Abstract
Background
The aim of this study was to describe the clinical characteristics of Chinese ADPKD inpatients and to identify the factors associated with disease severity.
Material/Methods
We included 167 hospitalized patients (inpatients) with ADPKD in this study. Multiple regression analyses were conducted to determine factors correlated with estimated glomerular filtration rate (eGFR). Patients were stratified into subgroups according to the presence of symptoms, in which clinical parameters were analyzed and compared.
Results
The mean age of hospitalized ADPKD patients was 48.7 years old, lumbar and/or abdominal pain was seen in 40.12% of patients, following by nephrolithiasis (38.92%), hematuria (30.54%), and urinary tract infection (24.55%). Serum thrombocyte level and hemoglobin exhibited significant positive correlations with eGFR. Symptomatic patients accounted for 71.26% of the studied population. Patients with hypertension had increased risk of presence of symptoms (OR=2.794, 95%CI=1.341–5.822). Low thrombocyte and hemoglobin levels were observed in patients with hematuria.
Conclusions
Thrombocyte level was positively correlated with eGFR but was not associated with presence of PKD-related symptoms, suggesting thrombocyte level might be an independent serum biomarker for disease progression. Hypertension was associated with increased risk of symptom occurrence, indicating the relationship between hypertension and disease progression. This study reveals the clinical characteristics of inpatients with ADPKD in China and provides clinicians with useful insights into this intractable disease.
MeSH Keywords: Signs and Symptoms; Glomerular Filtration Rate; Polycystic Kidney, Autosomal Dominant; Urologic Surgical Procedures
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease. Progressively enlarged cysts impair the renal parenchyma, resulting in renal function damage, and ultimately leading to end-stage renal disease (ESRD) [1]. The extrarenal manifestations of ADPKD include hepatic and pancreatic cysts, intracranial aneurysms, cardiac abnormalities, and inguinal hernia [2]. Diverse morbidities (incidence rates from 1/4000 to 1/400) have been reported in different regions of the world, and the European incidence of the disease was recently reported to be 1/2500 [1,3,4]. More than half of ADPKD patients develop ESRD at 50–60 years old, making ADPKD the fourth leading cause of ESRD [3].
ADPKD is caused by mutations in 2 different genes: approximate 85% in PKD1 and 15% in PKD2 [5,6]. However, recent Chinese studies reported a lower incidence (<10%) of PKD2 mutations [7–9]. In addition, approximately 10% of patients diagnosed with ADPKD via clinical features have no mutation detected in either PKD1 or PKD2 gene [5,10]. The latest study reported that mutations in the gene encoding the glucosidase IIα subunit (GANAB) induces ADPKD phenotypes and might be another causal gene [10–12]. PKD1 mutations lead to more severe symptoms and earlier onset of ESRD compared to PKD2 mutations [13]. In addition to genetic background, ADPKD progression is also associated with other factors such as ischemic kidney injury [14,15] and nephrotoxicity [16].
ADPKD patients usually do not exhibit obvious symptoms at the early stage of the disease. Along with the disease progression, patients present lumbar/abdominal pain caused by enlarged kidneys or accompanying nephrolithiasis, hematuria induced by vessel aneurysms or endothelial impairment, and fever due to renal cyst infection. Clinical manifestations and prognosis have been well characterized in developed countries, but ADPKD is often clinically overlooked at its early stage in most developing countries. In China, there are 1.5 million people with this life-threatening disease [17]. Although several recent studies have deepened the understanding of ADPKD in China [9,18–21], huge gaps still exist in ADPKD research between Western countries and China, particularly in central China.
Material and Methods
Study population
From January 2012 to December 2016, 178 inpatients at the First Affiliated Hospital of Anhui Medical University who were clinically diagnosed with ADPKD were included in this study. The diagnosis was based on the Japanese criteria for patients with unknown genotype (PKD1 or PKD2) [22]. Data of patients at admission time were extracted from medical records of the hospital, including general information, laboratory tests, imaging examinations, and clinical features. We excluded 12 patients because of incomplete clinical information. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation to evaluate renal function [23]. Patients with PKD-related symptoms at admission, including lumbar and/or abdominal pain, hematuria, urinary tract infection, and nephrolithiasis, were regarded as symptomatic. Surgical treatments for PKD included cyst decortication and cyst puncture. All clinical data were anonymized before use.
Statistical analysis
The data were statistically evaluated using SPSS V.22.0 (Chicago, IL, USA). Means, standard deviation, and percentage were used to describe the distribution of demographic and clinical parameters. To investigate the correlations between eGFR and major clinical parameters, Spearman’s rho test and partial correlation analysis were performed between eGFR and each parameter, then multiple linear regression analysis was performed to verify the correlation between eGFR and clinical parameters that had statistical significance (P<0.05) in the preceding partial correlation analysis. Shapiro-Wilk analysis was performed to determine normality of variables. Numerical variables with normal distribution and equal variance were evaluated by the t test, while those with non-normal distribution were compared by the Mann-Whitney U test. For multiple statistical tests using the same dataset as in Figure 1A and 1B, thrombocyte and hemoglobin levels in the 3 groups of ADPKD patients with impaired eGFR were compared with those in patients with eGFR over 90 mL/min/1.73 m2. P value was adjusted by Bonferroni correction with PAdjust=P value * 3 (number of independent tests). Categorical variables were assessed by chi-square test. Correlation between the presence of related symptoms and clinical parameters was assessed by logistic regression analysis. With 95% confident interval, P<0.05 was considered to be statistically significant.
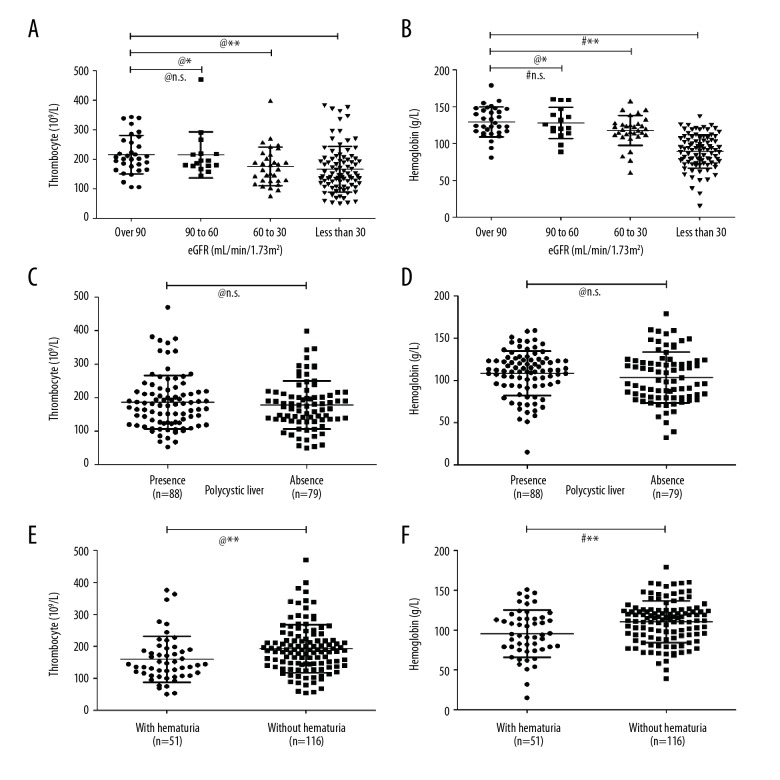
Figure 1.
Thrombocyte and hemoglobin levels in the ADPKD inpatients. (A, B) Thrombocyte and hemoglobin levels in 3 groups of ADPKD patients with impaired eGFR were compared with those in patients with eGFR over 90 mL/min/1.73 m2, Padjust was calculated by 3*P value according to Bonferroni correction. (C, D) Comparison of thrombocyte and hemoglobin levels in ADPKD patients with and without polycystic liver. (E, F) Comparison of thrombocyte and hemoglobin levels in ADPKD patients with and without hematuria. # t test; @ Mann-Whitney U test; * P<0.05; ** P<0.01.
Results
General characteristics of the ADPKD patients
In total, 167 ADPKD inpatients (72 males and 95 females) with evaluable clinical information were analyzed in the study. Clinical features of the patients are shown in Table 1. Patients admitted to hospital had an average age of 48.7 (16~78) years. Hypertension and polycystic liver disease were found in 84 (50.30%) and 88 (52.69%) cases, respectively. There were 119 (71.26%) who patients exhibited 1 or more PKD-related symptoms. Lumbar and/or abdominal pain was the most frequently symptom and was seen in 40.12% of patients, followed by hematuria (30.54%) and urinary tract infection (24.55%). Nephrolithiasis was found in 65 (38.92%) ADPKD patients. The remaining 48 (28.74%) patients without symptoms were hospitalized for further investigation because of an accidental finding of polycystic kidney via physical examination. Blood tests showed that the patients had an average leukocyte count of 6.75×109/L, thrombocyte count of 182.58×109/L, and hemoglobin level of 106 g/L. Mean serum creatinine level of ADPKD patients was 308.56 μmol/L and calculated eGFR was 42.81 mL/min/1.73 m2, indicating impaired renal function. In addition, 43 (25.75%) patients received surgical treatment during hospitalization.
Table 1.
Clinical features of the ADPKD patients in this study (mean ±SD or percentage).
| Total(n=167) | |
|---|---|
| Demographic features | |
| Age (years) | 48.66±10.57 |
| Age at diagnosis (years) | 41.60±12.34 |
| Gender(male/female) | 72/95 |
| Positive family history | 65 (38.92%) |
| Blood test parameters | |
| Leukocytes (109/L) | 6.75±3.03 |
| Thrombocytes (109/L) | 182.58±75.74 |
| Hemoglobin (g/L) | 105.97±28.15 |
| Total protein (g/L) | 67.43±7.98 |
| Albumin (g/L) | 39.81±6.90 |
| Globulin (g/L) | 27.63±4.35 |
| Creatinine (μmol/L) | 308.56±290.47 |
| eGFR (mL/min/1.73 m2) | 42.81±38.12 |
| Clinical features | |
| Hypertension (%) | 84 (50.30%) |
| Polycystic liver (%) | 88 (52.69%) |
| Presence of related symptoms (%) | 119 (71.25%) |
| Nephrolithiasis (%) | 65 (38.92%) |
| Flank pain (%) | 67 (40.12%) |
| Hematuria (%) | 51 (30.54%) |
| Urinary tract infection (%) | 41 (24.55%) |
| Surgical treatment (%) | 43 (25.75%) |
eGFR – estimated glomerular filtration rate.
Correlation between eGFR and clinical parameters in the ADPKD patients
eGFR is the fundamental indicator in assessment of renal disease progression and therapeutic effects. We therefore analyzed the correlations between eGFR and diverse clinical parameters in the ADPKD patients (Table 2). No correlation was found between eGFR and polycystic liver, leukocytes, albumin, globulin, or presence of related symptoms in the patients. Partial correlation analysis showed that age (P=0.029) and hypertension (P=0.021) were negatively correlated with eGFR, while female gender (P=0.035), thrombocyte (P=0.001), and hemoglobin (P<0.001) were positively correlated with eGFR. To further validate these 5 clinical parameters, multiple linear regression analysis was performed. The result revealed that the associations between eGFR and these parameters were still statistically significant (Table 2, right column), suggesting that hypertension, thrombocyte, and hemoglobin are independent correlation factors of eGFR in ADPKD patients.
Table 2.
Correlation analysis between eGFR and major clinical parameters in ADPKD patients.
| Parameters | eGFR | Multiple linear regression | ||
|---|---|---|---|---|
| Adjust R | P | β | P | |
| Age | −0.172 | 0.029* | −0.504 | 0.020* |
| Female gender | 0.167 | 0.035* | 9.259 | 0.045* |
| Hypertension | −0.182 | 0.021* | −10.497 | 0.018* |
| Polycystic liver | 0.041 | 0.601 | ||
| Leukocyte | −0.037 | 0.643 | ||
| Thrombocyte | 0.261 | 0.001** | 0.084 | 0.005* |
| Hemoglobin | 0.547 | <0.001** | 0.823 | <0.001** |
| Albumin | 0.052 | 0.507 | ||
| Globulin | −0.138 | 0.080 | ||
| Related symptoms | 0.082 | 0.305 | ||
Correlations between eGFR and major clinical parameters were assessed by Spearman rho test and adjusted by partial correlation analysis. Multiple linear regression was performed to validate these identified parameters.
P<0.05;
P<0.01.
Hypertension increased risk of PKD-related symptoms
In a total of 167 patients, 48 (28.74%) were admitted to hospital for further investigation because of physical examination-detected renal cysts while without symptoms. All of the other 119 (71.26%) patients presented PKD-related symptoms, including lumbar and/or abdominal pain, hematuria, urinary tract infection, and nephrolithiasis. Because symptoms are the main cause of hospitalization, we then evaluated the correlations between symptoms and clinical parameters in ADPKD patients (Table 3). The results showed that patients with hypertension account for the largest proportion of patients with related symptoms (P=0.017). Compared to patients without hypertension, patients with hypertension were more likely to have symptoms (OR=2.794, 95% CI=1.341–5.822). It is not surprising that leukocyte and hemoglobin levels were also correlated with presence of symptoms, because these symptoms are usually associated with inflammation and hemorrhage. Interestingly, thrombocyte count did not correlate with presence of symptoms, indicating that thrombocyte count is barely influenced by PKD-related events. Combined with the result that thrombocyte count is positively correlated with eGFR, thrombocyte count might be an independent serum biomarker related to disease progression.
Table 3.
Correlation analysis between symptoms and clinical parameters in ADPKD patients.
| With related symptoms | Without related symptoms | P | Logistic regression | ||
|---|---|---|---|---|---|
| P | OR | ||||
| Total | 119 | 48 | |||
| Age (years) | 48.98±10.05 | 47.85±11.85 | #0.658 | 0.286 | |
| Gender (male/female, %) | 45/74 | 27/21 | §0.038* | 0.161 | |
| Hypertension (%) | |||||
| Yes | 67 (56.3) | 17 (35.4) | §0.017* | 0.006** | 2.794 |
| No | 52 (43.7) | 31 (64.6) | |||
| Polycystic liver (%) | |||||
| Yes | 60 (50.4) | 28 (58.3) | §0.395 | 0.354 | |
| No | 59 (49.6) | 20 (41.7) | |||
| Leukocyte (109/L) | 7.05±3.35 | 5.98±1.87 | #0.126 | 0.027* | 1.194 |
| Thrombocyte (109/L) | 184.35±80.42 | 178.79±63.22 | #0.997 | 0.867 | |
| Hemoglobin (g/L) | 102.65±26.39 | 114.21±30.86 | #0.022* | 0.013* | 0.983 |
| Albumin (g/L) | 39.42±7.20 | 40.77±6.04 | #0.106 | 0.938 | |
| Globulin (g/L) | 28.08±4.78 | 26.50±3.84 | @0.034* | 0.342 | |
| eGFR (mL/min/1.73m2) | 42.16±3.56 | 44.43±5.31 | #0.728 | 0.260 | |
Correlations between the presence of related symptoms and clinical parameters were assessed by Student’s T-test, Mann-Whitney U test or Chi-Square test. Logistic regression was performed to validate these identified parameters.
Student’s T-test;
Mann-Whitney U test;
Chi-Square test.
P<0.05;
P<0.01.
Thrombocyte count is correlated with hematuria but not polycystic liver
Both thrombocyte and hemoglobin levels exhibited negative correlations with eGFR; therefore, we divided the patients into 4 groups according to eGFR to further assess correlations (Figure 1A, 1B). Patients with eGFR in the range of 60–30 and less than 30 mL/min/1.73 m2 had significantly lower hemoglobin levels compared to patients with eGFR over 60 mL/min/1.73 m2 (PAdjust(60 to 30 vs. over 90)=0.041, PAdjust(less than 30 vs. over 90)=0.001), which may be attributed to renal anemia. Thrombocyte showed a similar pattern to that of hemoglobin in ADPKD patients (PAdjust(60 to 30 vs. over 90) <0.001, PAdjust(less than 30 vs. over 90) <0.001). However, thrombocyte and hemoglobin levels showed no difference between patients with and without cystic liver (Figure 1C, P=0.749; Figure 1D, P=0.169). To assess potential effects of decreased thrombocytes, thrombocyte levels between patients with and without hematuria were analyzed. Although thrombocyte level showed no correlation with presence of overall symptoms, it did decline in patients with hematuria (Figure 1E, P=0.002), suggesting decreased thrombocyte level might associate with hemorrhagic complications. Hemoglobin level showed the same change in patients with hematuria (Figure 1F, P=0.001).
Discussion
ADPKD is now the fourth leading cause of ESRD, leading to poor outcomes and imposing a great economic burden on society [3]. It is usually asymptomatic at the early stage, and the number and volume of renal cysts increase with age in an exponential manner [24]. The progression of renal cysts results in a variety of clinical symptoms and complications, including abdominal pain, hematuria, urinary tract infection, and nephrolithiasis [25]. Our study found that approximately 40% of ADPKD patients had abdominal or lumbar pain as the chief complaint, and the incidence rate of hematuria and urinary tract infection were 30% and 25%, respectively, generally agreeing with the results of other global studies of ADPKD patients [21,26,27]. Hypertension is a common complication and often occurs early in ADPKD patients. It is also associated with disease progression to end-stage renal disease [28]. Although recent HALT-PKD studies showed that rennin-angiotensin-aldosterone system blockade does not alter the declining rate of eGFR [28,29], blood pressure control may be associated with reduced mortality in ADPKD patients [30]. In the present study, hypertension occurred in half of the patients and increased the risk of specific symptoms, including pain, hematuria, infection, and nephrolithiasis. In addition, the average age of ADPKD patients at diagnosis in HALT-PKD trials was 30 years old [31]. However, the average diagnosis age in our study was approximately 42 years old, indicating that many Chinese patients with ADPKD are diagnosed relatively late. Since early diagnosis and managements might benefit ADPKD patients, it should be encouraged and taught that all ADPKD patients in China need early clinical attention and diagnosis.
Renal anemia is a common complication in patients with chronic kidney disease. In our study, hemoglobin was significantly decreased in patients with low eGFR. We also found that thrombocyte count was correlated with eGFR, similar to hemoglobin. Patients with low eGFR showed declined thrombocyte levels, and mean thrombocyte levels of these ADPKD patients were lower than the mean value in a Chinese population, as reported by Li et al. [32]. Furthermore, there was a significant positive correlation between thrombocyte count and eGFR, consistent with a previous study [21]. It has been reported that ADPKD patients have decreased thrombocyte levels [33], but the underlying mechanism and its effect on the progression of ADPKD remains unclear. Recently, Setyapranata et al. [34] found that dialysis-treated ADPKD patients had lower thrombocyte levels than those dialysis-treated non-ADPKD patients with CKD. Because ADPKD is characterized by lesions in renal tubules and biliary ducts, while other CKDs mostly affect glomeruli, we speculate that thrombopoietin (TPO), the major thrombocyte regulator produced in the liver, kidneys, and skeletal muscle [35], might be involved in the low thrombocyte counts of ADPKD patients with exacerbated renal-hepatic cysts. However, we did not find any differences in thrombocyte counts between patients with and without polycystic liver, suggesting that renal lesions are the main pathological change in ADPKD patients and may have a dominant effect on thrombocyte counts and hemoglobin levels. In addition, thrombocyte count was significantly lower in patients with hematuria, suggesting that thrombocyte count is not only an independent correlative factor of renal function, but may also be associated with hemorrhagic complications of ADPKD. Treatments for renal anemia that focus on erythropoietin (EPO) have become more common in the new age of gene therapy [36], and further research on thrombocyte count might have clinical relevance, such as in preventing hemorrhagic events and improving quality of life.
Currently, treatment options for this incurable disease remain limited. As the most widely used surgical procedure, renal cyst decortication has only been shown to be effective in pain relief and it cannot delay the progression of ADPKD [37]. Several drugs (e.g., mTOR inhibitors, somatostatin analogues, and tyrosine kinase inhibitor) identified in animal models were shown to be ineffective in clinical trials [38–42]. Tolvaptan is the only drug approved for ADPKD treatment [26,43], but its clinical use is challenged by reports of adverse effects [44]. Other drugs, such as metabolism modulators [45–47], autophagy modulators [48], and roscovitine [49], were also assessed in preclinical trials. These promising drugs need further investigation and clinical assessment to develop effective therapeutics with minimal adverse effects for ADPKD patients.
Conclusions
We described the clinical features and manifestations of ADPKD inpatients in a single center in China. As the essential indicator of disease progression, eGFR is positively correlated with blood thrombocyte and hemoglobin levels. Furthermore, thrombocyte count was not associated with presence of PKD-related symptoms, and patients with hematuria exhibited a lower thrombocyte level, suggesting that thrombocyte count might be an independent serum biomarker related to disease progression and complications. But owing to the limitation of this observational study, the role of thrombocyte count in ADPKD needs to be confirmed in prospective studies, and the underlying mechanism needs further investigation. In addition, hypertension was found to increase the risk of symptom occurrence, highlighting the association between hypertension and disease progression. Recent studies revealed that TKV is a valuable clinical biomarker to predict disease progression [20,24,50,51], prompting us to perform the present study. Further investigations on other systemic manifestations such as cardiac abnormalities or intracranial aneurysms are needed, as is increased focus on comprehensive family history or screening of relatives.
Acknowledgements
We thank Professor Guanqing Wu for his kind help in manuscript editing.
Footnotes
Source of support: This manuscript was supported by the National Natural Science Foundation of China (81630019 to C Liang; 81400757 to S Fan), the National College Students Innovation and Entrepreneurship Training Program (201510366009 to S Fan), and the Sci-Tech Planning Projects of Anhui Province, China (1704e1002230 to C Liang)
Conflict of interest
None.
References
- 1.Harris PC, Torres VE. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest. 2014;124:2315–24. doi: 10.1172/JCI72272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rangan GK, Tchan MC, Tong A, et al. Recent advances in autosomal-dominant polycystic kidney disease. Intern Med J. 2016;46:883–92. doi: 10.1111/imj.13143. [DOI] [PubMed] [Google Scholar]
- 3.Ong AC1, Devuyst O2, Knebelmann B3, Walz G4 ERA-EDTA Working Group for Inherited Kidney Diseases. Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet. 2015;385:1993–2002. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 4.Willey CJ, Blais JD, Hall AK, et al. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. 2016;32:1356–63. doi: 10.1093/ndt/gfw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–60. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 6.Fedeles SV, Gallagher AR, Somlo S. Polycystin-1: A master regulator of intersecting cystic pathways. Trends Mol Med. 2014;20:251–60. doi: 10.1016/j.molmed.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B, Chen SC, Yang YM, et al. Identification of novel PKD1 and PKD2 mutations in a Chinese population with autosomal dominant polycystic kidney disease. Sci Rep. 2015;5:17468–79. doi: 10.1038/srep17468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Ma Y, Gu X, et al. Novel mutations in the PKD1 and PKD2 genes of Chinese patients with autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2018;43:297–309. doi: 10.1159/000487899. [DOI] [PubMed] [Google Scholar]
- 9.Jin M, Xie Y, Chen Z, et al. System analysis of gene mutations and clinical phenotype in Chinese patients with autosomal-dominant polycystic kidney disease. Sci Rep. 2016;6 doi: 10.1038/srep35945. 35945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porath B, Gainullin VG, Cornec-Le Gall E, et al. Harris PC Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease. Mutations in GANAB, encoding the glucosidase iialpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98:1193–207. doi: 10.1016/j.ajhg.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iliuta IA, Kalatharan V, Wang K, et al. Polycystic kidney disease without an apparent family history. J Am Soc Nephrol. 2017;28:2768–76. doi: 10.1681/ASN.2016090938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy BV, Chapman AB. A patient with a novel gene mutation leading to autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2017;12:1695–98. doi: 10.2215/CJN.02830317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornec-Le Gall E, Audrezet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006–13. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel V, Li L, Cobo-Stark P, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–90. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurbegovic A, Trudel M. Acute kidney injury induces hallmarks of polycystic kidney disease. Am J Physiol Renal Physiol. 2016;311:F740–51. doi: 10.1152/ajprenal.00167.2016. [DOI] [PubMed] [Google Scholar]
- 16.Happé H, Leonhard WN, van der Wal A, et al. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet. 2009;18:2532–42. doi: 10.1093/hmg/ddp190. [DOI] [PubMed] [Google Scholar]
- 17.Xue C, Zhou CC, Wu M, Mei CL. The clinical manifestation and management of autosomal dominant polycystic kidney disease in China. Kidney Dis (Basel) 2016;2:111–19. doi: 10.1159/000449030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Ma Y, Wang X, et al. Triptolide-containing formulation in patients with autosomal dominant polycystic kidney disease and proteinuria: An uncontrolled trial. Am J Kidney Dis. 2014;63:1070–72. doi: 10.1053/j.ajkd.2014.01.418. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Chen SC, Yang YM, et al. Identification of novel PKD1 and PKD2 mutations in a Chinese population with autosomal dominant polycystic kidney disease. Sci Rep. 2015;5 doi: 10.1038/srep17468. 17468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue C, Zhou C, Mei C. Total kidney volume: The most valuable predictor of autosomal dominant polycystic kidney disease progression. Kidney Int. 2018;93:540–42. doi: 10.1016/j.kint.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Ma Y, Wang X, et al. Clinical characteristics and disease predictors of a large Chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092232. e92232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horie S, Mochizuki T, Muto S, et al. Evidence-based clinical practice guidelines for polycystic kidney disease 2014. Clin Exp Nephrol. 2016;20:493–509. doi: 10.1007/s10157-015-1219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grantham JJ, Torres VE. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol. 2016;12:667–77. doi: 10.1038/nrneph.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akoh JA. Current management of autosomal dominant polycystic kidney disease. World J Nephrol. 2015;4:468–79. doi: 10.5527/wjn.v4.i4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres VE, Chapman AB, Devuyst O, et al. TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vikrant S, Parashar A. Autosomal dominant polycystic kidney disease: Study of clinical characteristics in an Indian population. Saudi J Kidney Dis Transpl. 2017;28:115–24. doi: 10.4103/1319-2442.198163. [DOI] [PubMed] [Google Scholar]
- 28.Schrier RW, Abebe KZ, Perrone RD, et al. HALT-PKD Trial Investigators. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–66. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2267–76. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patch C, Charlton J, Roderick PJ, Gulliford MC. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: A population-based study. Am J Kidney Dis. 2011;57:856–62. doi: 10.1053/j.ajkd.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Torres VE, Chapman AB, Perrone RD, et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012;81:577–85. doi: 10.1038/ki.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XS, Zhang JR, Meng SY, et al. Mean platelet volume is negatively associated with bone mineral density in postmenopausal women. J Bone Miner Metab. 2012;30:660–65. doi: 10.1007/s00774-012-0362-4. [DOI] [PubMed] [Google Scholar]
- 33.Bath PM, Saggar-Malik AK, Macdougall IC, et al. Original articles: increased platelet volume in patients with adult polycystic kidney disease. Platelets. 1995;6:336–39. doi: 10.3109/09537109509078468. [DOI] [PubMed] [Google Scholar]
- 34.Setyapranata S, Holt SG. Platelet counts in autosomal dominant polycystic kidney disease. Platelets. 2016;27:262–63. doi: 10.3109/09537104.2015.1071481. [DOI] [PubMed] [Google Scholar]
- 35.Foster DC, Sprecher CA, Grant FJ, et al. Human thrombopoietin: Gene structure, cDNA sequence, expression, and chromosomal localization. Proc Natl Acad Sci USA. 1994;91:13023–27. doi: 10.1073/pnas.91.26.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonomini M, Del Vecchio L, Sirolli V, Locatelli F. New treatment approaches for the anemia of CKD. Am J Kidney Dis. 2016;67:133–42. doi: 10.1053/j.ajkd.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Millar M, Tanagho YS, Haseebuddin M, et al. Surgical cyst decortication in autosomal dominant polycystic kidney disease. J Endourol. 2013;27:528–34. doi: 10.1089/end.2012.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–29. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 39.Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–40. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 40.Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–16. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 41.Caroli A, Perico N, Perna A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): A randomised, placebo-controlled, multicentre trial. Lancet. 2013;382:1485–95. doi: 10.1016/S0140-6736(13)61407-5. [DOI] [PubMed] [Google Scholar]
- 42.Tesar V, Ciechanowski K, Pei Y, et al. Bosutinib versus placebo for autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2017;28:3404–13. doi: 10.1681/ASN.2016111232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres VE, Chapman AB, Devuyst O, et al. REPRISE Trial Investigators. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377:1930–42. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 44.Horie S. Will introduction of tolvaptan change clinical practice in autosomal dominant polycystic kidney disease? Kidney Int. 2015;88:14–16. doi: 10.1038/ki.2015.143. [DOI] [PubMed] [Google Scholar]
- 45.Soomro I, Sun Y, Li Z, et al. Glutamine metabolism via glutaminase 1 in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2018 doi: 10.1093/ndt/gfx349. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flowers EM, Sudderth J, Zacharias L, et al. Lkb1 deficiency confers glutamine dependency in polycystic kidney disease. Nat Commun. 2018;9:814. doi: 10.1038/s41467-018-03036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiaravalli M, Rowe I, Mannella V, et al. 2-deoxy-d-glucose ameliorates PKD progression. J Am Soc Nephrol. 2016;27:1958–69. doi: 10.1681/ASN.2015030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu P, Sieben CJ, Xu X, et al. Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum Mol Genet. 2017;26:158–72. doi: 10.1093/hmg/ddw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billot K, Coquil C, Villiers B, et al. Casein kinase 1epsilon and 1alpha as novel players in polycystic kidney disease and mechanistic targets for (R)-roscovitine and (S)-CR8. Am J Physiol Renal Physiol. 2018 doi: 10.1152/ajprenal.00489.2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrone RD, Mouksassi MS, Romero K, et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep. 2017;2:442–50. doi: 10.1016/j.ekir.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu ASL, Shen C, Landsittel DP, et al. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2018;93:691–99. doi: 10.1016/j.kint.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]