Abstract
With the aim of developing novel anti-inflammatory scaffolds, a new series of pyrazole-substituted various nitrogenous heterocyclic ring systems at C-4 position were synthesized through different chemical reactions and validated by means of spectral and elemental data. The new obtained compounds were investigated for their anti-inflammatory activity using the carrageenan-induced paw edema standard technique and revealed that, compound 6b showed increased potency with % inhibition of edema 85.23 ± 1.92 and 85.78 ± 0.99, respectively, higher than the standard reference drugs indomethacin and celebrex (72.99% and 83.76%). Molecular modeling studies were initiated herein to validate the attained pharmacological data and provide understandable evidence for the observed anti-inflammatory behavior.
Keywords: 1,3-diaryl pyrazole derivatives; anti-inflammatory activity; synthesis
1. Introduction
The effective and quick preparation of biologically active compounds has encouraged Researchers to identify new strategies which could be beneficial to the pharmaceutical industry. Pyrazole analogs are a class of bioactive nitrogenous heterocycles, playing an essential role in the medicinal chemistry fields. Incorporation of different aryl and sulphonamides onto pyrazole nucleus have resulted in Celecoxib (1) and Rimonabant (2) which are anti-inflammatory drugs (Figure 1). Recently, Alegaon et al. in 2014 have reported some 1,3,4-trisubstituted pyrazole derivatives are potent anti-inflammatory activity and COX-2 selective inhibition [1,2]. In addition, numerous reports have appeared in the literature describing different bioactivities and good safety profiles of 1,3,4-trisubstituted pyrazole derivatives including: anti-inflammatory [3,4,5,6,7], analgesic, lipid peroxidation, ulcerogenic [8,9], antipyretic [10], antioxidant [11], antimicrobial, antiviral [12,13,14], anticancer [15,16,17,18], antimitotic [19], and immunosuppressive agents [20]. In addition, some pyrazole compounds have gained great attention as antibacterial and fungicidal isoforms of human cytosolic carbonic anhydrase I or II and antitumor properties [21,22,23]. Extension of our research towards the identification of an efficient synthesis of biologically active pyrazole compounds [24,25,26,27,28,29], we report herein the synthesis of novel derivatives of 1,3-diaryl pyrazoles and their anti-inflammatory activities.
Figure 1.
Structures of the selective COX-2 inhibitors, celecoxib and Rimonabant.
2. Results and Discussion
2.1. Chemistry
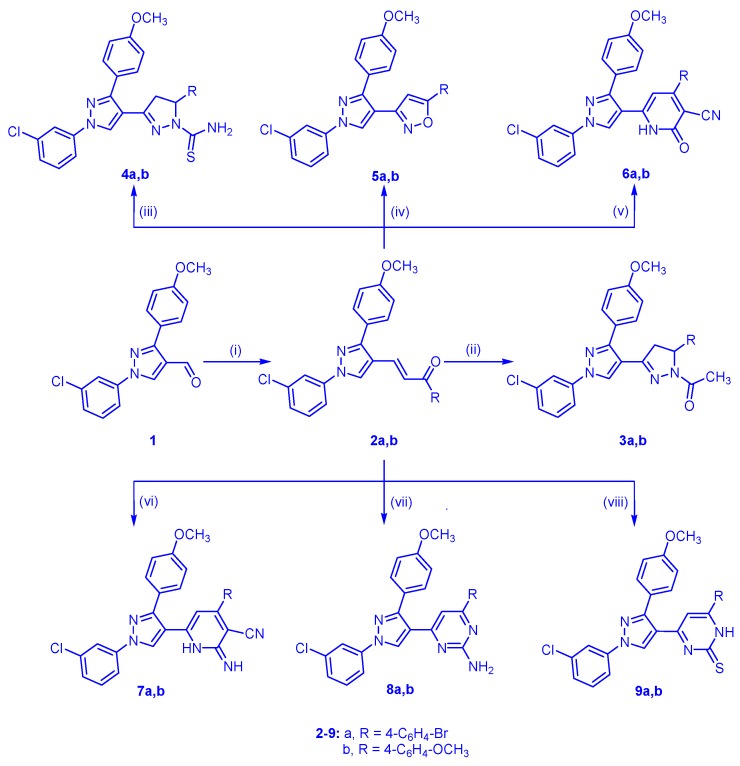
The reaction sequences outlined in Scheme 1 was used for the synthesis of the target compounds. Application of the Claisen Schmidt condensation on substituted acetophenones namely, 4-bromoacetophenone or 4-methoxyacetophenone and 1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazole-4-carboxaldehyde (1) in ethanolic sodium hydroxide solution afforded (E)-1-(4-substituted phenyl)-3-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (2a,b), which was used as starting materials. Cyclocondensation of the α,β-unsaturated ketone 2a,b with hydrazine hydrate in glacial acetic acid yielded the corresponding pyrazoline derivatives 3a,b. On the other hand, heating of 2a,b with thiosemicarbazide in ethanolic NaOH provided 1-thiocarbamoyl pyrazole derivatives 4a,b. In addition, condensation of compound 2a,b with hydroxylamine hydrochloride in refluxing ethanol in the presence of sodium hydroxide as alkaline medium afforded the corresponding isoxazoline 5a,b. Reaction of α,β-unsaturated ketone 2a,b with ethyl cyanoacetate, or malononitrile in presence of ammonium acetate, gave the corresponding 2-oxo(imino)pyridine derivatives 6a,b, and 7a,b, respectively. Furthermore, treatment of 2a,b with guanidine sulfate in ethanolic sodium hydroxide gave 2-aminopyrimidine derivatives 8a,b. Finally, treatment of 2a,b with thiourea in presence of sodium hydroxide gave the corresponding pyrimidine-2-thione derivative 9a,b (Scheme 1).
Scheme 1.
Synthetic route for polysubstituted pyrazole compounds 2–9. Reagents and Conditions: (i) RCOCH3 derivatives/30%Etanolic NaOH/r.t./12 h, 83%–85%; (ii) N2H4·H2O/AcOH/reflux/4–6 h, 73%–81%; (iii) NH2CSNHNH2/EtOH/NaOH/reflux/2–3 h, 71%–73%; (iv) NH2OH·HCl/ethanol/NaOH/reflux/2–3 h, 80%–85%; (v) NC-CH2COOEt/CH3COONH4+/ethanol//reflux/6 h, 75%–76%; (vi) CNCH2CN/CH3COONH4+/ethanol/reflux/6 h, 70%–73%; (vii) NH2NHNH2.H2SO4/ethanol/NaOH/reflux/5–7 h, 69%–71%; (viii) NH2CSNH2/ethanol/NaOH/reflux/6–8 h, 80%–81%.
2.2. Biological Evaluation
2.2.1. In Vivo Anti-Inflammatory Activity
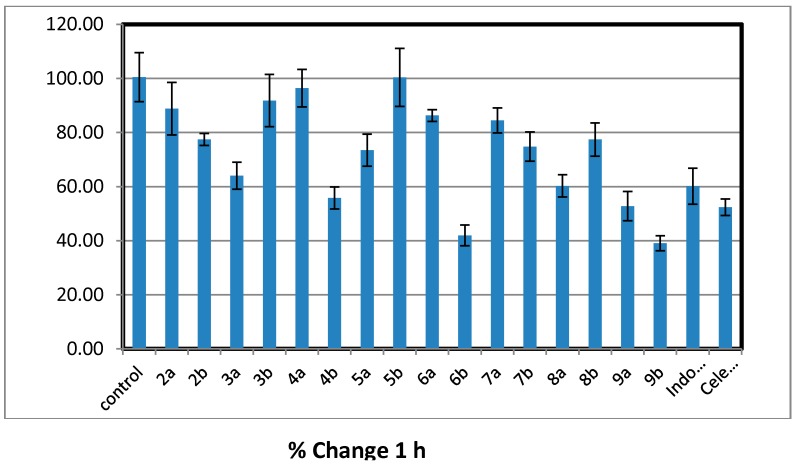
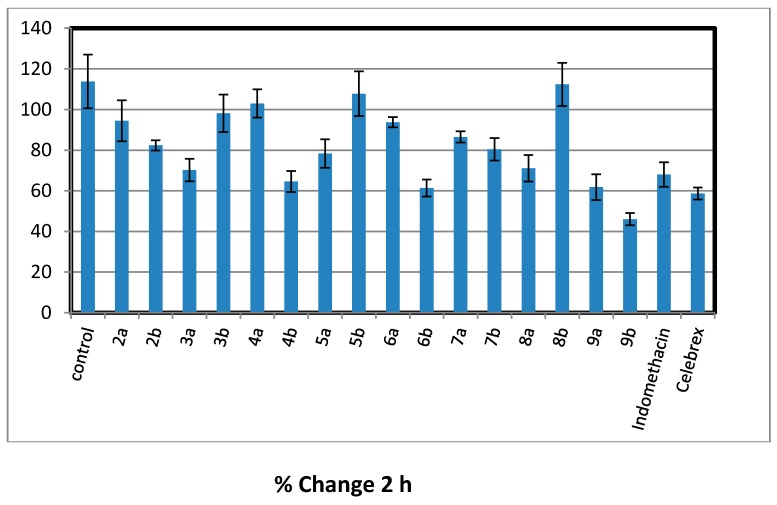
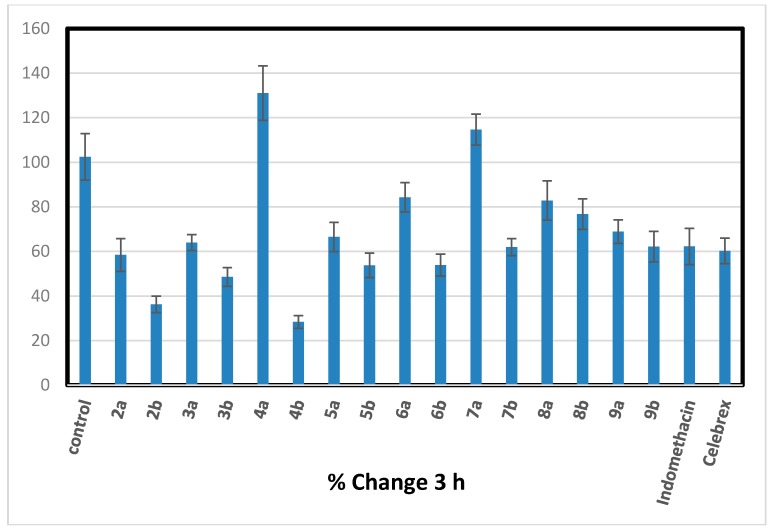
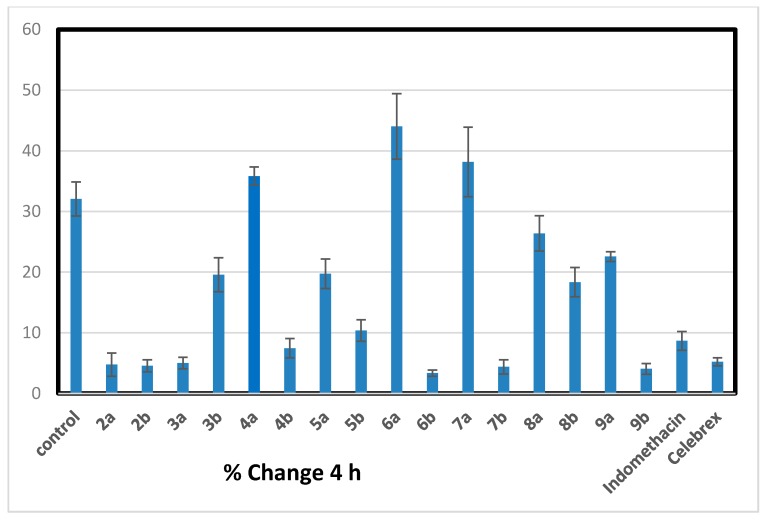
All the newly synthesized trisubstitutedpyrazole compounds 2–9 were evaluated for their in vivo anti-inflammatory activity using carrageenan induced rat paw edema method [30]. The primarily antiinflammatory activity results (Table 1) revealed that, six compounds (2a, 2b, 3a, 6b, 7b, and 9b) showed consistently excellent anti-inflammatory activity (84.39%–89.57% inhibition) 4 h after the carrageenan injection comparable to that of standard drugs indomethacin and celebrex (72.99% and 83.76%), respectively. Chalcones 2a and 2b showed approximately equal anti-inflammatory activity higher than the reference drugs (% inhibition of edema = 85.23 ± 1.92 and 85.78 ± 0.99), respectively. Considering of the target pyrazoles, it was noticed that the electron donating substituent (methoxy group) at position-4 of pyrazole moiety exhibited higher activity than their congeners with 4-electron withdrawing substituent (bromo group) except for acetylpyrazoline derivatives 3a,b. Cyclization of α,β-unsaturated ketone 2b bearing 4-methoxyphenyl at position-4 of pyrazole moiety, gave increased activity in compounds (6b, 7b, and 9b) with little decrease in acetyl pyrazoline derivative 3b and marked decrease in compounds (4b, 5b, and 8b). However, cyclization of chalcone 2a having 4-bromophenyl at position-4 of pyrazole moiety led to a drop in anti-inflammatory activity as in 3a–9a derivatives. The cyanopyridone derivative 6b seems to be the most effective prepared anti-inflammatory agent, revealing better activity (89.57% inhibition of edema) than both indomethacin and celecoxib (standard drugs). Insertion of cyanoiminopyridine moiety as in 7a,b (19.11% and 86.37% inhibition) instead of cyanopyridone in 6a,b (37.35% and 89.57 % inhibition) displayed a little decrease in the activity. While replacement of thiopyrimidine in 9a,b (29.61% and 87.42% inhibition) with aminopyrimidine moiety in 8a,b led to a drop in the activity (17.66% and 42.78% inhibition) (Figure 2, Figure 3, Figure 4 and Figure 5).
Table 1.
Anti-inflammatory activity of the tested compounds 2–9 using carrageenan-induced paw edema in rats.
| Drugs | %Change | % Inhibition | %Change | % Inhibition | %Change | % Inhibition | %Change | % Inhibition |
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | |||||
| control | 100.5 ± 9.07 | ---- | 113.8 ± 13.21 | ---- | 102.4 ± 10.42 | ---- | 32.05 ± 2.80 | ---- |
| 2a | 88.78 ± 9.70 | −11.62 | 94.47 ± 10.13 | −17.00 | 58.42 ± 7.31 * | −42.93 | 4.735 ± 1.92 * | −85.23 |
| 2b | 77.43 ± 2.25 | −22.92 | 82.37 ± 2.55 | −27.62 | 36.27 ± 3.73 * | −64.57 | 4.557 ± 0.99 * | −85.78 |
| 3a | 64.03 ± 4.96 * | −36.26 | 70.22 ± 5.50 * | −38.30 | 63.92 ± 3.59 * | −37.56 | 5.003 ± 0.94 * | −84.39 |
| 3b | 91.8 ± 9.65 | −8.61 | 98.19 ± 9.24 | −13.72 | 48.54 ± 4.24 * | −52.58 | 19.56 ± 2.79 | −38.96 |
| 4a | 96.39 ± 6.90 | −4.05 | 103 ± 6.95 | −9.51 | 131 ± 12.23 | 27.98 | 35.83 ± 1.50 | 11.80 |
| 4b | 55.82 ± 4.05 * | −44.43 | 64.57 ± 5.18 * | −43.27 | 28.38 ± 2.86 * | −72.28 | 7.442 ± 1.59 * | −76.78 |
| 5a | 73.41 ± 5.92 | −26.93 | 78.32 ± 7.00 | −31.18 | 66.51 ± 6.60 * | −35.02 | 19.73 ± 2.44 | −38.45 |
| 5b | 100.4 ± 10.72 | −0.06 | 107.8 ± 10.97 | −5.26 | 53.77 ± 5.47 * | −47.47 | 10.38 ± 1.77 * | −67.62 |
| 6a | 86.3 ± 2.17 | −14.09 | 93.78 ± 2.51 | −17.60 | 84.33 ± 6.57 | −17.62 | 44.02 ± 5.39 | 37.35 |
| 6b | 41.96 ± 3.82 * | −58.23 | 61.37 ± 4.23 * | −46.08 | 53.86 ± 4.94 * | −47.39 | 3.345 ± 0.52 * | −89.57 |
| 7a | 84.47 ± 4.65 | −15.92 | 86.53 ± 2.72 | −23.97 | 114.6 ± 6.95 | 12.00 | 38.18 ± 5.73 | 19.11 |
| 7b | 74.78 ± 5.38 | −25.56 | 80.48 ± 5.51 | −29.28 | 61.94 ± 3.79 * | −39.49 | 4.368 ± 1.18 * | −86.37 |
| 8a | 60.3 ± 4.08 * | −39.97 | 71.13 ± 6.50 * | −37.50 | 82.85 ± 8.84 | −19.07 | 26.39 ± 2.92 | −17.66 |
| 8b | 77.38 ± 6.13 | −22.97 | 112.4 ± 10.61 | −1.25 | 76.74 ± 6.85 | −25.03 | 18.34 ± 2.40 * | −42.78 |
| 9a | 52.79 ± 5.44 * | −47.45 | 61.83 ± 6.32 * | −45.67 | 68.91 ± 5.30 | −32.68 | 22.56 ± 0.81 | −29.61 |
| 9b | 39.06 ± 2.75 * | −61.11 | 46.06 ± 3.05 * | −59.53 | 62.15 ± 6.83 * | −39.29 | 4.033 ± 0.87 * | −87.42 |
| Indomethacin | 60.14 ± 6.64 * | −40.1295 | 67.97 ± 6.03 * | −40.2745 | 62.27 ± 8.14 * | −39.17 | 8.655 ± 1.53 * | −72.9943 |
| Celebrex | 52.39 ± 3.03 * | −47.8509 | 58.69 ± 2.95 * | −48.4341 | 60.25 ± 5.74 * | −41.1372 | 5.205 ± 0.65 * | −83.7597 |
Values represent the mean ± S.E. of six animals for each groups. * p < 0.05: Statistically significant from the control using one-way ANOVA (using Tukey as post hoc test).
Figure 2.
Percentage of inhibition after 1 h of carrageenan injection comparable to that of standard drugs, indomethacin and Celebrex.
Figure 3.
Percentage of inhibition after 2 h of carrageenan injection comparable to that of standard drugs, indomethacin and Celebrex.
Figure 4.
Percentage of inhibition after 3 h of carrageenan injection comparable to that of standard drugs, indomethacin and Celebrex.
Figure 5.
Percentage of inhibition after 4 h of carrageenan injection comparable to that of standard drugs, indomethacin and Celebrex.
2.2.2. Ulcerogenic Liability
Ulcerogenic liability of all prepared anti-inflammatory agents 2–9 was determined following the reported standard method [31] using indomethacin and Celebrex (in a dose 0.28 mmol/kg) as reference standards. It was noticed that all compounds revealed no ulcers, like celebrex, and they are considered safer than indomethacin itself which produced an ulcer count of 14 ± 1.2.
2.3. Molecular Modeling Study
Molecular modeling study of the highly observed anti-inflammatory active agent 6b was performed herein to understand the observed pharmacological data. Docking study was initiated using MOE 2008.10 program. It is used to predict the binding modes and orientation of compound 6b at the active site of the ATP binding site of COX-2 enzyme. The coordinates of this enzyme structure were obtained from the crystal structure of COX-2 with its inhibitor (PDB ID: 1CX2). The root mean square difference (RMSD) between the top docking pose and original crystallographic geometry of co-crystallized ligand SC-558 was 0.9 Å. The phenylsulphonamide moiety of this SC-558 is surrounded by hydrophobic residues Leu352, Tyr355, Phe518, Val523, and the backbone of Ser353. Beyond this hydrophobic pocket, the sulphonamide exhibits hydrophilic interaction with His90, GIn192, and Arg513 [32]. Celecoxib forms three hydrogen bonds with the hydrophilic side chains (His90 and Gln192) in the side pocket and the main chain carbonyl at residue Leu338 [33].
In Table 2, the compounds 2a, 2b, 3a, 4b, 5b, 6b, 7b and 9b with the highest and moderate anti-inflammatory activity were found to have high binding energy ranging from −6.25 to −8.11 kcal·mol−1 in comparison with reference ligands SC-558 and celecoxib (−6.30 and −6.55 kcal·mol−1 respectively). It was observed that most of the active compounds have arene-cation interaction between (Arg120 and Arg513) and the newly inserted 4-methoxyphenyl moiety.
Table 2.
Docking results of the compounds 2–9 with COX-2 enzyme in comparison with the ligands, SC-558 and celecoxib using MOE software version 2008.10.
| Compd. NO. | Docking Score (Kcal/mol) | Amino Acid Residues (Bond Length Å) | Atoms of Compound | Type of Bond |
|---|---|---|---|---|
| 2a | −6.78 | His90 (2.6); | O(CO) | H-acc |
| Arg120 (2.1); | O(OCH3) (parent) | H-acc | ||
| 2b | −6.93 | His90 (2.4); | O(CO) | H-accH-accArene-cation Arene-cation |
| Arg120 (2.3); | O(OCH3) (parent) | |||
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| 3a | −7.12 | Arg120 (1.8); | O(OCH3) (parent) | H-acc |
| 3b | −5.46 | Arg120 (2.1); | O(OCH3) (parent) | H-accArene-cation Arene-cation |
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| 4a | −5.22 | Arg120 (2.6); | O(OCH3) (parent) | H-acc |
| 4b | −7.24 | Arg120 (2.4); | O(OCH3) (parent) | H-accArene-cation Arene-cation |
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| 5a | −5.14 | Arg120 (2.7); | O(OCH3) (parent) | H-acc |
| 5b | −6.25 | Arg120 (2.1); | O(OCH3) (parent) | H-acc Arene-cation Arene-cation |
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| 6a | −5.43 | His90 (2.4); | NH(pyridone) | H-acc |
| His90 (2.6); | O(pyridone) | H-acc | ||
| Arg120 (2.5); | O(OCH3) (parent) | H-acc | ||
| 6b | −8.11 | His90 (2.7); | NH(pyridone) | H-accH-accH-accArene-cation Arene-cation |
| His90 (2.7); | O(pyridone) | |||
| Arg120 (3.1); | O(OCH3) (parent) | |||
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| 7a | −5.45 | His90 (2.6); | NH(iminopyridine) | H-accH-acc |
| Arg120 (2.3); | O(OCH3) (parent) | |||
| 7b | −6.98 | His90 (2.7); | NH(iminopyridine) | H-accH-accArene-cation Arene-cation |
| Arg120 (2.1); | O(OCH3) (parent) | |||
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| 8a | −5.20 | Arg120 (2.2); | O(OCH3) (parent) | H-acc |
| 8b | −6.26 | Arg120 (1.9); | O(OCH3) (parent) | H-accArene-cation Arene-cation |
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| 9a | −5.75 | Arg120 (2.5); | O(OCH3) (parent) | H-acc |
| 9b | −7.48 | Arg120 (1.9); | O(OCH3) (parent) | H-accArene-cation Arene-cation |
| Arg120; | C6H3-4-OCH3 (new) | |||
| Arg513 | C6H3-4-OCH3 (new) | |||
| SC-558 | −6.30 | His90 (2.2); | H(NH2) | H-acc |
| GIn192 (2.4); | H(NH2) | H-don | ||
| Arg513 (2.2); | H(NH2) | H-don | ||
| Val523; | C6H3-4-Br | Arene-Arene | ||
| Ala527; | C6H3-4-Br | Arene-Arene | ||
| Leu352; | Phenylsulphonamide | Arene-Arene | ||
| Ser353 | Phenylsulphonamide | Arene-Arene | ||
| Celecoxib | −6.55 | His90 (1.9); | H(NH2) | H-acc |
| GIn192 (2.1); | H(NH2) | H-don | ||
| Leu338 (2.3); | H(NH2) | H-acc | ||
| Val523; | C6H3-4-OCH3 | Arene-Arene | ||
| Ala527; | C6H3-4-OCH3 | Arene-Arene | ||
| Leu352; | Phenylsulphonamide | Arene-Arene | ||
| Ser353 | Phenylsulphonamide | Arene-Arene |
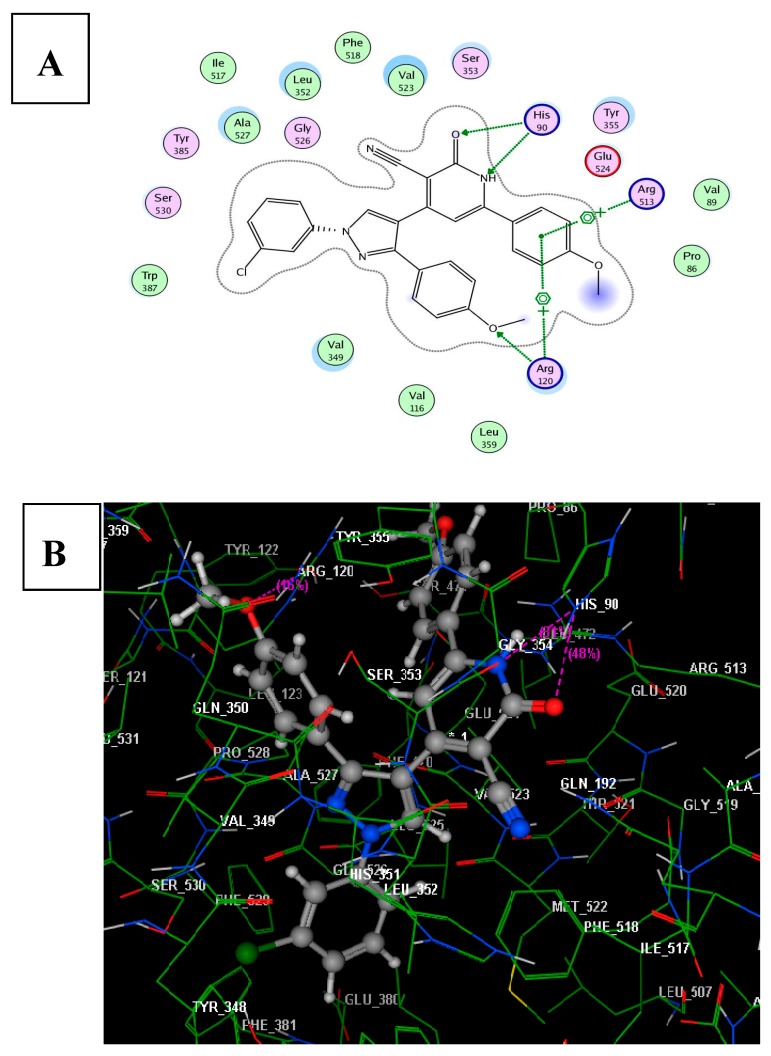
From Figure 6, it was found that the compound 6b revealed good fitting inside the binding site of the protein molecular surface and having minimum binding energy of −8.11 kcal·mol−1. There were two hydrogen bonds linking the sidechain of His90 with NH and oxygen of pyridone moiety as hydrogen acceptors (distance: 2.76 and 2.79 Å, respectively). The 4-methoxyphenyl moiety attached to pyridone formed two arene-cation interactions with Arg120 and Arg513. Furthermore, one H-bond acceptor was observed between the sidechain of Arg120 and 4-methoxyphenyl linked to pyrazole scaffold (distance: 3.19 Å). The previous results indicated that the insertion of 4-methoxyphenyl group to the pyridone moiety might reinforce the combination of compound 6b and the receptor, which might enhance the binding affinity, resulting in the increased anti-inflammatory activity of this compound.
Figure 6.
The proposed binding mode of compound 6b docked in the active site of COX-2; (A,B) showing 2D and 3D ligand-receptor interactions (hydrogen bonds are illustrated as dotted purple lines; C atoms are colored gray, N blue and O red).
3. Experimental Section
3.1. General Information
Melting points were measured in open capillary tubes using a Griffin apparatus and are uncorrected. Structures of compounds were confirmed by routine spectrometric analysis. Elemental analyses were carried results were within ±0.4% of the theoretical values. Infrared spectra were recorded on a 435 IR spectrophotometer (Shimadzu Bruker, Tokyo, Japan) using KBr discs. 1H-NMR and 13C-NMR spectra were obtained on a Gemini 500 MHz spectrophotometer (Varian, Polo Alto, CA, USA) or on a Bruker 500 MHz spectrophotometer, and measured in δ scale using TMS as an internal standard. Mass Spectra were recorded on a 5988 spectrometer (Hewlett Packard, Palo Alto, CA, USA). Analytical thin layer chromatography (TLC) was performed using silica gel aluminum sheets, 60 F254 (E. Merck, Darmstadt, Germany) for the progress of reactions and visualization with ultraviolet light (UV) at 365 and 254 nm.
3.1.1. 1-(4-Substitutedphenyl)-3-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (2a,b)
A mixture of pyrazolecarbaldehyde derivative 1 (0.01 mol) and substituted acetophenones, namely 4-bromoacetophenone or 4-methoxyacetophenone (0.01 mol), in of 30% ethanolic NaOH solution (40 mL) was stirred for 12 h at room temperature. The progress of reaction was monitored by TLC. After completion, the reaction mixture was poured into acidified ice cold water of pH ~2. The precipitated solid formed was filtered, washed with water and recrystallized from ethanol to afford the title compounds 2a,b.
1-(4-Bromophenyl)-3-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (2a). Yield 83%; m.p. 235–237 °C; IR (KBr, cm−1) ν: 1673 (C=O), 1589 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.81 (s, 3H, OCH3); 7.09–7.99 (m, 14H, ArH + CH=CH), 9.45 (s,1H, CH of pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.60, 114.34, 116.62, 117.75, 125.11, 125.95, 127.67, 129.08, 129.81, 131.74, 133.85, 134.47, 135.44, 141.08, 144.32, 151.54, 159.58, 191.96; MS (EI, 70 eV): m/z (%): 492 (11) [M]+; Anal. Calcd. for C25H18BrClN2O2 (493.78): C, 60.81; H, 3.67; N, 5.67; Found: C, 61.65; H, 3.77; N, 5.32.
1-(4-Methoxphenyl)-3-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (2b). Yield 85%; m.p. 152–154 °C; IR (KBr, cm−1) ν: 1680 (C=O), 1601 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.80, 3.85 (2s, 6H, 2OCH3); 7.10–8.09 (m, 14H, ArH + CH=CH), 9.43 (s,1H, CH of pyrazole); MS (EI, 70 eV): m/z (%): 447 (11, M+ + 3), 445 (29, M+ + 1), 410 (100); Anal. Calcd. for C26H21ClN2O3 (444.91): C, 70.19; H, 4.76; N, 6.30; Found: C, 70.35; H, 4.52; N, 6.55.
3.1.2. 1-(3-(4-Substituted phenyl)-5-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydro-pyrazol-1-yl ethanone (3a,b)
A solution of hydrazine hydrate (0.01 mol) was added to a solution of compounds 2a,b (0.01 mol) in glacial acetic acid (20 mL) and the mixture was refluxed for 4–6 h. The reaction mixture was cooled to room temperature and the precipitated solid was filtered, washed with water, dried, and recrystallized from ethanol to give the title compounds 3a,b.
1-(3-(4-Bromophenyl)-5-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydro-pyrazol-1-yl ethanone (3a). Yield 73%; m.p. 233–235 °C; 1H-NMR (DMSO-d6-δ ppm): 2.28 (s, 3H, COCH3), 3.16 (dd, 1H, CH), 3.77 (s, 3H, OCH3), 3.80 (dd, 1H, CH), 5.63 (dd, 1H, CH), 7.01–8.43 (m, 12H, Ar-H), 9.68 (s, 1H, CH-pyrazole); 13C-NMR (DMSO-d6-δ ppm): 24.0, 43.8, 55.6, 63.9, 114.3, 116.8, 117.6, 125.4, 125.9, 126.1, 127.6, 129.1, 129.8, 130.7, 131.1, 134.4, 137.8, 140.8, 150.4, 155.2, 159.1, 160.9, 168.2; MS (EI, 70 eV): m/z (%): 548 (43) [M]+; Anal. Calcd. for C27H22BrClN4O2 (549.85): C, 58.98; H, 4.03; N, 10.19; Found: C, 58.77; H, 4.37; N, 10.36.
1-(3-(4-Methoxyphenyl)-5-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydro-pyrazol-1-yl ethanone (3b). Yield 81%; m.p. 175–179 °C; 1H-NMR (DMSO-d6-δ ppm): 2.27 (s, 3H, COCH3), 3.13 (dd, 1H, CH), 3.76, 3.77 (2s, 6H, 2OCH3), 3.80 (dd, 1H, CH), 5.59 (dd, 1H, CH), 6.98–8.87 (m, 12H, Ar-H), 9.69 (s, 1H, CH-pyrazole) ppm; 13C-NMR (DMSO-d6-δ ppm): 24.01, 43.8, 55.6, 63.9, 114.3, 116.8, 117.6, 125.4, 125.9, 126.1, 127.6, 129.1, 129.8, 130.7, 131.1, 134.4, 137.8, 140.8, 150.4, 155.2, 159.1, 160.9, 168.2 ppm; MS (EI, 70 eV): m/z (%): 503 (11, M+ + 3), 501 (25, M+ + 1), 77 (100); Anal. Calcd. for C28H25ClN4O3 (500.98): C, 67.13; H, 5.03; N, 11.18; Found: C, 67.38; H, 4.95; N, 11.13.
3.1.3. 3-(4-Substitutedphenyl)-5-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-di-hydropyrazole-1-carbothioamide (4a,b)
Thiosemicarbazide was added to a mixture of chalcone 2a,b (0.01 mol) in absolute ethanol (30 mL) containing sodium hydroxide (1 g, 0.025 mol). The reaction mixture was heated under reflux for 2–3 h. The contents were reduced, cooled, and poured onto crushed ice containing a few drops of hydrochloric acid (until pH ~6). The resulting precipitate was collected by filtration and recrystallized from methanol to give the title compounds 4a,b.
3-(4-Bromophenyl)-5-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-di-hydropyrazole-1-carbothioamide (4a). Yield 71%; m.p. 284–286 °C; IR (KBr, cm−1) ν: 3414 (NH2), 1592 (C=C), 1067 (C=S); 1H-NMR (DMSO-d6-δ ppm): 3.24 (dd, 1H, CH), 3.78 (s, 3H, OCH3), 3.81 (dd, 1H, CH), 6.00 (dd, 1H, CH), 7.00–8.25 (m, 14H, Ar-H + NH2 exchangeable with D2O), 9.17 (s, 1H, CH of pyrazole); 13C-NMR (DMSO-d6-δ ppm): 40.5, 55.8, 56.2, 114.5, 114.7, 116.9, 118.0, 118.5, 124.3, 125.6, 126.1, 129.6, 129.9, 130.9, 131.6, 132.0, 134.4, 141.0, 150.1, 154.1, 159.7, 176.5; MS (EI, 70 eV): m/z (%): 569 (1, M + 4), 567 (2.7, M + 2), 565 (2.7, M+), 111 (100); Anal. Calcd. for C26H21BrClN5OS (566.9): C, 55.09; H, 3.73; N, 12.35; Found: C, 54.92; H, 3.52; N, 12.14.
3-(4-Methoxyphenyl)-5-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydro-pyrazole-1-carbothioamide (4b). Yield 73%; m.p. 223–225 °C; IR (KBr, cm−1) ν: 3444 (NH2), 1594 (C=C), 1092 (C=S); 1H-NMR (DMSO-d6-δ ppm): 3.24 (dd, 1H, CH), 3.75, 3.78 (2s, 6H, 2OCH3), 3.84 (dd, 1H, CH), 5.98 (dd, 1H, CH), 6.95–8.37 (m, 12H, Ar-H), 8.18 (s, 2H, NH2 exchangeable with D2O ), 9.17 (s, 1H, CH of pyrazole); 13C-NMR (DMSO-d6-δ ppm): 42.3, 55.2, 55.3, 61.4, 114.0, 114.1, 114.2, 116.4, 118.9, 125.6, 126.5, 128.0, 128.9, 129.5, 131.1, 134.8, 134.8, 140.5, 150.4, 151.6, 159.2, 160.4, 175.6; MS (EI, 70 eV): m/z (%): 519 (2.9, M+ + 2), 517 (7, M+), 369 (100); Anal. Calcd. for C27H24ClN5O2S (518.03): C, 62.60; H, 4.67; N, 13.52; Found: C, 62.52; H, 4.43; N, 13.26.
3.1.4. 4-(3-(4-Substituted phenyl)isoxazol-5-yl)-1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazole (5a,b)
A mixture of compounds 2a,b (0.01 mol) and hydroxylamine hydrochloride (0.01 mol) in ethanol (30 mL) containing sodium hydroxide solution (0.5 g NaOH in 0.5 mL water) was refluxed for 2–3 h. The reaction mixture was poured onto ice-water, neutralized with drops of concentrated Hydrochloric acid, and the solid precipitate formed filtered off, washed with water, and recrystallized from methanol to afford the desired compounds 5a,b.
4-(3-(4-Bromophenyl)isoxazol-5-yl)-1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazole (5a). Yield 85%; m.p. 168–170 °C; IR (KBr, cm−1) ν: 3064 (CH-Ar), 1601 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.77 (s, 3H, OCH3), 6.59–8.88 (m, 13H, Ar-H + CH-isoxazole), 9.18 (s, 1H, CH-pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.3, 113.7, 114.1, 114.2, 116.6, 118.6, 123.4, 126.5, 127.5, 128.6, 129.5, 129.7, 130.8, 131.3, 132.1, 134.1, 140.2, 151.5, 159.8, 164.3, 168.8; MS (EI, 70 eV): m/z (%): 509 (0.1, M+ + 4), 507 (0.2, M+ + 2), 505 (0.1, M+), 327 (100); Anal. Calcd. for C25H17BrClN3O2 (506.78): C, 59.25; H, 3.38; N, 8.29; Found: C, 59.48; H, 3.27; N, 8.17.
4-(3-(4-Methoxyphenyl)isoxazol-5-yl)-1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazole (5b). Yield 80%; m.p. 227–229 °C; 1H-NMR (DMSO-d6-δ ppm): 3.72, 3.78 (2s, 6H, 2OCH3), 7.00–8.87 (m, 13H, Ar-H + CH-isoxazole ring), 9.18 (s, 1H, CH-pyrazole); MS (EI, 70 eV): m/z (%): 461 (1.5, M+ + 4), 459 (4.8, M+ + H2), 327 (100); Anal. Calcd. for C26H20ClN3O3 (457.91): C, 68.20; H, 4.40; N, 9.18; Found: C, 68.41; H, 4.62; N, 9.23.
3.1.5. 6-(4-Substitutedphenyl)-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,2-dihydro-2-Oxopyridine-3-carbonitrile (6a,b) and 6-(4-substitutedphenyl)-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,2-dihydro-2-iminopyridine-3-carbonitrile (7a,b)
A mixture of compounds 2a,b (0.01 mol), ethyl cyanoacetate or malononitrile (0.01 mol), and ammonium acetate (6 g, 0.08 mol) was refluxed in ethanol (30 mL) for 6 h. The formed precipitate was collected by filtration, washed several times with water, dried and recrystallized from ethanol to afford the title compounds 6a,b and 7a,b, respectively.
6-(4-Bromophenyl)-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,2-dihydro-2-oxopyridine-3-carbonitrile (6a). Yield 75%; m.p. 217–219 °C; IR (KBr, cm−1) ν: 3335 (NH), 2192 (C≡N), 1687 (C=O), 1587 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.77 (s, 3H, OCH3), 6.71 (s, 1H, CH-pyridine), 6.96–8.05 (m, 13H, Ar-H + NH exchangeable with D2O), 9.07 (s, 1H, CH of pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.6, 114.7, 116.6, 117.5, 117.8, 118.7, 124.6, 125.3, 127.1, 129.4, 130.0, 130.8, 131.9, 132.4, 134.6, 140.5, 150.9, 152.3, 160.0, 162.9, 167.3; MS (EI, 70 eV): m/z (%): 558 (3.7, M+ + 2), 557 (1.7, M+ + 1), 91 (100); Anal. Calcd. for C28H18BrClN4O2 (557.83): C, 60.29; H, 3.25; N, 10.04; Found: C, 60.41; H, 3.32; N, 10.23.
6-(4-Methoxyphenyl)-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,2-dihydro-2-oxo-pyridine-3-carbonitrile (6b). Yield 76%; m.p. 198–200 °C; IR (KBr, cm−1) ν: 3417 (NH), 2216 (C≡N), 1726 (C=O), 1654 (C=N), 1597 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.35 (s, 1H, NH exchangeable with D2O), 3.77, 3.81 (2s, 6H, 2OCH3), 6.60 (s, 1H, CH-pyridine), 6.97–9.00 (m, 12H, Ar-H), 9.23 (s, 1H, CH of pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.3, 55.5, 100.2, 114.1, 114.1, 114.5, 115.7, 115.8, 116.2, 118.2, 122.6, 124.1, 126.6, 127.7, 129.0, 130.0, 130.3, 134.1, 140.0, 150.5, 155.2, 159.5, 160.3, 161.7, 161.9; MS (EI, 70 eV): m/z (%): 510 (4, M+ + 2), 508 (2, M+), 303 (100); Anal. Calcd. for C29H21ClN4O3 (508.96): C, 68.44; H, 4.16; N, 11.01; Found: C, 68.41; H, 4.22; N, 10.88.
6-(4-Bromophenyl)-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,2-dihydro-2-imino-pyridine-3-carbonitrile (7a). Yield 73%; m.p. 172–174 °C; IR (KBr, cm−1) ν: 3335, 3195 (2NH), 2192 (C≡N), 1587 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.59 (s, 1H, NH exchangeable with D2O), 3.78 (s, 3H, OCH3), 6.52 (s, 1H, CH-pyridine), 6.93–8.04 (m, 13H, Ar-H + NH exchangeable with D2O), 8.89 (s, 1H, CH-pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.6, 113.0, 114.1, 114.6, 115.5, 116.8, 118.3, 118.6, 123.1, 125.4, 126.7, 128.4, 129.3, 130.7, 131.2, 131.9, 132.1, 134.6, 140.8, 151.4, 159.8, 160.5, 164.2, 169.0; MS (EI, 70 eV): m/z (%): 507 (2.8, M+ + 2), 555 (7, M+), 149 (100); Anal. Calcd. for C28H19BrClN5O (556.84): C, 60.39; H, 3.44; N, 12.58; Found: C, 60.48; H, 3.27; N, 12.17.
6-(4-Methoxyphenyl)-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,2-dihydro-2-iminopyridine-3-carbonitrile (7b). Yield 70%; m.p. 246–248 °C; IR (KBr, cm−1) ν: 3405, 3334 (2NH), 2198 (C≡N), 1573 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.26 (s, 1H, NH exchangeable with D2O), 3.74, 3.79 (2s, 6H, 2 OCH3), 6.92–8.05 (m, 13H, Ar-H + CH-pyridine), 7.24 (s, 1H, NH exchangeable with D2O), 8.88 (s, 1H, CH-pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.1, 55.3, 109.5, 113.9, 114.0, 114.6, 115.1, 116.4, 117.8, 118.8, 122.5, 124.5, 126.1, 128.4, 129.6, 130.0, 131.4, 134.1, 140.3, 150.0, 159.3, 160.8, 161.0, 161.5, 169.3; MS (EI, 70 eV): m/z (%): 509 (10, M+ + 2), 507 (14, M+), 55 (100); Anal. Calcd. for C29H22ClN5O2 (507.97): C, 68.57; H, 4.37; N, 13.79; Found: C, 68.41; H, 4.42; N, 13.43.
3.1.6. 4-(4-Substitutedphenyl)-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)pyrimidin-2-amine (8a,b)
An aqueous solution of sodium hydroxide (40%, 5 mL) was added portion wise during a period of 3 h to a mixture of 1-substituted prop-2-en-1-ones 2a or 2b (0.01 mol) and guanidine sulphate (1.6 g, 0.01 mol) in ethanol (25 mL). After refluxing for 5–7 h, the solid product formed upon pouring onto ice/water containing a few drops of hydrochloric acid (until pH ~ 6) was collected by filtration, washed with water, then recrystallized from methanol.
4-(4-Bromophenyl)-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)pyrimidin-2-amine (8a). Yield 69%, m.p. 163–165 °C; IR (KBr, cm−1) ν: 3406 (NH2), 1581 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.80 (s, 3H, OCH3), 6.58 (s, 2H, NH2 exchangeable with D2O), 6.97–8.05 (m, 13H, Ar-H + CH-pyridine), 9.08 (s, 1H, CH-pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.1, 104.1, 114.0, 114.0, 116.1, 118.1, 124.0, 125.5, 126.3, 128.6, 129.9, 130.8, 131.2, 131.7, 133.9, 134.1, 140.7, 145.5, 159.4, 161.0, 162.7, 163.7; MS (EI, 70 eV): m/z (%): 535 (1.3, M+ + 4), 533 (7.6, M+ + 2), 531 (9, M+), 111 (100); Anal. Calcd. for C26H19BrClN5O (532.82): C, 58.61; H, 3.59; N, 13.14; Found: C, 58.48; H, 3.27; N, 13.17.
4-(4-Methoxyphenyl)-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)pyrimidin-2-amine (8b). Yield 71%, m.p. 224–226 °C; IR (KBr, cm−1) ν: 3324 (NH2), 1577 (C=C); 1H-NMR (DMSO-d6-δ ppm): 3.78, 3.81 (2s, 6H, 2 OCH3), 6.97 (s, 2H, NH2 exchangeable with D2O), 7.01–8.56 (m, 13H, Ar-H + CH-pyridine), 9.06 (s, 1H, CH-pyrazole; 13C-NMR (DMSO-d6-δ ppm): 55.3, 55.5, 103.6, 113.3, 114.0, 114.0, 115.8, 118.1, 125.2, 125.5, 126.6, 128.1, 128.9, 130.2, 131.2, 134.1, 140.7, 150.8, 160.4, 161.2, 163.5, 163.7; MS (EI, 70 eV): m/z (%): 485 (0.01, M+ + 2), 483 (0.02, M+), 135 (100); Anal. Calcd. for C27H22ClN5O2 (483.95): C, 67.01; H, 4.58; N, 14.47; Found: C, 67.41; H, 4.62; N, 14.23.
3.1.7. 4-(4-Substitutedphenyl)-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)pyrimidine-2-(1H)-thione (9a,b)
A mixture of 1-substituted prop-2-en-1-ones 2a or 2b (0.01 mol) and thiourea (0.76 g, 0.01 mol) in ethanol (30 mL) containing (1 g, 0.025 mol) sodium hydroxide was refluxed 6–8 h. The solid product formed upon pouring onto ice/water containing a few drops of hydrochloric acid (until pH ~6) was collected by filtration, washed with water, then recrystallized from methanol to yield the desired compounds 9a,b.
4-(4-Bromophenyl)-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)pyrimidine-2-(1H)-thione (9a). Yield 81%; m.p. 180–182 °C; IR (KBr, cm−1) ν: 3384 (NH), 1591 (C=C), 1174 (C=S); 1H-NMR (DMSO-d6-δ ppm): 3.77 (s, 3H, OCH3), 5.23 (s, 1H, NH exchangeable with D2O), 7.00–7.85 (m, 13H, Ar-H + CH-thiopyrimidine), 9.25 (s, 1H, CH-pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.2, 113.4, 114.0, 116.3, 117.1, 118.3, 122.0, 125.5, 126.8, 128.6, 129.2, 130.2, 131.1, 131.7, 134.0, 134.1, 140.6, 150.4, 160.8, 164.4, 176.1, 181.1; MS (EI, 70 eV): m/z (%); MS m/z (%): 535 (1.3, M+ + 4), 533 (7.6, M+ + 2), 531 (9, M+), 155 (100); Anal. Calcd. for C26H18BrClN4OS (549.87): C, 56.79; H, 3.30; N, 10.19; Found: C, 56.48; H, 3.27; N, 10.17.
4-(4-Methoxyphenyl)-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)pyrimidine-2-(1)-thione (9b). Yield 80%; m.p. 233–235 °C; IR (KBr, cm−1) ν: 3384 (NH), 1591 (C=C), 1174 (C=S); 1H-NMR (DMSO-d6-δ ppm): 3.78 (br s, 6H, 2 OCH3), 7.00–7.85 (m, 14H, Ar-H, CH-thiopyrimidine and NH exchangeable with D2O), 9.26 (s, 1H, CH-pyrazole); 13C-NMR (DMSO-d6-δ ppm): 55.2, 55.7, 101.9, 109.4, 114.0, 114.2, 115.0, 116.3, 117.9, 125.2, 125.8, 126.6, 127.4, 128.5, 130.0, 130.8, 135.1, 150.1, 159.7, 161.0, 165.1, 176.3, 180.3; MS (EI, 70 eV): m/z (%); MS m/z (%): 563 (0.7, M+ + 2), 561 (1.6, M+), 310 (100); Anal. Calcd. for C27H21ClN4O2S (501): C, 64.73; H, 4.22; N, 11.18; Found: C, 64.41; H, 4.62; N, 11.23.
3.2. Measurement of Anti-Inflammatory Activity
Compounds 2–9 were screened for their in vivo anti-inflammatory activity by the carrageenan-induced paw edema standard method [1]. Mature Swiss male albino rats were obtained from The Animal House, NRC, Cairo, weighing 150–200 g. Edema was induced in the left hind paw of all rats by subcutaneous injection of 0.1 mL of 1% (w/v) carrageenan in distilled water into their footpads. Rats were divided into six groups of six rats each. The first group was kept as control, and was given the respective volume of the solvent (1% of tween-80 in distilled water). The other groups were orally administered the drugs—indomethacin and celebrex (reference standards) and the tested compoundsafter dissolution in water and 1% tween 80 in dose of 0.28 mmol/kg, 1 h before carrageenan injection. The paw volume of each rat was measured using Vernier caliper; before carrageenan injection and then hourly for 4 h post-administration of the drugs. The edema rate and inhibition rate of each group were calculated as follows:
| Percentage change of Edema rate (E) % = [(Vt − Vo )/Vo] × 100 |
| Inhibition rate (I)% = [(Ec − Et)/Ec] × 100 |
where: Vo is the volume before carrageenan injection (mL). Vt is the volume at t hour after carrageenan injection (mL). Ec is the edema rate of control group. Et is the edema rate of the treated group.
3.3. Ulcerogenic Liability
After five hours of measuring the anti-inflammatory activity, the rats were sacrificed by decapitation. Their stomachs were removed, opened along the greater culvature, and the number of ulcers was assessed by the reported standard method [2]. The separate groups which received indomethacin and Celebrex (0.28 mmol/kg) as positive controls were used. The results were compared with tween-80 (1% solution) treated group as negative control.
3.4. Molecular Modeling Study
All the molecular modeling calculations and docking simulation studies were performed using Molecular Operating Environment (MOE®) [3] 2008.10. All the interaction energies and different calculations were automatically calculated.
3.4.1. Optimization of the Target Compound 6b
The target compound 6b was constructed into a 3D model using the builder interface of the MOE program. After checking their structures and the formal charges on atoms by 2D depiction, the following steps were carried out: the target compound was subjected to a conformational search. All conformers were subjected to energy minimization, all the minimizations were performed with MOE until a RMSD gradient of 0.01 Kcal/mole and RMS distance of 0.1 Å with MMFF94X force-field and the partial charges were automatically calculated. The obtained database was then saved as MDB file to be used in the docking calculations.
3.4.2. Optimization of the Enzymes Active Site
The X-ray crystallographic structure of COX-2 receptor complexed with 1-phenylsulfona-mide-3-trifluoromethyl-5-(4-bromophenyl)pyrazole, SC-558 (PDB ID: 1CX2) [4] was obtained from the Protein Data Bank through the internet. The enzyme was prepared for docking studies by removing the ligand molecule SC-558 from the COX-2 receptor active site. Hydrogen atoms were added to the system with their standard geometry. The atoms connection and type were checked for any errors with automatic correction. Selection of the receptor and its atom potential were fixed. MOE Alpha Site Finder was used for the active site search in the enzyme structure using all default items. Dummy atoms were created from the obtained alpha spheres. Re-docking of co-crystalline ligand to the receptor active site to insure the docking method was efficient and the active pocket was saved as a MOE file to be used for docking simulation of the selected compounds.
3.4.3. Docking of the Target Molecule 6b and Celecoxib to the Receptor Active Sites
Docking of the conformation database of the target compounds was done using MOE-Dock software. The following methodology was generally applied via loading of the enzyme active site file and the dock tool was initiated. The program specifications were adjusted to:
-
-
Dummy atoms as the docking site.
-
-
Triangle matcher as the placement methodology to be used.
-
-
London dG as scoring methodology to be used and was adjusted to its default values.
The MDB file of the ligand to be docked was loaded and dock calculations were run automatically. The obtained poses were studied and the poses showed best ligand-enzyme interactions were selected and stored for energy calculations. The 2D interaction and stereo view for compound 6b inside the active site of COX-2 kinase were obtained and saved as both MOE and photo files.
4. Conclusions
In summary, we have designed and synthesized a new series of 1,3,-diaryl pyrazole derivatives linked different nitrogenous heterocyclic ring systems at C-4 position including pyrazoles, isoxazole, pyridines, or pyrimidines and evaluated for their anti-inflammatory activity using standard acute carrageenan-induced paw edema method. From the obtained results, six compounds (2a, 2b, 3a, 6b, 7b, and 9b) showed consistently excellent anti-inflammatory activity (84.39–89.57% inhibition) 4 h after the carrageenan injection comparable to that of the standard drugs indomethacin and Celebrex (72.99% and 83.76%, respectively). The cyanopyridone derivative 6b seems to be the most effective product, displayed better activity (89.57% inhibition of edema) than both indomethacin and celecoxib (reference standards), and could be considered a promising selective anti-inflammatory lead for further development of more potent anticancer agents. The structures of the newly prepared compounds were elucidated using spectroscopic and elemental analysis.
Acknowledgments
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
Author Contributions
The listed authors contributed to this work as described in the following. Eman S. Nossier, carried out the synthetic work, interpreted the results, and prepared the manuscript; Hoda H. Fahmy gave the concepts of the work, interpreted the results, and prepared the manuscript; Nagy M. Khalifa interpreted the results and cooperated in the preparation of the manuscript; and Wafaa I. El-Eraky and Marawan A. Baset carried and interpreted the results of the biological activities. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all the compounds are available from the authors.
References
- 1.Alegaon S.G., Alagawadi K.R., Garg M.K., Dushyant K.B., Vinod D. 1,3,4-Trisubstituted pyrazole analogues as promising anti-inflammatory agents. Bioorg. Chem. 2014;54:51–59. doi: 10.1016/j.bioorg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Alegaon S.G., Hirpara M.B., Alagawadi K.R., Hullatti K.K., Kashniyal K. Synthesis of novel pyrazole-thiadiazole hybrid as potential potent and selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2014;24:5324–5329. doi: 10.1016/j.bmcl.2014.08.062. [DOI] [PubMed] [Google Scholar]
- 3.Chougala B.M., Samundeeswari S., Holiyachi M., Shastri L.A., Dodamani S., Jalalpure S., Dixit S.R., Joshi S.D., Sunagar V.A. Synthesis, characterization and molecular docking studies of substituted 4-coumarinylpyrano[2,3-c]pyrazole derivatives as potent antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2017;125:101–116. doi: 10.1016/j.ejmech.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Sayed M.A., Bayomi S.M., El-Sherbeny M.A., Abdel-Aziz N.I., El-Tahir K.H., Shehatou G.S., Abdel-Aziz A.A. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibition activities and molecular docking study of pyrazoline derivatives. Bioorg. Med. Chem. 2016;24:2032–2042. doi: 10.1016/j.bmc.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Abdelall E.K., Lamie P.F., Ali W.A. Cyclooxygenase-2 and 15-lipoxygenase inhibition, synthesis, anti-inflammatory activity and ulcer liability of new celecoxib analogues: Determination of region-specific pyrazole ring formation by NOESY. Bioorg. Med. Chem. Lett. 2016;26:2893–2899. doi: 10.1016/j.bmcl.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 6.Abdellatif K.R., Abdelall E.K., Fadaly W.A., Kamel G.M. Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of new 1,3,5-triarylpyrazoline and 1,5-diarylpyrazole derivatives as selective COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2016;26:406–412. doi: 10.1016/j.bmcl.2015.11.105. [DOI] [PubMed] [Google Scholar]
- 7.El-Feky S.A., Abd El-Samii Z.K., Osman N.A., Lashine J., Kamel M.A., Thabet H.K. Synthesis, molecular docking and anti-inflammatory screening of novel quinoline incorporated pyrazole derivatives using the Pfitzinger reaction II. Bioorg. Chem. 2015;58:104–116. doi: 10.1016/j.bioorg.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Hussain S., Kaushik D. Noval 1-substituted-3,5-dimethyl-4-[(substituted phenyl)diazenyl]pyrazole derivatives: Synthesis and pharmacological activity. J. Saudi Chem. Soc. 2015;19:274–281. doi: 10.1016/j.jscs.2012.04.002. [DOI] [Google Scholar]
- 9.Thore S.N., Gupta S.V., Baheti K.G. Novel ethyl-5-amino-3-methylthio-1H-pyrazole-4-carboxylates: Synthesis and pharmacological activity. J. Saudi Chem. Soc. 2016;20:259–264. doi: 10.1016/j.jscs.2012.06.011. [DOI] [Google Scholar]
- 10.CarmoMalvar D., Ferreira R.T., de Castro R.A., de Castro L.L., Freitas A.C., Costa E.A., Florentino I.F., Mafra J.C., de Souza G.E., Vanderlinde F.A. Antinociceptive, anti-inflammatory and antipyretic effects of 1.5-diphenyl-1H-Pyrazole-3-carbohydrazide, a new heterocyclic pyrazole derivative. Life Sci. 2014;95:81–88. doi: 10.1016/j.lfs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Sharath V., Kumar H.V., Naik N. Synthesis of novel indole based scaffolds holding pyrazole ring as anti-inflammatory and antioxidant agents. J. Pharm. Res. 2013;6:785–790. doi: 10.1016/j.jopr.2013.07.002. [DOI] [Google Scholar]
- 12.Mady M.F., Saleh T.S., El-Kateb A.A., Abd El-Rahman N.M., Abd El-Moez S.I. Microwave-assisted synthesis of novel pyrazole and pyrazolo[3,4-d]pyridazine derivatives incorporating diaryl sulfone moiety as potential antimicrobial agents. Res. Chem. Intermed. 2016;42:753–769. doi: 10.1007/s11164-015-2054-x. [DOI] [Google Scholar]
- 13.Yu L.G., Ni T.F., Gao W., He Y., Wang Y.Y., Cui H.W., Yang C.G., Qiu W.W. The synthesis and antibacterial activity of pyrazole-fused tricyclic diterpene derivatives. Eur. J. Med. Chem. 2015;90:10–20. doi: 10.1016/j.ejmech.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Mert S., Kasımoğulları R., İça T., Çolak F., Altun A., Ok S. Synthesis, structure-activity relationships, and in vitro antibacterial and antifungal activity evaluations of novel pyrazole carboxylic and dicarboxylic acid derivatives. Eur. J. Med. Chem. 2014;78:86–96. doi: 10.1016/j.ejmech.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Wang F-Q., Yang H., He B., Jia Y-K., Meng S-Y., Zhang C., Liu H-M., Liu F-W. A novel domino approach for synthesis of indolyltetrahydropyrano[4,3-c]pyrazole derivatives as anticancer agents. Tetrahedron. 2016;72:5769–5775. [Google Scholar]
- 16.Ganga Reddy V., Srinivasa Reddy T., Lakshma Nayak V., Prasad B., Reddy A.P., Ravikumar A., Taj S., Kamal A. Design, synthesis and biological evaluation of N-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-1,3-diphenyl-1H-pyrazole-4-carboxamides as CDK1/Cdc2 inhibitors. Eur. J. Med. Chem. 2016;122:164–177. doi: 10.1016/j.ejmech.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Metwally N.H., Abdelrazek F.M., Eldaly S.M. Synthesis and anticancer activity of some new heterocyclic compounds based on 1-cyanoacetyl-3,5-dimethylpyrazole. Res. ChemIntermed. 2016;42:1071–1089. doi: 10.1007/s11164-015-2074-6. [DOI] [Google Scholar]
- 18.Reddy T.S., Kulhari H., Reddy V.G., Bansal V., Kamal A., Shukla R. Design, synthesis and biological evaluation of 1,3-diphenyl-1H-pyrazole derivatives containing benzimidazole skeleton as potential anticancer and apoptosis inducing agents. Eur. J. Med. Chem. 2015;101:790–805. doi: 10.1016/j.ejmech.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Minu M., Singh D., Mahaddalkar T., Lopus M., Winter P., Ayoub A.T., Missiaen K., Tilli T.M., Pasdar M., Tuszynski J. Chemical synthesis, pharmacological evaluation and in silico analysis of new 2,3,3a,4,5,6-hexahydrocyclopenta[c]pyrazole derivatives as potential anti-mitotic agents. Bioorg. Med. Chem. Lett. 2016;26:3855–3861. doi: 10.1016/j.bmcl.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Lv X.H., Li Q.S., Ren Z.L., Chu M.J., Sun J., Zhang X., Xing M., Zhu H.L., Cao H.Q. (E)-1,3-diphenyl-1H-pyrazole derivatives containing O-benzyl oxime moiety as potential immunosuppressive agents: Design, synthesis, molecular docking and biological evaluation. Eur. J. Med. Chem. 2016;108:586–593. doi: 10.1016/j.ejmech.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Mert S., Alım Z., İşgör M.M., Beydemir S., Kasımoğulları R. The synthesis of novel pyrazole-3,4-dicarboxamides bearing 5-amino-1,3,4-thiadiazole-2-sulfonamide moiety with effective inhibitory activity against the isoforms of human cytosolic carbonic anhydrase I and II. Bioorg. Chem. 2016;68:64–71. doi: 10.1016/j.bioorg.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Guillen E., Gonzalez A., Basu P.K., Ghosh A., Font-Bardia M., Calvet T., Calvis C., Messeguer R., Lopez C. The influence of ancillary ligands on the antitumoral activity of new cyclometallatedPt(II) complexes derived from an ferrocene-pyrazole hybrid. J. Organomet. Chem. 2017;828:122–132. doi: 10.1016/j.jorganchem.2016.11.031. [DOI] [Google Scholar]
- 23.Zhang H., Zhu P., Liu J., Lin Y., Yao H., Jiang J., Ye W., Wu X., Xu J. Synthesis, in vitro and in vivo antitumor activity of pyrazole-fused 23-hydroxybetulinic acid derivatives. Bioorg. Med. Chem. Lett. 2015;25:728–732. doi: 10.1016/j.bmcl.2014.11.058. [DOI] [PubMed] [Google Scholar]
- 24.Fahmy H.H., Khalifa N.M., Ismail M.F., El-Sahrawy H.M., Nossier E.S. Biological Validation of Novel PolysubstitutedPyrazole Candidates with in Vitro Anticancer Activities. Molecules. 2016;21:271–284. doi: 10.3390/molecules21030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismail M.F., Khalifa N.M., Fahmy H.H., EL-Sahrawy H.M., Nossier E.S. Anticancer evaluation of novel 1,3,4-trisubstituted pyrazole candidates bearing different nitrogenous heterocyclic Moieties. Biomed. Res. 2016;27:1087–1093. [Google Scholar]
- 26.Fahmy H.H., Khalifa N.M., Ismail M.F., EL-Sahrawy H.M., Nossier E.S., Ali M.M. In vivo Cytotoxicity of Polysubstituted Pyrazole Derivatives against Liver Cancer Cell Line. Lat. Am. J. Pharm. 2016;35:1783–1790. [Google Scholar]
- 27.Fahmy H.H., Khalifa N.M., Ismail M.F., EL-Sahrawy H.M., Nossier E.S., Ali M.M. Biological Evaluation of Novel 1,3-Diaryl-1H-Pyrazoles Incorporating Different Heterocyclic Ring Systems as Potent Cytotoxic Agents. Lat. Am. J. Pharm. 2016;35:1740–1347. [Google Scholar]
- 28.Ismail M.F., Khalifa N.M., Fahmy H.H., Nossier E.S., Abdulla M.M. Design, Docking, and Synthesis of Some New Pyrazoline and Pyranopyrazole Derivatives as Anti-inflammatory Agents. J. Heterocycl. Chem. 2014;51:450–458. doi: 10.1002/jhet.1757. [DOI] [Google Scholar]
- 29.Fahmy H.H., Khalifa N.M., Nossier E.S., Abdalla M.M., Ismai M.M. Synthesis and anti-inflammatory evaluation of new substituted 1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H pyrazole derivatives. Acta Pol. Pharm. 2012;69:411–421. [PubMed] [Google Scholar]
- 30.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 31.Corell T., Jensen K.M., Splawinski J.A. New Anti-inflammatory Derivative of Imidazole which is Less Ulcerogenic than Indomethacin in Rats. Acta Pharmacol. Toxicol. 1979;45:232–239. doi: 10.1111/j.1600-0773.1979.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 32.Kurumbail R.G., Stevens A.M., Gierse J.K., McDonald J.J., Stegeman R.A., Pak J.Y., Gildehaus D., Iyashiro J.M., Penning T.D., Seibert K., et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 33.Wang J.L., Carter J., Kiefer J.R., Kurumbail R.G., Pawlitz J.L., Brown D., Hartmann S.J., Graneto M.J., Seibert K., Talley J.J. The novel benzopyran class of selective cyclooxygenase-2 inhibitors-part I: The first clinical candidate. Bioorg. Med. Chem. Lett. 2010;20:7155–7158. doi: 10.1016/j.bmcl.2010.07.053. [DOI] [PubMed] [Google Scholar]