muscle infiltration by M1 macrophages together with M2a macrophages, which may reduce cytotoxic activity of M1 macrophages (Villalta et al. 2009). The inflammatory environment in dystrophic muscle is comparable but not the same as in acute injury. Subsequent infiltration of M2c macrophages is associated with progression to the regenerative process. However, in acute injured muscle, the number of M2 macrophages decreases upon damage repair, while in mdx muscle their number increases with age and promotes fibrosis. Increased and persistent presence of macrophages modifies the microenvironment of dystrophic muscle, leading to amplified myofiber necrosis, and replacement of muscle with fibrotic and fat tissue.
In the mdx mouse, except neutrophils and macrophages, eosinophils play an important role in the innate immune response (Heredia et al. 2013; Madaro and Bouche 2014). Eosinophil invasion was found in Duchenne muscular dystrophy (DMD) patients and in mdx muscle, and was dependent on lymphocytes activity (Cai et al. 2000; Wehling-Henricks et al. 2008). In dystrophic muscle, eosinophils modulate injury and regeneration by promoting the transition from a Th1 to Th2 inflammatory environment. IL-4, the key cytokine produced by eosinophils, may support muscle regeneration; however, the primary targets of this cytokine are fibro-adipogenic progenitors (FAPs) (Heredia et al. 2013)—described below.
In normal steady-state conditions, lymphocytes are not involved in skeletal muscle regeneration, due to lack of ability of muscle fibers to induce a T-cell response as they do not express HLA class I or HLA class II antigens or co-stimulatory molecules (Karpati et al. 1988; Maffioletti et al. 2014). However, inducible expression of HLA class I and class II antigens on muscle fibers is generated in inflammatory muscle diseases. In this context, muscle cells act as nonprofessional antigen-presenting cells and attract T lymphocytes to the injury site and trigger a T-cell mediated immune response by modulation of the inflammatory cytokine milieu (Wiendl et al. 2003). Thus, the adaptive immune response is generally associated with chronic muscle dystrophies and persistence of T lymphocytes in dystrophic muscle exerts an influence on the muscle fiber environment and muscle cell function (Madaro and Bouche 2014; Spencer et al. 2001). However, the recruitment of regulatory T cells CD4+/CD25+/FOXP3+ to the injury site promotes muscle regeneration by direct contact with muscle precursor cells, as confirmed in a Rag2−/− γ-chain−/− mouse model (Castiglioni et al. 2015).
Thus, the immune response in muscular dystrophies introduced above in an experimental mdx mouse model and in clinical observations suggests that inflammation is considered as a feature of muscle repair and regulation of innate and adaptive immune responses may support muscle regeneration. This process may be supported by immunomodulatory activity of MSCs, which release anti-inflammatory factors and may create a favorable environment for muscle stem/progenitor cells for their differentiation and muscle repair.
MSCs of Bone Marrow Origin
It is well known that MSCs in the BM environment constitute a part of the bone marrow stroma and create a specific niche supporting hematopoiesis (Klimczak and Kozlowska 2016; Majumdar et al. 1998). The regenerative potential of plastic-adherent stromal cells of BM origin was described for the first time by Friedenstein et al. (1966, 1974) by introducing their ability to regenerate or support ectopic bone, stroma and hematopoietic tissues. Further studies documented that MSCs have heterogeneous nature as they are linked to the development of various mesenchymal tissues. Caplan (1991) documented that an isolated adult bone marrow population of MSCs could give rise to a variety of tissues of mesenchymal origin by differentiating along separate and distinct lineage pathways. As they are associated with the formation of mesenchymal tissues during embryonic development, these cells were called MSCs (Caplan 1991). Subsequent studies performed by Caplan and co-workers, and other research groups, documented that MSCs are not only present in the BM compartment but relative abundance of MSCs was found throughout the body, and most of them are of perivascular origin (Caplan 2008; Caplan and Correa 2011; Crisan et al. 2008; da Silva Meirelles et al. 2009; Dellavalle et al. 2011).
Since that time extensive research on MSCs of BM origin has been performed to characterize their biology and surface epitopes. Heterogenicity of MSCs which reside in the human BM is exemplified by expression of a variety of surface epitopes including integrin receptors (CD29, CD49α), cell adhesion molecules (CD44, CD54, CD58, CD62L, CD105, CD106, CD146, CD166), enzymes (CD39, CD73), growth factor receptors (CD140b, CD271, CD340, CD349) intermediate filaments (nestin, vimentin, desmin, neurofilament) and embryonic antigens (SSEA-1), but none of these molecules have been specific for bone marrow-derived MSCs (Meirelles Lda and Nardi 2009; Rasini et al. 2013). The widespread capacities of MSCs in tissue repair are also accomplished by their ability to secrete a variety of bioactive proteins as part of their local trophic and immunomodulatory properties. MSCs are able to secrete growth factors and chemokines to induce proliferation and angiogenesis. Mitogenic factors produced by MSCs such as TGF-α, TGF-β, HGF, epithelial growth factor (EGF), basic FGF (FGF-2) and IGF induce epithelial and endothelial cell divisions. Moreover, IGF, EGF and VEGF secreted by MSCs may recruit endothelial precursor cells and initiate vascularization (Chen et al. 2008; Murphy et al. 2013).
The most interesting features of the biology of MSCs are their anti-inflammatory and immunomodulatory properties. The anti-inflammatory activity of MSCs is accomplished by their ability to secrete a variety of growth factors and anti-inflammatory cytokines affecting many types of immune cells including T cells, natural killer cells, B cells, monocytes, macrophages and dendritic cells. In response to inflammatory cytokines, such as IL-1, IL-2, IL-12, TNF-α, and IFN-γ, MSCs secrete a set of immunomodulatory factors including prostaglandin 2 (PGE2), TGF-β1, HGF, stromal-derived factor (SDF)-1, NO, indoleamine 2,3-dioxygenase, IL-4, IL-6, and IL-10, thereby limiting T-cell proliferation and function, and increasing T regulatory cell development and their activity (English et al. 2009; Miyagawa et al. 2017; Murphy et al. 2013). MSCs are also able to influence T-cell differentiation, and imbalance between Th1 and Th2 subpopulations of T lymphocytes in chronically inflamed microenvironments may be reversed by MSCs. MSCs promote transition of Th1 to Th2 type of T cells, thus reducing IFN-γ production by Th1 cells and increasing secretion of the more immunotolerant cytokines IL-4 and IL-10 by Th2 cells (Kong et al. 2009). Moreover, reduction of IFN-γ activity, and MSC-derived IL-4 and IL-10 have an influence on macrophages activity in inflamed tissue by conversion of macrophages from M1 (pro-inflammatory) to M2 (anti-inflammatory) (Murphy et al. 2013).
Superiority of MSCs as a therapeutic tool is due to the low or moderate expression of HLA class I antigens and lack or low expression of HLA class II antigens, making MSCs “invisible” to the recipient immune system in an allogeneic scenario. However, a pro-inflammatory environment and IFN-γ production may increase expression of their HLA class II antigens (Le Blanc et al. 2003; Siegel et al. 2009). Immunomodulatory activity of MSCs towards dendritic cells is associated with their capacity to produce anti-inflammatory factors (PGE2, TGF-β), which inhibit activation and maturation of dendritic cells, impairing their function. Crosstalk between MSCs and dendritic cells downregulates expression of co-stimulatory molecules (CD80, CD86), thus reducing the ability of dendritic cells to stimulate T cells (Nauta et al. 2006; Ramasamy et al. 2007).
Biological properties of MSCs provide an even wider tool with regenerative potential, as previously documented by their well-predicted therapeutic application in tissue regeneration of post-infarct heart (Jung et al. 2017; van den Akker et al. 2013). The immunomodulatory potential of MSCs is not only desired in the well-known therapy of graft-versus-host disease, and serious complications in patients after hematopoietic stem cell transplantation (Copland et al. 2015; Le Blanc et al. 2004; Lin and Hogan 2011), but also proved to be beneficial in therapy for skeletal, muscular (Maeda et al. 2017) and neural regeneration (Mokarram et al. 2012).
Summarizing the above data, MSCs of BM origin may have great curative potential in vivo due to their trophic, paracrine and immunomodulatory function. MSCs isolated from BM could be used as progenitors for tissue regeneration and tissue engineering to repair or replace damaged tissue of mesenchymal origin. Activated MSCs are not only able to differentiate into a specific cell lineage but may also establish a regenerative microenvironment by immunomodulatory activity regulating the local immune response.
Skeletal Muscle Stem/Progenitor Cells
Satellite Cells and Environmental Conditions in Muscle Regeneration
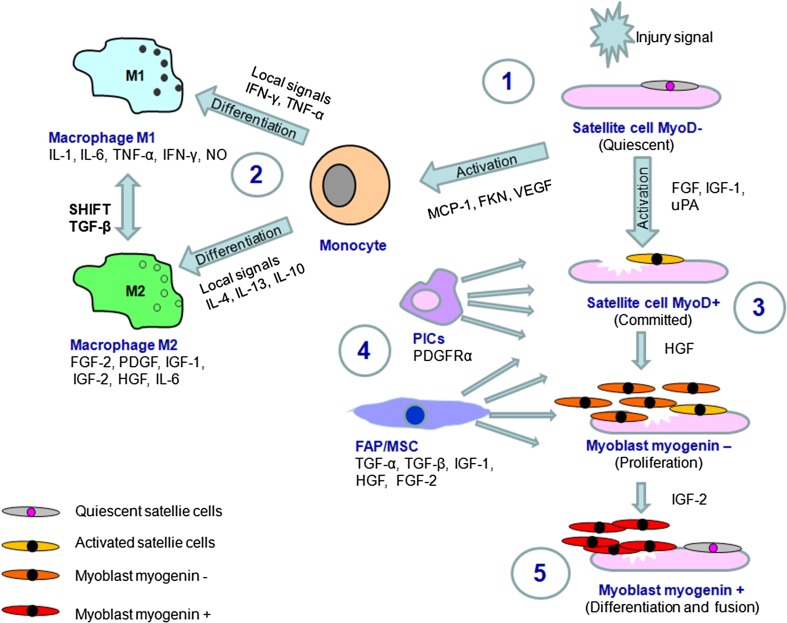
Satellite cells are a well-known muscle-resident cell population involved in muscle growth and regeneration (Boonen and Post 2008; Judson et al. 2013; Srikuea et al. 2010; Yin et al. 2013). They are located in the specific muscle stem cell niche, between muscle fiber and the basal lamina, and they are naturally quiescent until an activation signal from the local environment is delivered. SCs participate in self-renewal and myogenesis after their activation. In the most common situations of muscle injury, SC activation is initiated by a signal delivered from myofibers in stress conditions. When SCs move out from the quiescence niche, they interact with stromal components that support development of their myogenic differentiation program and promote cell survival. Injured muscle fibers produce a number of growth factors including FGF, HGF and IGF, which are involved in the proliferation and differentiation of SCs (Boonen and Post 2008; Srikuea et al. 2010). Quiescent SCs naturally express the transcriptional factor Pax7 and most of them (but not all) express Pax3 as well as Myf5, but they not express MyoD or myogenin (Lagha et al. 2008) (Fig. 1). Moreover, quiescent SCs naturally express the fibroblast growth factor receptor gene Fgfr4 and the myogenic fate determining gene Myf5, and both are controlled with Pax3/Pax7 family transcription factors (Lagha et al. 2008; Pannerec et al. 2013). An activation signal, directed under Pax3/Pax7 regulatory transcriptional control, leads to the activation and proliferation cascade and induces expression of muscle-specific transcriptional factors including MyoD, Myf5 and myogenin, which lead to proliferation of SCs, and are subsequently involved in differentiation of SCs into myoblasts (Lagha et al. 2008). Proliferating SCs are referred to as MPCs. The terminal differentiation process of MPCs is associated with downregulation of transcriptional factor Pax7 and with myogenin and MyoD expression. Damaged muscle also expresses SDF-1 (ligand for CXCR4) which reacts with the CXCR4 chemokine receptor present on the SC surface. Upregulation of SDF-1 on injured muscle facilitates SC migration into the site of injury (Yin et al. 2013). During the proliferation phase, committed SCs secrete chemokines and factors exhorting other adjacent cells to promote their survival and differentiation. The regeneration process, in addition, is supported by growth factors secreted by cells arriving from the SC niche. Activity of SCs is also regulated by their interactions with cells of myeloid origin including macrophages (Fig. 1), which constitute the stromal cell type present at the site of muscle injury (Boonen and Post 2008).
Fig. 1.
Activation and differentiation of muscle-resident satellite cells. Factors from local injury site activate quiescent satellite cells (1). Activated satellite cells and factors secreted by injured muscle attract monocyte into site of damage (2). Under environmental signal monocyte differentiates into M1 (pro-inflammatory) or M2 (supportive for muscle regeneration) macrophages (2). Activated satellite cells shift into differentiation cascade via committed MyoD+ cells (3) into myoblasts expressing myogenin. Non-satellite cells FAP/MSC and PICs secrete trophic factors into environment (4) supporting committed MyoD+ satellite cells activity. After proliferation and terminal differentiation myoblasts fuse to pre-existing injured myofibre or fuse one to another to form new myotubes, thus completing regenerative process (5). Quiescent satellite cell pool is renewed. FKN: fractalkine; MCP-1: monocyte chemotactic protein 1
Regenerative-friendly macrophages of M2a and M2c phenotype usually reach the muscle about the second day after injury (Tidball and Villalta 2010). This is also the time when the SCs activate the myogenic program, migrate from their niche and increase in numbers in the proliferation process. There are several factors that allow activated SC migration into the site of injury. The process of migration is not too easy, because quiescent SCs are localized under the basal lamina and need to migrate to the injured site through the ECM. Remodeling of the ECM is facilitated by matrix metalloproteinase (MMP)-2 and MMP-9, which can be secreted by SCs and are upregulated during muscle regeneration (Boonen and Post 2008). Inhibition of MMP activity affects migration of muscle-derived stem cells in vitro (Bellayr et al. 2013). Moreover, migration of activated SC from their niche is also regulated by syndecan-3 expression in SCs as proved in studies on a syndecan-3 null mouse model (Pisconti et al. 2016). After migration and expansion near the site of injury, activated SCs are ready to form myoblasts—cells involved directly in the formation of myotubes or fusion with damaged myofibers. While approaching the phase of differentiation, SCs strongly express insulin-like growth factor binding protein-5, which makes factors from the IGF family able to bind. Especially the factor IGF-2 is known to provide strong signaling promoting SCs to enter the phase of myogenic differentiation (Boonen and Post 2008; Goldspink 2005; Harridge 2003). Newly formed, young myoblasts at first are myogenin negative—this prevents their differentiation into myofibers or fusion with the old ones, and allows an increase of the number of myoblasts. Then, when the number of myoblasts becomes correspondingly greater, there appears myogenin leading directly into myoblast differentiation (Yin et al. 2013). Later, in the phase when myogenic cells fuse to existing damaged fibers or fuse with one another to form myofibers de novo, IGF silences the process of cell proliferation and differentiation. Also, TGF-β modulates maturation of new fibers, influencing synthesis of collagen related with tendon. Long-term muscle integrity is permitted by the ability of SCs to return to quiescence to maintain the SC pool (Fig. 1).
In muscular dystrophies, SCs actively participate in muscle regeneration; however, each cell cycle shortens the telomeres of SCs, leading to cell senescence and a rapid decrease of the SC pool (Decary et al. 2000). In the DMD environment, SCs preserve regenerative capacity, but the dystrophic niche is unfavorable for efficient muscle regeneration, as proved in an mdx mouse model (Boldrin et al. 2015). Recent studies on muscle stem cells of mdx mouse documented that dystrophin is also expressed in activated SCs and controls the determination of SC polarity and asymmetric divisions. The authors suggested that impaired regeneration of dystrophic muscle in DMD patients is aggravated by intrinsic SC dysfunction and the consequent limited regenerative lifetime of dystrophin-deficient SCs (Dumont et al. 2015).
Therefore, biological properties of SCs of dystrophic muscle are insufficient to maintain the regenerative potential in the dystrophic environment, and employment of SCs from a healthy donor may take over the biological function of damaged SCs.
Non-satellite Cells in Muscle Regeneration
SCs after activation need strong paracrine support from their niche, because without it they will not be able to survive. Data have shown that the majority (about 95%) of transplanted SCs die without anti-apoptotic signals, and that without pro-differentiation and pro-proliferative factors muscle regeneration will not be possible (Chazaud et al. 2003; Skuk and Tremblay 2000). Apart from SCs, a number of non-satellite stem cells including mesenchymal stem/stromal cells MSC, PW1+/PAX7− interstitial cells, FAPs/MSCs, muscle SP cells and muscle resident pericytes are active and participate in the muscle regeneration process (Boppart et al. 2013). The myogenic potential of non-satellite progenitor cells was recognized in a cell population located in the muscle interstitium in the neonate (Mitchell et al. 2010). These cells revealed multilineage potential and belong to mesenchymal progenitor/stromal cells, as confirmed by the wide range of gene expression common to MSCs (Pannerec et al. 2013).
Fibrocyte/adipocyte progenitors (FAPs) are bipotent cells able to differentiate into fibroblasts and adipocytes in vitro—hence the term fibrocyte/adipocyte. Also, there is another term used to describe them, FAPs/MSCs, because in fact they are MPCs (mesenchymal progenitor cells)—bipotent progenitors obtained through one of the MSC maturation pathways (Faralli and Dilworth 2014; Joe et al. 2010). Under steady-state conditions, FAPs do not contribute directly to regeneration of muscle fibers. The direction of FAP differentiation can be regulated by the microenvironment, especially by upregulation of IL-6 and IGF-1, which may have a great pro-differentiate influence on SCs and myoblasts as documented in studies on a mouse model (Joe et al. 2010). The phenotype of FAPs, which may acquire a more myogenic than adipogenic fate, is recognized as Sca+/integrin−, but FAPs definitely are not involved in muscle regeneration by direct differentiation and forming myofibers (Joe et al. 2010; Judson et al. 2013). In the case of muscle injury, eosinophils infiltrating the injured area secrete IL-4 and IL-13, which activate FAPs and inhibit FAPs adipogenic conversion after muscle injury (Heredia et al. 2013). Activated FAPs have the ability to form a fibrotic muscle scaffold supportive in the muscle regeneration process (Faralli and Dilworth 2014; Joe et al. 2010).
However, the situation is not clear when the compensatory response of degenerating muscles is associated with formation of fibrotic scars and excessive fat infiltration (Serrano et al. 2011). The study revealed that FAPs in mdx mice might actually lead to fibrosis or fat deposition in muscle; furthermore they may contribute to diminution of myofiber contractility, retarding its metabolism. Paradoxically, FAPs can be involved in treatment of dystrophy as well (Mozzetta et al. 2013). The study revealed that treating FAPs of young mdx mice with trichostatin A (TSA), a member of HDACi (histone deacetylase inhibitors), can block their fibrotic and adipogenic differentiation, and promote myogenic fate, by changing the organization of chromatin structure (Saccone et al. 2014). RNA analysis showed a decrease of adipogenic genes and upregulation of myogenic genes in FAPs after TSA treatment (Mozzetta et al. 2013; Saccone et al. 2014). This pharmacological influence on FAPs can be applied for regeneration of dystrophic muscles and may prevent the deleterious effect associated with fibro/adipogenic changes of dystrophic muscles.
PW1+/PAX7− interstitial cells Interstitial stem cells, positive for stress mediator PW1 expression and negative for transcriptional factor PAX7, called PICs, constitute an important muscle-resident stem cell population involved in perinatal skeletal muscle growth and during the adult muscle regeneration process (Mitchell et al. 2010).
These muscle interstitial cells are characterize by the expression of the muscle-specific progenitor marker CD34, and they are negative for endothelial markers, as proved by CD31 negative staining (Bosnakovski et al. 2008). Studies have shown that these cells contribute to regenerative myogenesis and SC generation, as documented in vitro when co-cultured with myoblasts or in vivo when transplanted into the regenerating muscle environment (Mitchell et al. 2010). PW1+ interstitial cells express a variety of genes common to MSCs (Oct3/4, Sox2, Nanog) (Cottle et al. 2017), and a subset of PICs expressing PDGF receptor (PDGFR)α overlap the cell surface expression and function of FAPs (Pannerec et al. 2013).
Side population cells These are muscle-resident progenitors located in the skeletal muscle interstitium next to blood vessels, which makes them distinguishable from bone marrow-derived SP cells and from SCs. They are a heterogeneous population of muscle-resident progenitors which contribute to both muscle and vascular regeneration (Asakura et al. 2002; Majka et al. 2003). Myogenic differentiation was induced in co-culture with primary myoblasts or through the induced expression of PAX7 or MyoD (Asakura et al. 2002). The majority of muscle SP cells express the endothelial marker CD31+, making them an attractive candidate to induce vasculogenesis, necessary for proper muscle regeneration. However, a fraction of muscle-origin SP cells, CD31−/CD45−, isolated from injured muscle, also express proangiogenic factors including angiopoietin-1 and VEGF, and factors associated with mesodermal/mesenchymal nature of cells, e.g. PDGFRα. Thus, studies on muscle-resident SP cells suggest that SP cells within the muscle constitute a sub-fraction of mesenchymal-like stem cells and/or pericytes, and both directly and indirectly contribute to muscle repair.
Pericytes Muscle resident pericytes are the next muscle progenitors of mesenchymal origin with a cell marker signature identical to MSCs (Birbrair et al. 2014; Caplan and Correa 2011; Crisan et al. 2008; Dellavalle et al. 2011; Traktuev et al. 2008). In fact, most pericytes are a quiescent type of MSCs residing on the surface of blood vessels and appear once in every 100 endothelial cells. Multipotential character of pericytes, sorted by CD146+, CD34−, CD45− and CD56− expression, was confirmed by their osteogenic, chondrogenic, adipogenic and myogenic potential (Caplan 2007). However, pericytes are heterogeneous, and the phenotype and biological activity of pericytes depend on their tissue localization. Differentiation potential of pericytes might be induced by the environment of cells surrounding them, so while residing on the surface of blood vessels penetrating (for example) smooth muscle, pericytes will gain inner potential to form smooth muscle, and preserve a lot of attributes characteristic for all pericytes independent of their tissue localization (Birbrair et al. 2014; Cappellari and Cossu 2013). Studies by Birbrair et al (2013), performed on double transgenic mice, documented that in the skeletal muscle there are two types of pericytes, type 1 (Nestin-GFP−/NG2-DsRed+) and type 2 (Nestin-GFP+/NG2-DsRed+), and only the latter is a marker allowing cells to enter the myogenic differentiation process. Moreover, type 2 vessel associated pericytes express the transcriptional factor PAX7 and in appropriate conditions can accomplish satellite cell position and function. In contrast, type 1 pericytes in skeletal muscle express PDGFRα, which is the major contributor to ectopic adipocyte formation in muscular dystrophies and in older adults (Birbrair et al. 2014). An in vitro study performed by Crisan et al. (2008) revealed that pericytes isolated from human tissues might have even greater myogenic potential than myoblasts.
However, in addition to their direct contribution to muscle tissue regeneration, pericytes have another very special function. When new blood vessel formation is necessary, for muscle regeneration or in ischemic conditions, pericytes play a significant role. Neoangiogenesis takes place in response to trophic factors such as VEGF, FGF-2, PDGF and PIGF (placental growth factor) secreted by myofibers, fibroblasts and inflammatory cells, which are essential for muscle survival in such conditions.
Unfortunately, pericytes, similarly to FAPs, are also suspected to cause fibrosis and fat deposition in dystrophic muscles and both of them express the PDGFRα receptor (Birbrair et al. 2014; Olson and Soriano 2009; Uezumi et al. 2011). Naturally, in normal conditions PDGFRα is an essential factor in many processes, including cell development and angiogenesis, but some studies have revealed that prolonged activation of this receptor, induced by mutation, may actually cause multiple organ fibrosis in the adult mouse body (Olson and Soriano 2009). The pathological contribution of mesenchymal progenitors, FAPs and pericytes, may be induced by TGF-β—a factor which stimulates expression of collagen I and III type and connective tissue growth factor (Uezumi et al. 2011).
Summarizing activity of muscle-resident non-satellite cells in muscle regeneration, it is important to note that they play dual roles: in healthy muscle they have an influence on SC differentiation and a significant function in myogenesis, but in unfavorable conditions, such as in muscular dystrophies, they contribute to fibrosis and adipose tissue accumulation. Therefore, manipulation of biological activity of non-satellite cells may support therapeutic strategies to treat muscular dystrophies.
Combined Therapy with MSCs of BM Origin and SCs of Skeletal Muscle Origin for Potential Application for Muscular Dystrophy Treatment
Cellular therapies in muscular dystrophies are not a new idea. Studies on progression of muscular dystrophy documented that rapid occurrence of the dystrophic phenotype in the dystrophin/utrophin double knock-out mice model was associated with a rapid depletion of the functional MPCs. The authors suggests that preventing the depletion of the MPC pools could be a novel approach to delay the histopathologic feature associated with the skeletal muscles of DMD patients (Lu et al. 2014). Several studies have been performed to introduce different cellular therapies in muscular dystrophies; however, the rate of success has been limited (reviewed by Cossu and Sampaolesi 2007; Farini et al. 2009; Meng et al. 2011; Price et al. 2007). The cells most often applied for cellular therapies in muscular dystrophies are myoblasts, muscle precursor cells or stem cells with ability to differentiate into muscle cells. Early clinical studies, performed over 20 years ago, with adoptive transfer of myoblasts, isolated from skeletal muscle of healthy donors, seemed to show a promising strategy to restore skeletal muscle function; however, limited positive results were reported (Karpati et al. 1993; Law et al. 1993; Mendell et al. 1995; Miller et al. 1997; Skuk et al. 2004). These poor results using myoblast transfer may be explained by immunosuppression, an inadequate number of transplanted cells and limited distribution of transplanted cells, as myoblasts have limited migratory capability and limited proliferative potential (Mouly et al. 2005; Skuk et al. 2004). Moreover, allogeneic myoblast delivery may induce a strong immune response, in consequence leading to allograft rejection.
Promising results of cell-based therapy in DMD treatment were reported using the “high density injection” protocol for intramuscular cell delivery of muscle precursor cells from allogenic (sibling) healthy donors under a tacrolimus regimen (Skuk et al. 2006, 2007). Restoration of donor-derived dystrophin expression was observed in 27.5% of myofibres 1 month after cell transplantation, and 34.5% 18 months after intramuscular cell delivery; however, a significant improvement in strength was not observed. Success in local therapeutic cell delivery via intra-femoral arterial perfusion of skeletal muscle was also reported in studies using human induced pluripotent stem cells of myogenic origin in an immunodeficient mouse model NSG-mdx(4cv) for DMD (Matthias et al. 2015). Four weeks after intra-arterial cell delivery human cells were detected in the interstitial space of myofibers within the perfused muscle, and some of them fused with the recipient myofibers and expressed dystrophin. A clinical study on intra-arterial HLA-matched donor mesoangioblast transplantation in five DMD patients documented the presence of a low level of donor DNA in muscle biopsies in 4/5 patients and donor-derived dystrophin in one patient. A study showed that intra-arterial cell delivery is a feasible and relatively safe procedure, but functional improvement was not observed (Cossu et al. 2015). On the other hand, an experimental study showed that specific muscle-resident human dystrophin-positive mesoangioblasts from healthy donors co-cultured with dystrophin-negative myoblasts from DMD patients in vitro in a microengineered model of DMD resulted in cell fusion and functional differentiation of myotubes and dystrophin expression (Serena et al. 2016). Therapeutic potential of muscle-derived stem/progenitor cells of human origin was also tested on athymic mouse and rat models. Highly myogenic (CD34−/CD45−/CD29+) fraction revealed an active contribution to muscle fiber regeneration whereas multipotent stem cell (CD34+/CD45−) revealed multiple differentiation potential as confirmed by engraftment to the interstitium and differentiation into Schwann cells, perineurial/endoneurial cells, vascular endothelial cells and pericytes. Moreover, co-transplantation of both populations of cells, separately expanded, showed favorable results in skeletal muscle regeneration. Therefore, synergistic effect of co-transplantation of highly myogenic and multipotent stem cells (both of human muscle-origin) may be promising tool for muscle regeneration in autologous conditions in non-genetic muscular injuries (Tamaki et al. 2015).
The recognition of stem cell-myogenic precursors such as SCs, muscle-derived stem cells, SP cells, BM-derived stem cells, mesoangioblasts, muscle-derived CD133+ stem cells and pericytes seems to be a promising strategy for their application to overcome difficulties related to more differentiated myoblasts (Farini et al. 2009). Stem cell-myogenic precursors are more primitive than myoblasts and are able to proliferate and could be distributed through the blood vessels to the whole body musculature to treat severely affected patients (Farini et al. 2009; Peault et al. 2007). Moreover, some populations of myogenic precursors such as CD133+ cells or pericytes may be isolated not only from skeletal muscle but also from bone marrow and blood. Very recent studies on human myogenic cells introduced a population of human myogenic reserve cells (about 38.0%) which are not able to fuse in two-day culture in the differentiation medium, and are distinct from those which differentiate into myoblasts and fuse (about 62.0%). The human myogenic reserve cells generated in vitro significantly augmented the number of myogenic progenitor cells expressing PAX7, as compared to human myoblasts, after intramuscular transplantation in immunodeficient mice (Laumonier et al. 2017).
Based on the current knowledge and our own experience on the biology of MSCs of BM origin and stem/progenitor cells of skeletal muscle origin (Klimczak et al. 2016) we propose combined cellular therapy with MSCs of BM origin and muscle stem/progenitor cells for treatment of patients suffering from DMD. We hypothesize that both cell populations will fuse with damaged muscle cells and repopulate the muscle with dystrophin, improving muscle function (Fig. 2). Apart from stem/progenitor cells of muscle origin, also MSCs of BM origin have been shown to be able to participate in myogenesis as they are able to differentiate into mesodermal cells, including myoblasts (Bhagavati and Xu 2004; Fairclough et al. 2011). In addition, MSCs have pro-angiogenic potential and they might participate in blood vessel formation by direct differentiation into endothelial cells and/or as supporting niche cells for vascular (re-)generation, which is critical for proper muscle function (Lin and Lue 2013; Watt et al. 2013).
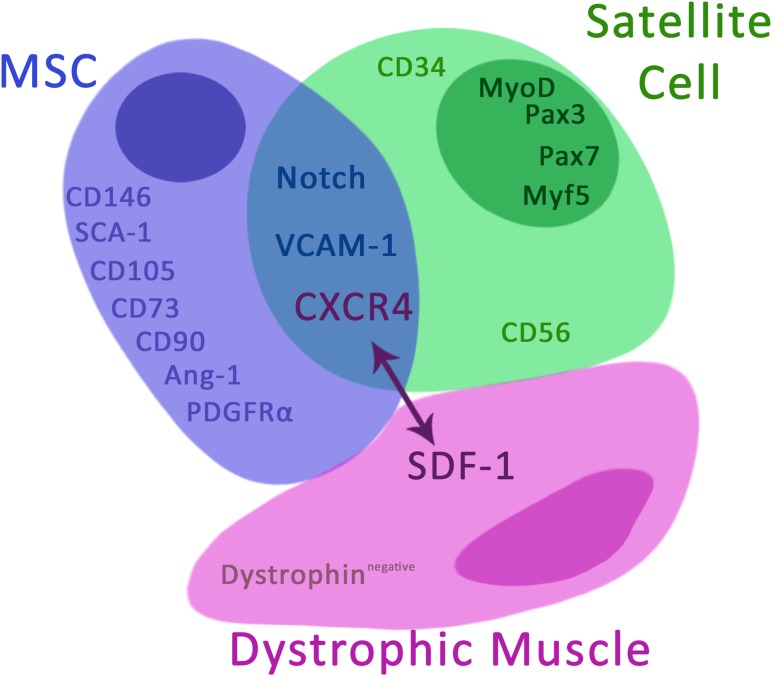
Fig. 2.
Hypothetical mechanism of dystrophic muscle regeneration by combined cellular therapy with MSC of bone marrow origin and muscle stem/progenitor cells. The local MSCs delivery into dystrophic muscles will create the microenvironment supporting homing of myogenic precursors and enhance tropism of stem cells of myogenic origin with the CXCR4+ expression to the injured muscle expressing SDF-1. Collaborative activities of MSC and satellite cells enhance regenerative potential of stem/progenitor cells of muscle-origin by direct contact and by secretion of trophic factors which influence on muscle stem/progenitor cells proliferation and myogenic differentiation
Moreover, immunosuppressive properties of MSCs may inhibit the inflammatory process at the site of stem cell delivery. Muscle degeneration in DMD is associated with chronic inflammation associated with active production of TNF-α by infiltrating M1 macrophages (Ichim et al. 2010). MSCs have the potential to convert inflammatory M1 type of macrophages (pro-inflammatory, anti-angiogenic and inhibitors of tissue growth) to M2 phenotype (anti-inflammatory, pro-remodeling and tissue healing) by secretion of IL-4 and IL-10, cytokines which support a shift from M1 to M2 type. This effect is required for skeletal muscle and neural healing and regeneration (Murphy et al. 2013; Rigamonti et al. 2014).
Muscle-derived stem/progenitor cells are heterogeneous population of muscle precursors with different phenotype depending on stage of differentiation. Most stem cell-myogenic precursors (about 70%), such as SP cells and SCs, are CD34+ cells which express VLA-4 (ligand for VCAM-1), and CXCR4, a chemokine receptor specific for SDF-1 (also named CXCL12) (Ichim et al. 2010; Perez et al. 2009). SDF-1 is upregulated in dystrophic muscle, whereas VCAM-1 is upregulated on the vessel endothelium in the dystrophic muscle environment. Experimental studies confirmed that intra-arterial delivery of myogenic precursors expressing CXCR4+ enhances their ability to extravagate into dystrophic muscle (Gavina et al. 2006). However, intravascular infusion of a large number of non-hematopoietic stem/progenitor cells of muscle origin will be risky for patients. Some studies suggest that a more therapeutically relevant method would be administration of MSCs directly into dystrophic muscle where they may contribute to formation of new muscle fibers and muscle neovascularization. In the dystrophin-deficient mdx mouse model, transplantation of primary human MSCs genetically modified with Pax3 into tibia muscle of mdx deficient mice resulted in donor-origin myofiber formation, but functional recovery was not achieved. The authors emphasized the lack of evidence that human MSC-Pax3 contributes to the satellite cell compartment in vivo. Fusion of donor cells with host myofibers rather than reprogramming of MSCs into myogenic progenitors may contribute to dystrophin-positive myofiber formation. Based on this observation, the authors suggest that multiple cell injections might be required to trigger functional recovery of dystrophic muscle (Gang et al. 2009). This proposal is in line with our hypothesis that MSCs alone or muscle progenitors alone are not able to restore dystrophin-deficient muscle function and local cell delivery may be a more effective method. Local MSC delivery into dystrophic muscles will create a microenvironment supporting homing of myogenic precursors and enhance tropism of stem cells of myogenic origin with CXCR4+ expression to the injured muscle expressing SDF-1 (Fig. 2). Studies have shown that signaling through CXCR4/SDF-1 interaction stimulates satellite cell migration (Ratajczak et al. 2003; Sherwood et al. 2004). However, in Sherwood and co-workers’ studies, functional heterogenicity between muscle-resident progenitor cells was introduced in a mouse model. Bone marrow-derived myofiber-associated cells do not form myogenic colonies when cultured alone, but some of them are able to generate myoblasts and myotubes when co-cultured with myogenic cells. They are non-hematopoietic cells and the authors suggest that these cells are MSCs of BM origin (Sherwood et al. 2004). These studies again confirmed our hypothesis that MSCs of BM origin can contribute to myogenic recovery when co-transplanted with muscle-resident progenitor cells.
Our hypothesis on the regenerative potential of MSCs of BM origin in muscular dystrophies is also supported by very recent studies by Maeda et al. (2017). The authors documented that transplantation of MSCs of BM origin into peritoneal cavities of a mdx mouse model strongly suppressed dystrophic pathology in diaphragms of mdx deficient mice, which resulted in significant lifespan extension.
Moreover, MSC plasticity will cause that in the vicinity of injured muscle these cells will differentiate into myoblasts producing dystrophin, which may enhance the regenerative effect on dystrophic muscles. This is supported by studies performed ex vivo on rat origin cells documenting that myogenic differentiation of MSCs is facilitated by co-culture with primary myoblasts stimulated by basic FGF and dexamethasone (Beier et al. 2011). Subsequent studies by the same research group in a rat model showed that myogenic differentiation of MSCs of BM origin upon mono- and co-cultivation with myoblasts in the presence of HGF and/or IGF-1 was successful on a biocompatible 3D nanofiber scaffold. However, HGF and/or IGF-1 stimulation was not essential for successful myogenic differentiation (Witt et al. 2017).
The studies described above clearly suggest that in contrast to transplantation of only myoblasts with limited migratory potential, simultaneous co-transplantation of MSCs of BM origin and myogenic stem/progenitor cells of skeletal muscle origin seems to be the most effective method for cellular therapies because they possess the ability to proliferate and expand, to fuse with dystrophic muscular cells, and to migrate to affected muscle.
These clinical and experimental results demonstrate that it is important to find a method to reconstruct functional muscles, severely affected by fat and fibrotic tissue, in order to restore strength either by local application of specific stem cell-myogenic precursors or by systemic cell delivery. Thus, the development of cell-based therapies for muscular dystrophies by the delivery of two populations of normal stem/progenitor cells (MSCs of BM origin and SCs with myogenic potential isolated from healthy skeletal muscle) would be a promising tool to treat muscular dystrophies. Biological properties of MSCs of BM origin and stem/progenitor cells of skeletal muscle origin justify combined therapy by using both cell populations because to date there is no alternative method to treat patients suffering from DMD.
Concluding Remarks and Future Directions
Therapeutic approaches for DMD have extensively been developed in recent years. Unfortunately, most of the strategies such as gene therapy to replace the mutated gene or to repair the endogenous gene [exon skipping or skipping premature termination (PTC124)] are effective only for specific mutations or for nonsense mutations and cannot be applied to all DMD patients (Cossu and Sampaolesi 2007; Farini et al. 2009). Recent studies, using muscle-derived stem cells of DMD patients transduced with dystrophin constructs and transplanted into an immunodeficient mouse model of DMD, documented dystrophin production functional in vivo (Meng et al. 2016). After extensive experimental procedures and clinical trials, researchers proposed that the most promising strategies for the treatment of muscular dystrophies will be a combination of different approaches including stem cell therapy in combination with gene therapy [reviewed by (Crist 2017; Pini et al. 2017)].
The therapeutic effect of individually transplanted MSCs of BM origin or stem/progenitor cells of muscle origin is limited due to the complexity of muscular dystrophies and biological properties of stem/progenitor cells. Muscle-origin stem/progenitor cells are currently not applicable to treat muscular dystrophies due to difficulties to keep stemness ex vivo. We propose therefore combined therapy including two populations of stem/progenitor cells of bone marrow and muscle origin by local intramuscular co-transplantation. MSCs of BM origin may create a more favorable environment for muscle progenitor cells in dystrophic muscle by secretion of immunosuppressive cytokines and enhance the regenerative potential of stem/progenitor cells of muscle origin by direct contact, and by secretion of trophic factors which control muscle stem/progenitor cell proliferation and myogenic differentiation. Collaborative activities of MSCs and muscle stem/progenitor cells may influence further the changes in the dystrophic microenvironment. These changes concern different molecules, cells and structures that constitute the dynamic niche supporting the regenerative potential of stem/progenitor cells. Moreover, immunosuppressive properties of MSCs may reduce alloreactivity of muscle stem/progenitor cells in allogenic conditions.
Thus, characterization of a stem cell population effective for muscle regeneration, and timing, dosage and route of delivery may hold potential for treatment of genetic-related muscular dystrophies—but this remains a distant goal.
Acknowledgements
This work was supported by the National Science Center Grant N N407 121940.
Abbreviations
- FGF
Fibroblast growth factor
- DMD
Duchenne muscular dystrophy
- ECM
Extracellular matrix
- EGF
Epithelial growth factor
- FAPs/MSCs
Fibro/adipogenic progenitors/mesenchymal stem cells
- HGF
Hepatocyte growth factor
- IGF
Insulin growth factor
- MMP
Matrix metalloproteinase
- MSC
Mesenchymal stem cell
- MPC
Myogenic precursor cell
- NO
Nitric oxide
- PGE-2
Prostaglandin 2
- PIC
PW1+/PAX7− interstitial cell
- PDGFR
Platelet-derived growth factor receptor
- SC
Satellite cell
- SP
Side population
- SDF-1
Stromal-derived factor-1
- uPA/uPAR
Urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor
- VEGF
Vascular endothelial growth factor
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, et al. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JP, Bitto FF, Lange C, et al. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol Int. 2011;35:397–406. doi: 10.1042/CBI20100417. [DOI] [PubMed] [Google Scholar]
- Bellayr I, Holden K, Mu X, et al. Matrix metalloproteinase inhibition negatively affects muscle stem cell behavior. Int J Clin Exp Pathol. 2013;6:124–141. [PMC free article] [PubMed] [Google Scholar]
- Bhagavati S, Xu W. Isolation and enrichment of skeletal muscle progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 2004;318:119–124. doi: 10.1016/j.bbrc.2004.03.192. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, et al. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, et al. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 2014;6:245. doi: 10.3389/fnagi.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L, Zammit PS, Morgan JE. Satellite cells from dystrophic muscle retain regenerative capacity. Stem Cell Res. 2015;14:20–29. doi: 10.1016/j.scr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonen KJ, Post MJ. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue Eng Part B Rev. 2008;14:419–431. doi: 10.1089/ten.teb.2008.0045. [DOI] [PubMed] [Google Scholar]
- Boppart MD, De Lisio M, Zou K, et al. Defining a role for non-satellite stem cells in the regulation of muscle repair following exercise. Front Physiol. 2013;4:310. doi: 10.3389/fphys.2013.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Li W, et al. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells. 2008;26:3194–3204. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Spencer MJ, Nakamura G, et al. Eosinophilia of dystrophin-deficient muscle is promoted by perforin-mediated cytotoxicity by T cell effectors. Am J Pathol. 2000;156:1789–1796. doi: 10.1016/S0002-9440(10)65050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellari O, Cossu G. Pericytes in development and pathology of skeletal muscle. Circ Res. 2013;113:341–347. doi: 10.1161/CIRCRESAHA.113.300203. [DOI] [PubMed] [Google Scholar]
- Castiglioni A, Corna G, Rigamonti E, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One. 2015;10:e0128094. doi: 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland IB, Qayed M, Garcia MA, et al. Bone marrow mesenchymal stromal cells from patients with acute and chronic graft-versus-host disease deploy normal phenotype, differentiation plasticity, and immune-suppressive activity. Biol Blood Marrow Transplant. 2015;21:934–940. doi: 10.1016/j.bbmt.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13:520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Cossu G, Previtali SC, Napolitano S, et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med. 2015;7:1513–1528. doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle BJ, Lewis FC, Shone V, et al. Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Res Ther. 2017;8:158. doi: 10.1186/s13287-017-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Crist C. Emerging new tools to study and treat muscle pathologies: genetics and molecular mechanisms underlying skeletal muscle development, regeneration, and disease. J Pathol. 2017;241:264–272. doi: 10.1002/path.4830. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Sand TT, Harman RJ, et al. MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A. 2009;15:221–229. doi: 10.1089/ten.tea.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary S, Hamida CB, Mouly V, et al. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Deng B, Wehling-Henricks M, Villalta SA, et al. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, von Maltzahn J, et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med. 2015;21:1455–1463. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(high) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough RJ, Bareja A, Davies KE. Progress in therapy for Duchenne muscular dystrophy. Exp Physiol. 2011;96:1101–1113. doi: 10.1113/expphysiol.2010.053025. [DOI] [PubMed] [Google Scholar]
- Faralli H, Dilworth FJ. Dystrophic muscle environment induces changes in cell plasticity. Genes Dev. 2014;28:809–811. doi: 10.1101/gad.241182.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farini A, Razini P, Erratico S, et al. Cell based therapy for Duchenne muscular dystrophy. J Cell Physiol. 2009;221:526–534. doi: 10.1002/jcp.21895. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Darabi R, Bosnakovski D, et al. Engraftment of mesenchymal stem cells into dystrophin-deficient mice is not accompanied by functional recovery. Exp Cell Res. 2009;315:2624–2636. doi: 10.1016/j.yexcr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Gavina M, Belicchi M, Rossi B, et al. VCAM-1 expression on dystrophic muscle vessels has a critical role in the recruitment of human blood-derived CD133+ stem cells after intra-arterial transplantation. Blood. 2006;108:2857–2866. doi: 10.1182/blood-2006-04-018564. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology. 2005;20:232–238. doi: 10.1152/physiol.00004.2005. [DOI] [PubMed] [Google Scholar]
- Harridge SD. Ageing and local growth factors in muscle. Scand J Med Sci Sports. 2003;13:34–39. doi: 10.1034/j.1600-0838.2003.20235.x. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Fischbeck KH, Brown RH, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne’s or Becker’s muscular dystrophy. N Engl J Med. 1988;318:1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Ichim TE, Alexandrescu DT, Solano F, et al. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010;260:75–82. doi: 10.1016/j.cellimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RN, Zhang RH, Rossi FM. Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs? FEBS J. 2013;280:4100–4108. doi: 10.1111/febs.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung N, Rupp H, Koczulla AR, et al. Myocardial homing of mesenchymal stem cells following intrapericardial application and amplification by inflammation—an experimental pilot study. Can J Physiol Pharmacol. 2017;95:1064–1066. doi: 10.1139/cjpp-2016-0373. [DOI] [PubMed] [Google Scholar]
- Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol. 1988;23:64–72. doi: 10.1002/ana.410230111. [DOI] [PubMed] [Google Scholar]
- Karpati G, Ajdukovic D, Arnold D, et al. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993;34:8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- Klimczak A, Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int. 2016;2016:4285215. doi: 10.1155/2016/4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimczak A, Kozlowska U, Jurek T, et al. Phenotypical differences of mesenchymal stromal/stem cells isolated from human bone marrow and skeletal muscle. Bone Marrow Transplant Suppl. 2016;51(1):128. [Google Scholar]
- Kong QF, Sun B, Bai SS, et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol. 2009;207:83–91. doi: 10.1016/j.jneuroim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lagha M, Sato T, Bajard L, et al. Regulation of skeletal muscle stem cell behavior by Pax3 and Pax7. Cold Spring Harb Symp Quant Biol. 2008;73:307–315. doi: 10.1101/sqb.2008.73.006. [DOI] [PubMed] [Google Scholar]
- Laumonier T, Bermont F, Hoffmeyer P, et al. Human myogenic reserve cells are quiescent stem cells that contribute to muscle regeneration after intramuscular transplantation in immunodeficient mice. Sci Rep. 2017;7:3462. doi: 10.1038/s41598-017-03703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PK, Goodwin TG, Fang Q, et al. Cell transplantation as an experimental treatment for Duchenne muscular dystrophy. Cell Transplant. 1993;2:485–505. doi: 10.1177/096368979300200607. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Lin Y, Hogan WJ. Clinical application of mesenchymal stem cells in the treatment and prevention of graft-versus-host disease. Adv Hematol. 2011;2011:427863. doi: 10.1155/2011/427863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Lue TF. Defining vascular stem cells. Stem Cells Dev. 2013;22:1018–1026. doi: 10.1089/scd.2012.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Poddar M, Tang Y, et al. Rapid depletion of muscle progenitor cells in dystrophic mdx/utrophin/ mice. Hum Mol Genet. 2014;23:4786–4800. doi: 10.1093/hmg/ddu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaro L, Bouche M. From innate to adaptive immune response in muscular dystrophies and skeletal muscle regeneration: the role of lymphocytes. Biomed Res Int. 2014;2014:438675. doi: 10.1155/2014/438675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Yonemochi Y, Nakajyo Y, et al. CXCL12 and osteopontin from bone marrow-derived mesenchymal stromal cells improve muscle regeneration. Sci Rep. 2017;7:3305. doi: 10.1038/s41598-017-02928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti SM, Noviello M, English K, et al. Stem cell transplantation for muscular dystrophy: the challenge of immune response. Biomed Res Int. 2014;2014:964010. doi: 10.1155/2014/964010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka SM, Jackson KA, Kienstra KA, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, et al. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Campbell KP. Deficiency of dystrophin-associated proteins: a common mechanism leading to muscle cell necrosis in severe childhood muscular dystrophies. Neuromuscul Disord. 1993;3:109–118. doi: 10.1016/0960-8966(93)90002-2. [DOI] [PubMed] [Google Scholar]
- Matthias N, Hunt SD, Wu J, et al. Skeletal muscle perfusion and stem cell delivery in muscle disorders using intra-femoral artery canulation in mice. Exp Cell Res. 2015;339:103–111. doi: 10.1016/j.yexcr.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281–4298. doi: 10.2741/3528. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Meng J, Muntoni F, Morgan JE. Stem cells to treat muscular dystrophies—where are we? Neuromuscul Disord. 2011;21:4–12. doi: 10.1016/j.nmd.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Meng J, Counsell JR, Reza M, et al. Autologous skeletal muscle derived cells expressing a novel functional dystrophin provide a potential therapy for Duchenne muscular dystrophy. Sci Rep. 2016;6:19750. doi: 10.1038/srep19750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RG, Sharma KR, Pavlath GK, et al. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Pannerec A, Cadot B, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- Miyagawa I, Nakayamada S, Nakano K, et al. Induction of regulatory T cells and its regulation with insulin-like growth factor/insulin-like growth factor binding protein-4 by human mesenchymal stem cells. J Immunol. 2017;199:1616–1625. doi: 10.4049/jimmunol.1600230. [DOI] [PubMed] [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, et al. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Perie S, et al. Myoblast transfer therapy: is there any light at the end of the tunnel? Acta Myol. 2005;24:128–133. [PubMed] [Google Scholar]
- Mozzetta C, Consalvi S, Saccone V, et al. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 2013;5:626–639. doi: 10.1002/emmm.201202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannerec A, Formicola L, Besson V, et al. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140:2879–2891. doi: 10.1242/dev.089326. [DOI] [PubMed] [Google Scholar]
- Patsalos A, Pap A, Varga T, et al. In situ macrophage phenotypic transition is affected by altered cellular composition prior to acute sterile muscle injury. J Physiol. 2017;595:5815–5842. doi: 10.1113/JP274361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Perez AL, Bachrach E, Illigens BM, et al. CXCR4 enhances engraftment of muscle progenitor cells. Muscle Nerve. 2009;40:562–572. doi: 10.1002/mus.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini V, Morgan JE, Muntoni F, et al. Genome editing and muscle stem cells as a therapeutic tool for muscular dystrophies. Curr Stem Cell Rep. 2017;3:137–148. doi: 10.1007/s40778-017-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisconti A, Banks GB, Babaeijandaghi F, et al. Loss of niche-satellite cell interactions in syndecan-3 null mice alters muscle progenitor cell homeostasis improving muscle regeneration. Skelet Muscle. 2016;6:34. doi: 10.1186/s13395-016-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FD, Kuroda K, Rudnicki MA. Stem cell based therapies to treat muscular dystrophy. Biochim Biophys Acta. 2007;1772:272–283. doi: 10.1016/j.bbadis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Qiao C, Li J, Zhu T, et al. Amelioration of laminin-alpha2-deficient congenital muscular dystrophy by somatic gene transfer of miniagrin. Proc Natl Acad Sci USA. 2005;102:11999–12004. doi: 10.1073/pnas.0502137102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Fazekasova H, Lam EW, et al. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- Rasini V, Dominici M, Kluba T, et al. Mesenchymal stromal/stem cells markers in the human bone marrow. Cytotherapy. 2013;15:292–306. doi: 10.1016/j.jcyt.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Majka M, Kucia M, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- Rigamonti E, Zordan P, Sciorati C, et al. Macrophage plasticity in skeletal muscle repair. Biomed Res Int. 2014;2014:560629. doi: 10.1155/2014/560629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone V, Consalvi S, Giordani L, et al. HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles. Genes Dev. 2014;28:841–857. doi: 10.1101/gad.234468.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saclier M, Yacoub-Youssef H, Mackey AL, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- Serena E, Zatti S, Zoso A, et al. Skeletal muscle differentiation on a chip shows human donor mesoangioblasts’ efficiency in restoring dystrophin in a duchenne muscular dystrophy model. Stem Cells Transl Med. 2016;5:1676–1683. doi: 10.5966/sctm.2015-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Mann CJ, Vidal B, et al. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol. 2011;96:167–201. doi: 10.1016/B978-0-12-385940-2.00007-3. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87(9 Suppl):S45–S49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- Skuk D, Tremblay JP. Progress in myoblast transplantation: a potential treatment of dystrophies. Microsc Res Tech. 2000;48:213–222. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<213::AID-JEMT9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulet M, et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther. 2004;9:475–482. doi: 10.1016/j.ymthe.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, et al. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Montecino-Rodriguez E, Dorshkind K, et al. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- Srikuea R, Pholpramool C, Kitiyanant Y, et al. Satellite cell activity in muscle regeneration after contusion in rats. Clin Exp Pharmacol Physiol. 2010;37:1078–1086. doi: 10.1111/j.1440-1681.2010.05439.x. [DOI] [PubMed] [Google Scholar]
- Tamaki T, Uchiyama Y, Hirata M, et al. Therapeutic isolation and expansion of human skeletal muscle-derived stem cells for the use of muscle-nerve-blood vessel reconstitution. Front Physiol. 2015;6:165. doi: 10.3389/fphys.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin J, Temmerman L, Sampson RD, et al. Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther. 2015;23:1189–1200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Ito T, Morikawa D, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(Pt 21):3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- van den Akker F, de Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: immunomodulatory properties and the influence of Toll-like receptors. Mediat Inflamm. 2013;2013:181020. doi: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T, Mounier R, Patsalos A, et al. Macrophage PPARgamma, a lipid activated transcription factor controls the growth factor GDF3 and skeletal muscle regeneration. Immunity. 2016;45:1038–1051. doi: 10.1016/j.immuni.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta SA, Nguyen HX, Deng B, et al. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Melton DW, Porter L, et al. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol. 2014;184:1167–1184. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt SM, Gullo F, van der Garde M, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25–53. doi: 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling-Henricks M, Sokolow S, Lee JJ, et al. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum Mol Genet. 2008;17:2280–2292. doi: 10.1093/hmg/ddn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiendl H, Lautwein A, Mitsdorffer M, et al. Antigen processing and presentation in human muscle: cathepsin S is critical for MHC class II expression and upregulated in inflammatory myopathies. J Neuroimmunol. 2003;138:132–143. doi: 10.1016/s0165-5728(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Witt R, Weigand A, Boos AM, et al. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017;18:15. doi: 10.1186/s12860-017-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zhang RZ, Sabatelli P, et al. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol. 2008;67:144–154. doi: 10.1097/nen.0b013e3181634ef7. [DOI] [PubMed] [Google Scholar]