Abstract
Background:
Diabetes care is predominately done at home by the patient. When clinics do not have a reliable, easy process for obtaining this patient data, clinical decisions must be made with incomplete verbal recall reports. Unused or inaccessible glucose data represent a large information gap affecting clinical decision making. This study’s purpose was to design an optimized glucose device download system with a standardized report and to evaluate its efficiency.
Methods:
Observations and evaluations of glucose data retrieval occurred at two clinics; an additional clinic utilized the optimized process doing only post process timings. Patients/families and clinicians were surveyed about their experiences with the system and the standardized report (AGP). The study was approved by all the sites’ IRBs.
Results:
Optimized systems saved staff at least 3 min per patient. Standardized AGP reports and an optimized data system made the work flow of glucose data easier to complete. The AGP report was preferred by patients, families, and clinicians.
Conclusions:
An optimized system takes advantage of patient lobby downtime to download glucose devices and ensures that diabetes clinical decisions are made utilizing all available data. Staff and patients liked the software lobby system and found it a valuable time-saving tool.
Keywords: AGP, diabetes care, standardized systems, time savings
Optimized diabetes care requires an understanding of glucose patterns. While HbA1c rates help give an average of glucose control, the only way to truly understand the patient’s daily experience is through glucose tracking using self-monitoring blood glucose meters (SMBG) or continuous glucose monitoring (CGM).1-3 These data inform insulin adjustments, hypoglycemia identification, and modifications to nutritional intake and activity. Although clinicians find glucose data important and useful, most clinics struggle with data acquisition.
Diabetes devices cause numerous operational problems for clinics. In the United States, there are 98 different glucose monitoring devices available.4 Each manufacturer has proprietary software and device-to-computer connector cable for downloading data. Each software displays unique reports. Clinical staff on average have 5 or 6 software programs on their PCs and numerous connection cables. The staff must match the cable with the device and the software to enable them to print a report for use in the visit. This complex process of softwares, cables, and reporting makes these systems difficult for staff to use and impossible to ask patients to use in downloading data. In busy clinical settings, device downloads take a great deal of staff time and may still not be successful.
Additional struggles occur for staff when the softwares are not reliably functioning. Manufacturers of the devices and softwares inconsistently updated without warning to the clinics. The software update requirements can cause the system to stop working not only for the software from one manufacturer, but several at the same time due to port connections. This inconsistent device downloads reliability leads to random interruptions and inaccessible data during a clinical day. In many clinics, staff are unable to update and fix the software due to restricted IT administrative permissions; requiring longer downtimes. To support the highest quality diabetes care, glucose data must be accessible and usable.
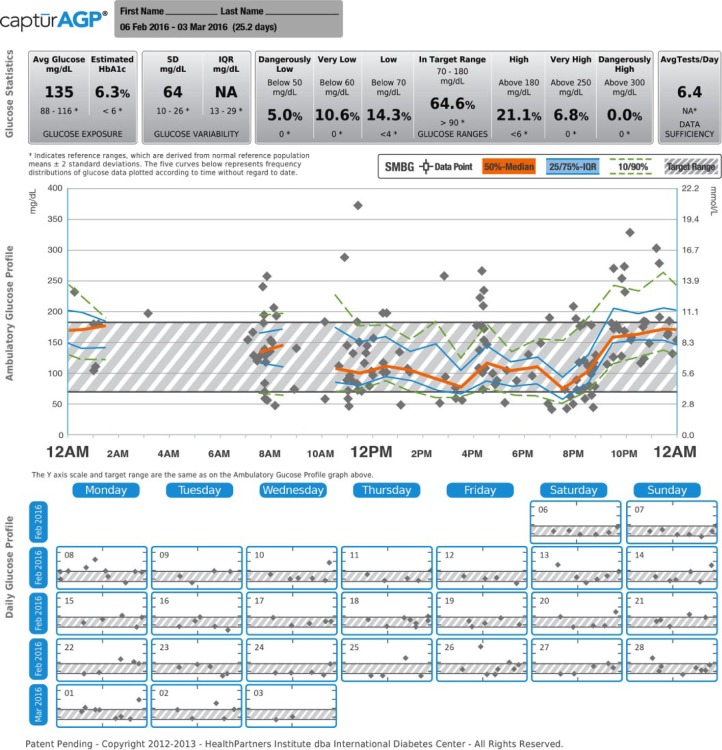
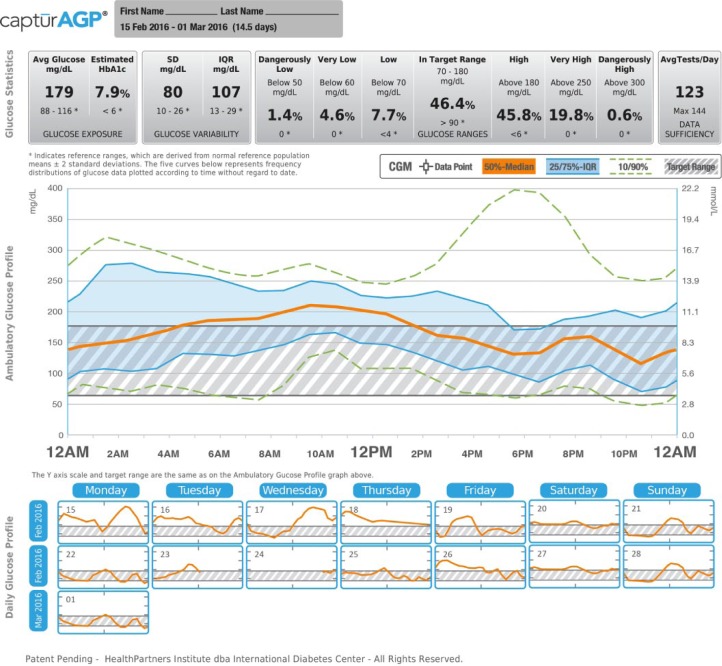
Recognizing the importance of these data, many professional and advocacy groups have suggested that a standardized report would help both clinicians and patients to better utilize glucose data.5-7 The International Diabetes Center (IDC) developed the single page standardized ambulatory glucose profile (AGP) report (Figures 1 and 2).8,9 This single-page report, endorsed internationally, reduces the glucose reporting challenges by replacing the device-specific reports with standardized graphic displays of glucose trends.6,8,10,11 The AGP report is generated using the CaptūrAGP software and a middleware device downloader system. A middleware device downloader system is a software that allows the downloading of multiple manufacturers’ devices using a single software such as Glooko + Diasend, Tidepool, and previously Sweetspot. The CaptūrAGP software is a research version of the AGP reporting that was built by the IDC prior to the licensed AGP reports now available in Diasend middleware and in several device specific softwares for no cost to clinicians beyond the cost of middleware subscriptions (more information at AGPreport.org). The CaptūrAGP v3 software is a secure cloud-based interactive program that produces graphical and statistical representations of a patient’s daily glucose patterns and key characteristics of glycemic control (time in range and variability). AGP reduces the clinician and patient burden by making glucose data easily comparable across devices.
Figure 1.
CaptūrAGP report—continuous glucose monitoring (CGM).
Figure 2.
CaptūrAGP report—self-monitoring blood glucose (SMBG).
Overall study aims were to (1) chart an optimized work flow for use of glucose data that could save time for clinic staff, (2) enhance patient/clinician interactions by providing standardized glucose reports in a timely, easy-to-use format, (3) determine what report features clinicians prefer and use, and (4) determine what report features patients/families prefer and use. Aims 3 and 4 are addressed in more detail in a subsequent manuscript. Aims 3 and 4 overall found that patients and clinicians felt the AGP report to be useful and preferred to other methods of looking at glucose data (on the devices screen, device specific reports, on the web).
Methods
Study Design
This study was a prospective, intervention time in motion (TIM) study with pre- and postintervention data collection. This study was approved by IRBs at each site. Two sites were selected for the TIM study, a midwestern pediatric practice and a southern US mixed adult/pediatric practice. A third pediatric study site in the northeastern United States also implemented the redesigned work flow, but did not complete the full set of pre TIM measurements and is therefore not included in the results. All sites are part of a national network of clinics specializing in diabetes care, the T1D Exchange, and have different clinic configurations.12 These sites were chosen to ensure that a varied population of patients tested the system.
Pilot Test
Before deploying the system in the clinical sites for the TIM study, a pilot test at one pediatric site was completed, using two systems. The middleware systems allow clinics to download a wide variety of manufacturer’s devices using a single software package instead of using each manufacturer’s proprietary software. The systems chosen represented two of the three options on the market in 2014. Diasend uses a cellular-enabled transmitter box without requiring a computer. SweetSpot was a web-based software on a computer. All optimized systems included a cable box, affectionately called the “octopus,” which hid a 10-port USB hub; the box simplified the cables, keeping them organized.
Pilot testing included 12 pediatric participants ages 7+ and their parents per set up (total of 24 participants). Tests were conducted to verify that the system interfaces between middleware and CaptūrAGP were reliable and efficient. Participants were a convenience sample of patients. Since efficiency and effectiveness of reporting were key to the study, this pilot was done using the RITE method (Medlock, Wixon, Terrano, Romero, & Fulton, 2002).13 RITE is similar to the traditional quality improvement cycle of Plan-Do-Study/Check-Act and the software “agile development” cycle. The RITE method included multiple feedback cycles, with users providing feedback about system problems. Fixes were implemented and then the next user tried the improved system. This process was repeated until all technological challenges were resolved.
As patients entered the clinic, they were asked if they would like to try a new download work flow before or after their clinic visit. After obtaining informed parental consent and participant assent, one staff member began timing the download steps and another guided the patient to the download desk behind a privacy divider in the endocrinology lobby. The desk included a PC with device cables (Sweetspot) or a transmitter box (Diasend) with cables as well as a text and pictorial download guide. Staff observed and only intervened when the download did not succeed multiple times. Staff only needed to step in twice; both times when the SMBG device was difficult to place in transmit mode. Once shown how to place in transmit mode, patients were able to activate the device and complete the process. Upon successful completion of the download, staff showed the patient/family member the glucose report.
Redesigning the Download Work Flow
Redesigning the download work flow involved three main steps: assessing the traditional work flow, using the information gained to modify the work flow, and testing the revised work flow. Assessing the traditional work flow involved documenting and timing the work flow at two sites using Toyota Lean (Kaizen) work flow mapping.14,15 Mapping included observing multiple staff completing the work flow, drawing a map of the work site, and labeling each step. Once a complete map was ready, the amount of time required by each step was recorded multiple times and the average TIM calculated. Work flow mapping began when a patient arrived and ended with glucose report creation. Previous work at the site had shown that clinician interpretation of the glucose report with patients, is highly variable based on the complexity of the patient’s health; therefore, this was excluded from the TIM measurement.
The goal of redesigning the work flow was to reduce the time staff spent generating glucose reports. The work flow was revised with input from staff and vetted with clinicians to ensure that no critical steps were missed. Once approved, the optimized design with fewer steps was implemented and timed. Two sites participated in the work flow redesign and timing using the Sweetspot and Diasend systems. To assess the revised work flow, participants were surveyed about their download experiences using a self-administered questionnaire developed for this study. The results of this paper include the time in motion results of the first 2 sites. Twenty patients were recruited per site by the clinical team based on who they felt would benefit from more intensive SMBG testing schedules or a CGM trial period. Staff (reception staff, medical assistants, clinic managers, and clinicians) comments were collected during interviews. AGP reports were printed prior to the rooming to ensure clinicians were able to utilize reports during visits.
All TIM data were collected using Excel based templates. For the pilot testing and traditional versus optimized test, subject and staff comments were recorded and summarized into recurrent themes. Survey data were summarized using SAS v9.3.16
Results
Pilot
Patients/families expressed interest in trying the systems and were generally successful following download instructions even though few patients had ever tried downloading their devices previously. Devices that required mode changing using multiple buttons to prep for downloading were the most difficult for patients. Participants reported that both systems were easy to use, but were unsure about whether they had successfully downloaded even when notified by the systems of success. Every participant was interested in trying the system at future visits and were able to complete downloads within <5 min average.
Traditional Work
Traditional work flows relied heavily on staff. Site 1 had front desk staff download devices while checking patients in. At other sites (2 and 3) during check in, staff collected the devices and handed them off to lab or informatics staff for download. Average time for downloads was 16 min 14 s (minimum 8 min 19 s; maximum 24 min 6 s) (Table 1). The variability in these timings was due to the number of staff involved, the distance from patient/device check in to download stations, and the volume of other work being completed at the same time. Patient check in was not measured since the standard work includes things like insurance and demographics checks as well as paperwork that are not relevant to this glucose device process.
Table 1.
Traditional Work Flow Time in Motion.
| Traditional process—staff
download in back office | |||
|---|---|---|---|
| Task completed | Site 1 | Site 2 | Average |
| Patient checks in at reception desk | Not measured | ||
| Staff obtains device(s) for downloading | 3 min 12 s | 4 min 39 s | 3 min 56 s |
| Staff logs into download software and downloads data | 4 min 15 s | 4 min 34 s | 4 min 25 s |
| Staff prints report and hands device(s) back to patient | 52 s | 14 min 53 s | 7 min 53 s |
| Staff time total | 8 min 19 s | 24 min 6 s | 16 min 14 s |
Staff had the following complaints about device downloads:
Each manufacturer has unique software and several cables
Each software has a unique report; for patients with several devices, different reports exist
Software updates occur inconsistently, resulting in unpredictable downtime and delays waiting for IT
These softwares are incompatible with operating system, search engine, firewall, and/or USB protocols as well as with each other; fixes often break another software
Software troubleshooting is complicated
Staff also noted that these downloads occur concurrently with other responsibilities and are burdensome even when the work flow works. When the work flow breaks down due to software updates or missing cables, visits have to be completed without glucose data. Clinicians felt that the missing data lead to missed education and treatment opportunities, but they could not delay patient schedules to do extensive troubleshooting and generate the report.
Optimized Work
The optimized work flow placed a device download kiosk in the lobby and asked patients to complete downloads. Participants were given written instructions with photos and color-coded cables to assist with downloading since they had never seen these systems previously. The average time for downloads was 4 min 44 s (minimum 3 min 17 s; maximum 6 min 27 s) (Table 2). SweetSpot took longer (6 min 27 s) versus Diasend (3 min 17 s) because the software required typing the study ID, false date of birth, and selecting their device from a drop down menu. Staff were able to log into the CaptūrAGP system in the back office and print the one-page report in an average of 48 s. Automatic printing is possible, but not used in this study. In the web version of the AGP report, the report can be viewed and manipulated on screen. In this study to allow patients to use the AGP educational guide and report while answering the surveys, the reports were printed.
Table 2.
Optimized Time in Motion.
| Optimized process—Patient
download front office with AGP report | |||
|---|---|---|---|
| Task completed | Diasend site 1 | Sweetspot site 2 | Average |
| Patient sits down at station | Not measured | ||
| Patient connects device to cable for downloading | 42 s | 3 min 20 s | 1 min 52 s |
| Device download is complete | 61 s | 1 min 52 s | 1 min 27 s |
| Patient removes device and leaves station | 31 s | 42 s | 37 s |
| Patient time total | 2 min 14 s | 5 min 54 s | 3 min 56 s |
| Staff log into download software website | Not measured | ||
| Staff finds device data file and enters demographics | 30 s | 0 s | 15 s |
| Staff logs into CaptūrAGP | 12 s | 12 s | 12 s |
| Staff finds patient data in CaptūrAGP | 17 s | 17 s | 17 s |
| Staff opens patient record to print report | 2 s | 2 s | 2 s |
| Staff print AGP report | 2 s | 2 s | 2 s |
| Staff time total | 1 min 3 s | 33 s | 48 s |
| Overall effort | 3 min 17 s | 6 min 27 s | 4 min 44 s |
A conservative estimate of time savings for staff was at least 3 min per patient. In a clinic with 20 patients per day, optimized downloading would save 1 hour of staff time/day. Over the course of a year in a clinic of 4 clinicians, this time savings would translate to saving 0.5 full time employee (FTE). With medical assistants nationally earning $31 500 before benefits and RNs earning much more; time saved translates to sizeable dollars.17 Staff positively responded: saves staff time (64%), adds value to practice (82%), and downloads were easy for patients (42%). Staff felt downloads were too hard for patients (52%). Middleware softwares prices for clinics are $0-$150 per month dependent on the options and systems desired.
Sixty subjects downloaded; half used their own SMBG devices, half used CGM devices. None of the subjects had used CGMs previously; Dexcom Gen 4 (or Pediatric) CGM after enrollment. Most participants used their SMBG 3-6 times daily looking at the device screen to review the data (Table 3). They reported that the data are useful (95%) and are most often used to determine the amount or when to administer insulin (Table 3). Participants also reported most often using glucose data to make immediate treatment decisions, but only sometimes, rarely, or never using these data to examine glucose trends (65%-70%) (Table 3).
Table 3.
Optimized Work Flow Subject Characteristics.
| Subject characteristics—Optimized work flow | Site 1, n (%) | Site 2, n (%) |
|---|---|---|
| Gender (female) | 11 (55) | 12 (60) |
| Current age | ||
| 0-5 years old | 0 (0) | 0 (0) |
| 6-18 years old | 18 (90) | 5 (25) |
| 19-30 years old | 2 (25) | 3 (15) |
| Over 30 years old | 0 (0) | 12 (60) |
| Age at diagnosis | ||
| 0-5 years old | 7 (35) | 4 (20) |
| 6-18 years old | 12 (60) | 5 (25) |
| 19-30 years old | (0) | 4 (20) |
| Over 30 years old | (0) | 2 (10) |
| No response | 1 (5) | 5 (25) |
| Average SMBG tests per day | ||
| 1-2 tests | 0 (0) | 4 (20) |
| 3-4 tests | 9 (45) | 12 (60) |
| 5-6 tests | 9 (45) | 3 (15) |
| 7-9 tests | 2 (10) | 1 (5) |
| Reporting tool used traditionally | ||
| Logbook | 2 (10) | 3 (15) |
| Device screens | 14 (70) | 12 (60) |
| Do not use a tool | 1 (5) | 2 (10) |
| Download device at home, work or school | 4 (20) | 1 (5) |
| Download report at clinic | 12 (60) | 14 (70) |
| Other (my pump screen includes my SMBG data) | 1 (5) | 0 (0) |
| The reporting tool I use is useful | ||
| Yes, it is useful | 19 (95) | 19 (95) |
| Data useful to change exercise | 5 (25) | 4 (20) |
| Data useful to change eating | 8 (40) | 15 (75) |
| Data useful to change amount of insulin used | 18 (90) | 18 (90) |
| Data useful to change when insulin used | 5 (25) | 7 (35) |
| Use glucose data for immediate changes | ||
| Always | 13 (65) | 12 (60) |
| Often | 5 (25) | 5 (25) |
| Sometimes | 1 (5) | 3 (15) |
| Rarely | 1 (5) | 0 (0) |
| Never | 0 (0) | 0 (0) |
| Use data to look for trends | ||
| Always | 3 (15) | 3 (15) |
| Often | 4 (20) | 3 (15) |
| Sometimes | 8 (40) | 7 (35) |
| Rarely | 4 (20) | 6 (30) |
| Never | 1 (5) | 1 (5) |
Participants reported that the download was easy to complete (97% CGM; 94% SMBG) with the exception of figuring out how the connection cables connected their device to the PC (11% CGM; 6% SMBG). The patients’ perceptions of ease of use contradict the staff opinions. Of respondents, 3% reported that instructions were unclear. SMBG respondents (97%) were more confident than the CGM respondents (94%) that their device(s) had downloaded successfully.
Planned IT implementations were not one size fits all; site security protocols and resources were different. Large health systems security scrutiny was more intense than sites with administrative control of their own IT. Hardware requirements were different; some had standardized hardware replacement schedules and strict requirements for printer configurations, some simply add equipment as old equipment fails. Each site had unique IT challenges, all which were overcome, but unpredictable at site enrollment.
Limitations
This study was limited in the number of sites as well as participants included. While interviewing the 7 sites in the overall study, many expressed concern about the space they would need in a lobby to house a download station. While the number of sites were limited, the optimized work flow was developed from the most common two set ups for not only these clinics, but others in the T1D Exchange.12 With new systems on the market (Tidepool, Glooko) not all available middleware systems were tested, but the systems are similar enough to those used in this study that the findings are expected to be consistent.
Discussion
Incorporating device data into routine care is burdensome due to the download process and reporting inconsistencies; however it is critical for fully informed diabetes care decision making. However, there are ways to lessen the burden of data acquisition, such as implementing a lobby download kiosk, moving to a standardized report, and when available moving patients to new synching devices; all of which are effective in saving staff time and ensuring patient best care. Using the patient downtime in the lobby for downloading does not negatively affect patient experience and in many cases activates patients to new levels of engagement in their own care per staff report.
Implementing a lobby download kiosk was an effective way utilize patient down time and save staff time. Participants were happy to try downloading with minimal guidance, even when they had never downloaded device(s) before. It is reasonable to expect that repeated downloading would result in faster downloads. Confusion persists over which cord to use and where to aim Bluetooth and infrared for downloads. The comments highlighted the confusion caused by each device having its own cord (even a single manufacturer may have several cables). E-transfer devices (cellular, Bluetooth) now available on the market that will greatly reduce these frustrations as more patients transition to these devices. Patients regularly change between manufacturers’ products, not due to their own choice, but due to changing insurance coverage for supplies and devices.
Middleware software was key to optimization. Clinics reported recurrent problems keeping software up to date. At many clinic systems staff do not have administrative rights to computers and must call IT for each update. Middleware software applications remove the burden of multiple updates and ease the burden on staff; using a single system to download devices.
Conclusions
As devices shift to web synced technologies (Bluetooth and cellular), cabled downloading will no longer be required. However the issue of multiple reports will still exist. Using the standardized, single-page CaptūrAGP report allows for glucose data to be device ambiguous; easing interpretation. Data standardization ensures that all members of the care team are using the same data; time can then be spent not searching each report for information needed but instead using the data.
Time saved translates to more time for shared decision making and education. Using the patient down time in the lobby also engages them in utilizing the glucose data as active participants in their glucose monitoring. Given that diabetes outcomes require ongoing management of not only immediate treatment (hypoglycemia and hyperglycemia) but also ongoing trend management, better trend reports and utilization of these reports allow easier management. The standardized report allows providers to be more efficient in the clinic by having the data they need in an easy-to-read format for patient education and decision making.
One site said, “We had a very efficient process for downloading data prior to this trial. What we learned was that our process needed improvement. This trial encouraged us to experiment with different technology including remote access with patient data linked to our clinic.”
Acknowledgments
Thank you to IDC staff (Beth Olson, Noel Goldman, and Kyle Thompson); David Wesley (Sursumcorda); the clinics; patients/families; and Jeanne Mettner, for editorial assistance.
Footnotes
Abbreviations: AGP, ambulatory glucose profile; CGM, continuous glucose monitoring; IDC, International Diabetes Center; IRB, institutional review board; SMBG, self-monitoring blood glucose; TIM, time in motion.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DMM’s nonprofit employer has received research grants from Abbott Diabetes Care and Dexcom for which there was no personal compensation. RB’s nonprofit employer has received consultant payments on his behalf from Abbott Diabetes Care, Amylin, Animas, Bayer, Boehringer Ingelheim, Calibra, Eli Lilly, the Helmsley Trust, Hygieia, Johnson & Johnson, Medtronic, Novo Nordisk, Roche, Sanofi, Takeda, and Tandem with no personal compensation; research grants from Abbott Diabetes Care, Animas, Bayer, Becton Dickinson, Boehringer Ingelheim, Bigfoot, Calibra, DexCom, Eli Lilly, Helmsley Trust, Hygieia, Johnson & Johnson, Medtronic, Merck, NIH, Novo Nordisk, Roche, Sanofi, Takeda, and Tandem with no personal compensation. RB’s employer receives royalties from the Betty Crocker Diabetes Cookbook, and RB holds stock in Merck. AC’s nonprofit employer has received research grants from Abbott Diabetes Care, Animas, Bayer, Becton Dickinson, Boehringer Ingelheim, Bigfoot, Calibra, DexCom, Eli Lilly, Helmsley Trust, Hygieia, Johnson & Johnson, Medtronic, Merck, NIH, Novo Nordisk, Roche, Sanofi, Takeda, and Tandem with no personal compensation. KCA is a speaker for Sanofi, NovoNordisk, AstraZeneca, and Valeritas and does research for Type 1 DM exchange, Medtronic, Roche. For the remaining authors none were declared.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Work was funded through a research grant from the Leona M. and Harry B. Helmsley Charitable Trust.
References
- 1. Bergenstal RM. Glycemic variability and diabetes complications: does it matter? Simply put, there are better glycemic markers! Diabetes Care. 2015;38(8):1615-1621. [DOI] [PubMed] [Google Scholar]
- 2. Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, Holl R. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12(1):11-17. [DOI] [PubMed] [Google Scholar]
- 3. Renard E. Monitoring glycemic control: the importance of self-monitoring of blood glucose. Am J Med. 2005;118(9):12-19. [DOI] [PubMed] [Google Scholar]
- 4. McElwee-Malloy M. The right meter for you. Diabetes Forecast. 2016;69(2):50-63. [PubMed] [Google Scholar]
- 5. Evans M, Cranston I, Bailey CJ. Ambulatory glucose profile (AGP): utility in UK clinical practice. Br J Diabetes. 2017;17(1):26-33. [Google Scholar]
- 6. Matthaei S, DeAlaiz RA, Bosi E, Evans M, Geelhoed-Duijvestijn N, Joubert M. Consensus recommendations for the use of ambulatory glucose profile in clinical practice. Br J Diabetes. 2014;14(4):153-157. [Google Scholar]
- 7. Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care. 1987;10(1):111-117. [DOI] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile (AGP). Diabetes Technol Ther. 2013;15(3):198-211. [DOI] [PubMed] [Google Scholar]
- 9. Mullen D, Richter S, Bergenstal R. Understanding patient and clinician glucose reporting preferences in type 1 diabetes: ambulatory glucose profile (AGP). Paper presented at: European Association for the Study of Diabetes annual meeting; 2015; Stockholm, Sweden. [Google Scholar]
- 10. Matthaei S. Assessing the value of the ambulatory glucose profile in clinical practice. Br J Diabetes. 2014;14(4):148-152. [Google Scholar]
- 11. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(suppl 2):S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. T1D Exchange. Available at: T1DExchange.org. Accessed March 16, 2016.
- 13. Medlock MC, Wixon D, Terrano M, Romero R, Fulton B. Using the RITE method to improve products: a definition and a case study. Paper presented at: Usability Professionals’ Association; 2002; Orlando, FL. [Google Scholar]
- 14. Abdulmalek FA, Rajgopal J. Analyzing the benefits of lean manufacturing and value stream mapping via simulation: a process sector case study. Intern J Prod Econ. 2007;107(1):223-236. [Google Scholar]
- 15. Bahensky JA, Roe J, Bolton R. Lean sigma—will it work for healthcare? J Healthc Inf Manag. 2005;19(1):39-44. [PubMed] [Google Scholar]
- 16. SAS Institute Inc., Cary, NC, USA. 2015. [Google Scholar]
- 17. US Bureau of Labor Statistics. Occupational employment and wages, May 2016: 31-9092 medical assistants. March 31, 2017. https://www.bls.gov/Oes/current/oes319092.htm. Accessed April 5, 2017. [Google Scholar]