Abstract
Background:
The increasing popularity of wearable technology necessitates the evaluation of their accuracy to differentiate physical activity (PA) intensities. These devices may play an integral role in customizing PA interventions for primary prevention and secondary management of chronic diseases. For example, in persons with type 1 diabetes (T1D), PA greatly affects glucose concentrations depending on the intensity, mode (ie, aerobic, anaerobic, mixed), and duration. This variability in glucose responses underscores the importance of implementing dependable wearable technology in emerging avenues such as artificial pancreas systems.
Methods:
Participants completed three 40-minute, dynamic non-steady-state exercise sessions, while outfitted with multiple research (Fitmate, Metria, Bioharness) and consumer (Garmin, Fitbit) grade wearables. The data were extracted according to the devices’ maximum sensitivity (eg, breath by breath, beat to beat, or minute time stamps) and averaged into minute-by-minute data. The variables of interest, heart rate (HR), breathing frequency, and energy expenditure (EE), were compared to validated criterion measures.
Results:
Compared to deriving EE by laboratory indirect calorimetry standard, the Metria activity patch overestimates EE during light-to-moderate PA intensities (L-MI) and moderate-to-vigorous PA intensities (M-VI) (mean ± SD) (0.28 ± 1.62 kilocalories· minute-1, P < .001, 0.64 ± 1.65 kilocalories· minute-1, P < .001, respectively). The Metria underestimates EE during vigorous-to-maximal PA intensity (V-MI) (–1.78 ± 2.77 kilocalories · minute-1, P < .001). Similarly, compared to Polar HR monitor, the Bioharness underestimates HR at L-MI (–1 ± 8 bpm, P < .001) and M-VI (5 ± 11 bpm, P < .001), respectively. A significant difference in EE was observed for the Garmin device, compared to the Fitmate (P < .001) during continuous L-MI activity.
Conclusions:
Overall, our study demonstrates that current research-grade wearable technologies operate within a ~10% error for both HR and EE during a wide range of dynamic exercise intensities. This level of accuracy for emerging research-grade instruments is considered both clinically and practically acceptable for research-based or consumer use. In conclusion, research-grade wearable technology that uses EE kilocalories · minute-1 and HR reliably differentiates PA intensities.
Keywords: aerobic, circuit, continuous, exercise, wearable technology, anaerobic
Promoting increased levels of physical activity (PA) to prevent and or treat a number of chronic diseases (obesity, diabetes, cardiovascular disease) dates back at least to the 1950s.1-7 However, accurately monitoring an individual’s PA patterns (frequency, intensity, duration) outside of laboratory conditions remains a major research hurdle.8-10
The ability to monitor PA intensity via heart rate (HR), movement from accelerometry and/or breathing rate and thereby estimating energy expenditure (EE) with reasonable accuracy using wearable technology could potentially improve decision making around insulin adjustments for exercise.11-13 Exercise monitoring could also inform the nutritional needs of individuals since monitoring daily EE could help inform energy and macronutrient needs.14 At the very least, these emerging technologies (ie, body-worn wearables) can help persons and their caregivers determine if the current PA guidelines are being met.15 The success of any PA intervention is dependent on an individuals’ physical and physiological attributes, level of motivation, ability to stay committed, and the ability to accurately quantify the PA that the individual is participating in to meet specific EE and intensity thresholds.12,16 Effective EE thresholds for reductions in the incidence for type 2 diabetes has been documented to be as low as 500 kilocalories per week of PA,14 while increases of 2200 kilocalories per week is associated with atherosclerotic plaque reduction and perhaps even type 2 diabetes remission.16-18 This evidence supports the importance of quantifying an individual’s daily PA duration and intensity, using EE and HR for customizing PA prescriptions. The purpose of this study was to determine the accuracy of select research-grade and consumer-based exercise wearables throughout a wide dynamic range of exercise intensities and modes. It was hypothesized that EE, HR, and breathing rate recorded from this technology would accurately differentiate PA intensity.
Methods
Study Participants
The experimental protocol conformed to the standards set by the Declaration of Helsinki and was approved by York University’s Research Ethics Board. Twenty-five participants were recruited, including eight persons with T1D. The inclusion criteria indicated that participants must be over the age of 16 years, otherwise healthy and active with a diagnosis of T1D > 1 year. Participants with T1D were using continuous subcutaneous insulin infusion technology (ie, insulin pump) and were classified as having moderate to high levels of regular PA based on a self-reported questionnaire. Participants with T1D were deemed in fair to good glycemic control, based on a HbA1c ≤ 8.0%, and did not have other ailments that would preclude them from participating in the study (eg, cardiomyopathy, neuropathy, other diabetes-related complications). Participants were screened by a qualified exercise physiologist using the evidence-based tools, the PAR-Q+ and ePARmed-X+ (www.eparmedx.com) for contraindications and risk stratification. Pre-exercise HR and blood pressure were measured using the BpTRU100 (Surgo Surgical Supplies, Toronto, Ontario) automated device to ensure participants were within an acceptable range prior to the initiation of the exercise protocols; blood pressure < 160/90 mmHg. Six measurements were taken consecutively with a 1-minute rest interval in between, and averaged.
Experimental Protocol
Participants reported to the York University Human Performance Laboratory on three or four separate occasions. The first visit consisted of an incremental-to-maximal effort treadmill test for the determination of aerobic fitness or power (VO2 max) using the criterion open circuit spirometry discrete system (n = 18) or Fitmate (n = 19) metabolic unit (Cosmed, Rome, Italy). The next two visits for persons with T1D involved 40-minute exercise sessions: one ranging from moderate-to-vigorous intensity (M-VI) plus vigorous-to-maximum intensity (V-MI) circuit sessions and one continuous, L-MI steady-state session. The three additional visits for persons without T1D involved one M-VI plus V-MI circuit session, one continuous L-MI steady-state sessions, and one additional incremental-to-maximal effort treadmill test to determine the reliability amongst the criterion open circuit spirometry discrete system and Fitmate. A further description of the visits and the types of exercise performed are detailed below.
All experimental visits were conducted at the same time of day for each participant (either 11:00 am or 4:00 pm). Participants with T1D were instructed to take their usual bolus of insulin with breakfast (7:00 am) or lunch (12:00 pm) to ensure beginning the exercise protocols with minimal active or “on-board” insulin. The exercise was terminated if blood glucose levels dropped < 3.9 mmol•L-1 at any point during the session. If necessary, participants were provided with 15-20 grams of dextrose to treat hypoglycemia (Dex4©, AMG Medical Inc, Québec, Canada). The goal of the multiple visits was to determine wearable accuracy during steady and non-steady-state physical activities including incremental-to-maximum exercise on a treadmill (visit 1; see below) as well as cycling at varying intensities and a circuit training protocol with a variety of movements and activities (visits 2 and 3; see below). Participants were randomly assigned to the order of these various activities to minimize potential order effects.
Visit 1
Anthropometric data including height, body mass, body fat percentage, skinfolds, and waist circumference were collected using standardized protocols. Body mass was measured upon each visit (Seca Alpha Scale, Modell 770, Germany). Body fat percentage was measured, without shoes, using bioelectrical impedance analysis (Tanita scale, model TBF-612, Arlington Heights, IL). Height was measured without footwear, using a wall-mounted stadiometer. Waist circumference was measured following the standard National Institutes of Health protocol, with a tape measure around the waist, on the skin, at the level of the iliac crest.19 Skinfolds (triceps, biceps, subscapularis, iliac crest, and medial calf) were measured using Harpenden fat calipers (Baty International, Burgess Hill, UK) according to the PALM protocol to determine body adiposity.15
Participants were instrumented with (1) a Fitmate Pro portable metabolic unit with a Polar heart rate chest strap (Polar Electro KP 4, Kemple, Finland) for “ground truth” measurements of VO2, breathing rate (breaths·min-1), and HR (bpm), (2) a Zephyr Bioharness (Medtronic, Annapolis, MD) “sports wearable” chest strap for the measurements of HR and breathing rate, and (3) a Metria IH1 clinical research wearable arm patch (Vancive/Vandrico Inc, Vancouver, BC, Canada), for the measurement of EE (kilojoules). The Metria arm patch was worn on the study participants’ nondominant upper arm and functions similarly to the BodyMedia SenseWear research arm band. The Metria data were extracted using SenseWear software and exported as a Microsoft Excel output file. Subsets of the participants were also instrumented with the Garmin Vivo Fit2 chest strap and wristband (Garmin Ltd, Olathe, KS) or Mio Fuse (Mio Global, Vancouver, BC, Canada) wristband for additional measurements of HR (bpm) and total EE (kilocalaries) during the PA sessions. The Garmin VivoFit2 measures HR through a conventional chest strap, while the Mio fuse measures HR through reflection photoplethysmography at the wrist. All devices were noninvasive, and persons with T1D exercised while wearing their own insulin pumps. The Garmin Vivofit2 and Mio Fuse EE data were recorded as total kcals for the given activity bout, as per manufacturers design.
Either the open circuit spirometry criterion discrete component system (see below for a description) (n = 18) or Fitmate (n = 19) metabolic unit were used for the determination of participants’ VO2 max. A subset of participants (n = 14, 7 males and 7 females) had their VO2 max determined using both systems on separate occasions to assess validity of the portable Fitmate system. There were no significant differences between the two measurement systems for the VO2 max values (P = .565). The correlation between the two system for the incremental-to-maximum VO2 values was r = .97 ± .03. This permitted the Fitmate to be used as the criterion device during the remaining exercise sessions.
The incremental-to-maximal effort treadmill test for the determination of VO2max followed the same loading sequence for all participants. The protocol included a 2 min warm-up, and then progressive exercise workloads increased every two minutes (treadmill speed and/or elevation). The participants were instructed to remain on the treadmill until their work tolerance was compromised, at which point they received a 2-minute low intensity active recovery. Following the recovery period, the participants completed another incremental workload that was then followed by a 2-minute recovery. This discontinuous sequence was repeated until the VO2 of the subsequent workload was, equal to, or lower than the previous, indicating attainment of VO2max.12,15,20,21 The VO2 was determined from measurements obtained during the last 30-seconds of each workload via direct analysis of mixed expired gases.
The discrete component system consisted of; a 120L Tissot gasometer (Warren E Collins LTD, Braintree, MA), rapid response oxygen and carbon dioxide gas analyzers (Applied Electrochemistry, Model S-3A and CD-3S, Sunnyvale, CA), a hose, two-way y-valve (Ewald Koegal Co, San Antonio, TX), mouthpiece, and nose plugs.15 Once the expired gases were collected they were then analyzed using the gas analyzers. The collected variables; minute ventilation, fractions of expired carbon dioxide and oxygen, were then used to calculate the participants’ VO2. The other variables of interest included breathing frequency, HR (bpm), and EE (kilocalories) was estimated from VO2 L·min-1, where for every liter of consumed oxygen the EE is approximately 4.86 kilocalories per minute.22,23
The Fitmate portable metabolic unit, worked similarly to the discrete component system by using expired gas analysis, but differed in gas collection and calculation techniques. The participant was outfitted with a face mask that was held in place by a headpiece. While the participant was exhaling, a flowmeter and oxygen sample line, attached to the face mask, collected data on breathing frequency, volume of air, and the fractional concentration of oxygen. Unlike the discrete component system, the Fitmate only analyzes the expired oxygen and assumes the associated carbon dioxide concentration based on proprietary formulas. The Fitmate metabolic unit internally calculates the participants’ breath-by-breath VO2, which was averaged over the last 30 seconds for each workload. The other variables of interest included breathing frequency, HR (bpm), and EE (kilocalories·minute-1) was estimated from VO2 L·min-1, where for every liter of consumed oxygen the EE is approximately 4.86 kilocalories·minute-1.22,23 The criterion pulse (heart) rate was measured throughout using a Polar HR monitor (Polar Electro, Kempele, Finland).
Visits 2 and 3: Circuit/Intermittent Moderate-to-Vigorous (M-VI) and Vigorous-to-Maximum Intensity (V-MI) Exercise Protocol
Participants began by walking on a treadmill at 3.5 mph and 2% incline for 4 minutes followed by the sequenced exercises in the circuit: 45 seconds of marching on the spot with high knees and swinging the arms with 5-8 lb dumbbells in each hand (5 lb for females, 8 lb for males), squat with a front sweep using a 4 kg medicine ball swinging between the legs and over the head for 60 seconds, 4 jumping jacks, a quadruped motion (palms flat on the floor, extending 1 arm and opposite leg simultaneously) for 30 seconds, 2 jumping jacks, 4 push-ups, a 20-second forearm plank, marching on the spot with high knees for 30 seconds, squats with an 8 kg medicine ball placed on at chest height shelf each time for 60 seconds, 4 push-ups, and a 20-second forearm plank. Participants were then directed to cycle on an ergometer (Monark 874 E, Sweden) for 4 minutes at 60 rpm with 2.5 kg of resistance and then completed the circuit two more times. In between the second and last circuit, participants walked on the treadmill for 4 minutes at 3.5 mph and 2% incline. Participants completed the circuit protocol by cycling at 60-70 rpm for approximately 10 minutes until 40 minutes of exercise was reached. The intensity of the circuit ranged from M-VI (50-75% of VO2max) and V-MI (75-100% of VO2max).
Continuous Light-to-Moderate Intensity (L-MI) Exercise Protocol
During the steady-state treadmill protocol, participants were outfitted with the same measurement equipment as the circuit protocol. Participants exercised at ≤50% of VO2max for a total of 40-minutes.
Statistical Analysis
The primary outcome variable was kcal·min-1 (EE). A power analysis was conducted using SAS v 9.4, 80% power, and the respective correlations (0.74, 0.84, 0.73) the study was sufficiently powered given the following results; L-MI 159 pairs, M-VI 19 pairs, V-MI 13 pairs. The statistical analysis conducted was a matched pair t-test. The data were aligned by documenting the Global Start Time (initiation of exercise protocol) of the exercise session. The Fitmate was the standard comparator given the correlation testing compared to the gold standard discrete. For EE, the Metria was compared against the Fitmate. The Fitmate gave breathe-by-breathe data, which was averaged to align with the minute-by-minute Metria data.
Results
Table 1 shows the anthropometric, physical and physiological fitness profiles for all study participants (14 males, 11 females) including eight persons with T1D, 18-55 years of age (25 ± 7.6, mean ± SD).
Table 1.
Anthropometric, Physical, and Physiological Fitness Profiles of Participants.
| Variable | Male |
Female |
||
|---|---|---|---|---|
| ND (n = 10) |
T1D (n = 4) |
ND (n = 7) |
T1D (n = 4) |
|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age (years) | 21 ± 1.6 | 23 ± 9.2 | 24 ± 6.0 | 34 ± 13.5 |
| Height (cm) | 175.3 ± 5.9 | 180.3 ± 5.9 | 164.4 ± 3.5 | 169.5 ± 10.6 |
| Body mass (kg) | 79.9 ± 7.9 | 76.5 ± 3.4 | 64.4 ± 8.3 | 71.3 ± 9.9 |
| BMI (kg/m2) | 26.0 ± 2.2 | 23.6 ± 2.2 | 23.8 ± 2.7 | 24.7 ± 1.5 |
| Body fat (%) | 19.3 ± 5.1 | 13.1 ± 6.9 | 30.1 ± 6.2 | 31.2 ± 2.7 |
| Sum of 5 skinfolds (mm) | 53.3 ± 27.1 | 35.3 ± 10.5 | 82.1 ± 27.8 | 79.1 ± 13.8 |
| WC (cm) | 84.9 ± 7.9 | 80.5 ± 3.3 | 83.2 ± 5.2 | 87.3 ± 6.6 |
| VO2max (mL·kg-1·min-1) | 49.9 ± 4.3 | 55.9 ± 6.7 | 42.0 ± 7.7 | 38.1 ± 1.9 |
| Peak HR (bpm) | 200 ± 3.7 | 203 ± 3.6 | 199 ± 3.9 | 182 ± 15.7* |
Significant difference was observed (P = .045) in peak exercise HR between females without diabetes and females with T1D, due to the older age of one of the female study participants.
Table 2 contains a summary of the wearable bias (ie, under/over-reporting), and the corresponding practical applications during a thirty-minute workout. The over/under estimation is reported in reference to the Fitmate portable metabolic unit derived EE plus breathing rate, and the PA tracker HR from the Polar monitor. The clinically or practical acceptability is based on the results from the Bland-Altman analyses, respective to the PA intensity and is reported in EE (kilocalories·minute-1), HR (bpm), and breathing rate.
Table 2.
Physical Activity Intensity, Device Estimation, Statistical and Practical Application.
| Metria energy expenditure compared to Fitmate metabolic unit | |||||
|---|---|---|---|---|---|
| Intensity | Over-/underestimate | Statistical significance | 95% CI (kcals·minute-1) | Percentage error | Practical application (error) for a 30-min
workout (kcals) |
| Light-to-moderate | Over | P < .001 | −2.91, 3.46 | 4.44 | 8.4 |
| Moderate-to-vigorous | Over | P < .001 | −2.59, 3.87 | 6 | 19.2 |
| Vigorous-to-maximal | Under | P < .001 | −7.22, 3.65 | −13 | −53.4 |
| Bioharness heart rate compared to Polar unit | |||||
| Intensity | Over-/underestimate | Statistical significance | 95% CI (bpm) | Percentage error | Practical application (error) for a 30-min workout (kcals) |
| Light-to-moderate | Under | P < .001 | −16, 15 | −0.5 | −0.6 |
| Moderate -to-vigorous | Under | P < .001 | −26, 17 | −3.1 | −4.73 |
| Vigorous -to-maximal | Over | P > .05 | −21, 24 | 0.82 | 1.45 |
| Bioharness breathing rate compared to Fitmate metabolic unit | |||||
| Intensity | Over-/underestimate | Statistical significance | 95% CI (breaths·minute-1) | Percentage error | Practical application (error) for a 30-min workout (breaths·minute-1) |
| Light-to-moderate | Under | P < .05 | −18, 12 | −11.33 | −3 |
| Moderate -to-vigorous | Under | P < .001 | −33, 14 | −24.3 | −10 |
| Vigorous -to-maximal | Under | P < .001 | −22, 11 | −11.6 | −6 |
Figure 1 shows the Bland-Altman plots for the Fitmate versus the Metria during the L-MI, M-VI, and V-MI portions of the incremental-maximum effort VO2 protocol for EE, respectively (1A, 1B, 1C). Panel 1A demonstrates that the Metria has a bias of 0.28 ± 1.62 kilocalories·minute-1 with 95% confidence limits of agreement from –2.91 to +3.46 kilocalories·minute-1 during the L-MI PA. Panel 1B shows that the Metria has a bias of 0.64 ± 1.65 kilocalories·minute-1 with 95% confidence limits from –2.59 to +3.87 kilocalories·minute-1 during the M-VI PA. Panel 1C shows that the Metria has a bias of –1.78 ± 2.77 kilocalories·minute-1 with 95% confidence limits from –7.22 to +3.65 during the V-MI PA.
Figure 1.
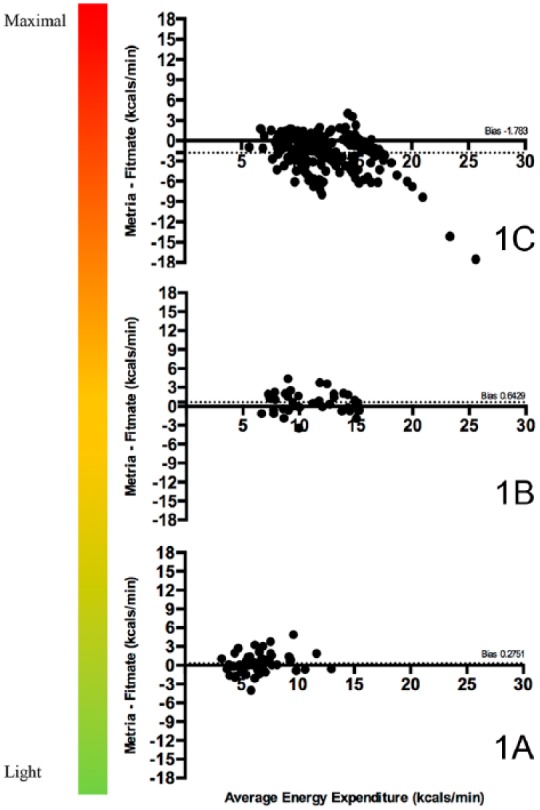
Bland-Altman plots for the Fitmate versus the Metria during the L-MI, M-VI, and V-MI portions of the incremental-maximum effort VO2 protocol for EE, respectively (1A, 1B, 1C).
Figure 2 shows the Bland-Altman plots for the Polar HR monitor (laboratory standard) versus the Bioharness during the L-MI, M-VI, and V-MI portions of the incremental-maximum effort VO2 protocol (2A, 2B, 2C). Panel 2A shows that the Bioharness has a small but statistically significant (P < .001) bias of –1 ± 8 bpm with 95% limits of agreement from –16 to +15 bpm during L-MI PA. Panel 2B shows that the Bioharness has a bias of –5 ± 11 bpm with 95% limits of agreement from –26 to 17 bpm indicating that the Bioharness underestimates the HR by –5 bpm during M-VI PA. Panel 2C demonstrates that the Bioharness has a bias of 2 ± 12 bpm with 95% limits of agreement from 21 to 24 bpm indicating that the Bioharness overestimates the HR by 2 bpm during the V-MI PA.
Figure 2.
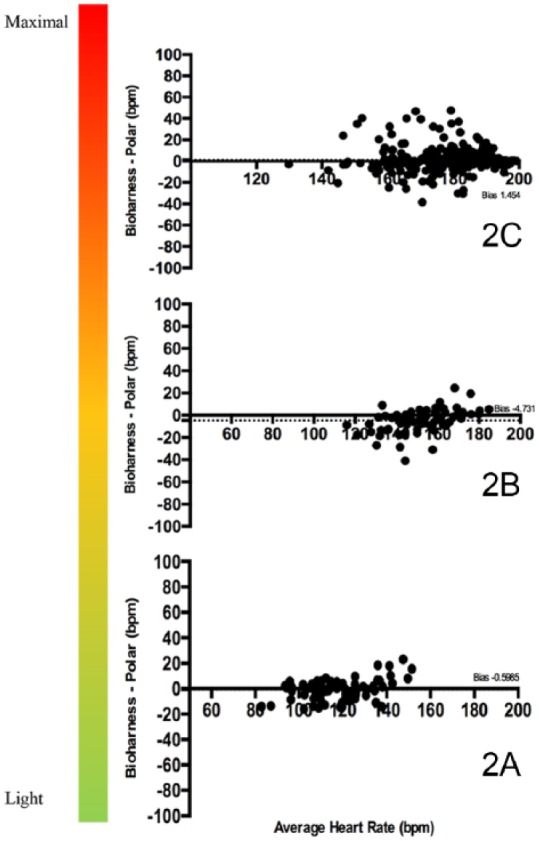
Bland-Altman plots for the Polar HR monitor versus the Bioharness during the L-MI, M-VI, and V-MI portions of the incremental-maximum effort VO2 protocol (2A, 2B, 2C).
In addition to the Metria and Bioharness, the Garmin VivoFit 2 and Mio Fuse (consumer grade wrist-worn activity monitors) were used during the continuous L-MI treadmill and circuit sessions in a subset of participants. These devises do not provide EE expenditures as continuous outputs, but they do provide estimates of total EE over a given workout. The accuracy of total EE (kilocalories) reported from both devices was determined using a paired t-test analysis. The results are summarized in Table 3 and Table 4, respectively. A significant difference was observed in the total EE for the Garmin device, compared to the Fitmate (P < .001) during the continuous L-MI treadmill activity. No significant differences were observed in total EE for either device compared to the Fitmate metabolic unit during the circuit activity. The statistical analyses of the consumer-grade devices (ie, Garmin VivoFit 2, Mio Fuse) should be interpreted with caution given the small sample size.
Table 3.
Accuracy and Reliability of Consumer-Based Wearables for Energy Expenditure During Continuous Light Activity (Mean ± SD).
| Continuous light-to-moderate protocol | ||||||
|---|---|---|---|---|---|---|
| n | Fitmate | Garmin | n | Fitmate | Mio | |
| Grouped | 5 | 355.19 ± 39.67 | 174.00 ± 28.53* | 6 | 255.30 ± 55.80 | 277.00 ± 136.31 |
A significant difference was observed for the Garmin device compared to the Fitmate (P < .001).
Table 4.
Accuracy of Consumer-Based Wearable Technology for Energy Expenditure During Circuit (Mean ± SD).
| Circuit moderate-to-vigorous and vigorous-to-maximum intensity protocol | ||||||
|---|---|---|---|---|---|---|
| n | Fitmate | Garmin | n | Fitmate | Mio | |
| Grouped | 7 | 343.38 ± 50.81 | 310.43 ± 201.80 | 5 | 297.92 ± 56.00 | 431.00 ± 234.29 |
No significant differences were observed for either of the devices compared to Fitmate (P < .05).
Discussion
The results from this limited investigation of four exercise wearables tentatively support the use of EE in kilocalories·minute-1 and HR recorded from select research-grade wearable technology to practically and reliably differentiate PA intensity within the widely accepted 10% error as a cutoff for clinical accuracy.8-10,24,25 The novel approach of this investigation was examining device accuracy continuously during dynamic non-steady-state exercise. This yielded a large data set for bias plots and regression analyses. This was accomplished by comparing each participants’ minute-by-minute data, throughout the full duration of the exercise sessions, as opposed to the conventional single steady-state average time point.
Our analyses demonstrated that the Bioharness HR was only significantly different (typically less than a few bpm) from the Polar unit during dynamic non-steady-state exercise at L-MI and M-VI PA. These small differences in HR and breathing rate should be deemed both clinically and practically acceptable. Multiple Bland-Altman analyses also demonstrated that these research-grade devices were operating well within acceptable ranges for EE and HR.
It is important to note the varying PA intensities in this study were confirmed via blood lactate samples and from ratings of perceived exertion (RPE) from a subset of participants (n = 8). At rest, an individual’s blood lactate typically ranges between 0.5 to 1.0 mmol/L.26,27 As PA intensity increases, there is an increase of metabolites and by-products due to the greater reliance on the anaerobic system. At maximal and near maximal effort, blood lactate values can reach up to 25.0 mmol/L.26,27 We found that the blood lactate values during the continuous L-MI PA were 1.6 ± 1.1 mmol/L, n = 48, while the levels during the intermittent M-VI and V-MI circuit were 6.0 ± 4.0 mmol/L, n = 49 samples. Therefore, the blood lactate values confirmed the PA intensity threshold used to classify the excise intensity as light, moderate-to-vigorous and vigorous-to-maximal in our study.26,27 The participants’ reported RPE using the original Borg scale (6-20) and HR values also confirmed the intensity.28
Despite the statistical difference compared to the criterion measures for EE and HR, the estimates of the research-grade devices should be deemed acceptable for clinical use, given that the discrepancies were all well below 10%.8-10,24,25 As shown in Table 2, the EE as measured by the Metria had a mean error of 1.78 kilocalories·minute-1, which would not impose a substantial difference in a 30-minute V-MI PA session, where the EE can be upward of 13 kilocalories·minute-1. In reference to HR, a mean underestimation of 0.6 bpm in HR should also not be considered clinically relevant- nor can most equipment measure to this degree of specificity. At all exercise intensities, HR fell within an operative 10% clinical error. At most, an underestimation of 5 bpm at a M-VI PA where the HR can range between 140 and 160 bpm, is still well within an acceptable margin of error.
Although there was no statistical significance between the criterion versus the Garmin VivoFit 2 and Mio Fuse derived total EE kilocalories for continuous L-MI steady-state and circuit M-VI plus V-MI PA (Tables 3 and 4), these findings should be interpreted with caution given our small sample size. There are a number of investigations which report similar findings and sample sizes.8-10,24,25 Further studies are needed to determine the accuracy of these and other wrist worn activity monitors during dynamic non-steady-state exercise.
Limitations
During this investigation, a number of setbacks occurred relating to product design and hardware malfunction. At times, device malfunction could have been due to either mechanical or natural and inevitable flaws (eg, battery life), causing some devices to not operate as intended. The cause of malfunction in the Fitmate could have been associated with oxygen sensor issues, damaged oxygen sample line, or file saving errors. The issues were largely unavoidable during the M-VI circuit, as the participants had worn the Fitmate in a closed and secured backpack which was not observable during the data collection. Malfunction in the Metria was discovered only upon data upload (this device is no longer commercially available). The main limitation of the Metria was its inability to communicate with an external component until it was removed from the body (ie, it is a blinded retrospective activity and sleep monitor). Nonetheless, there was only a 10% failure in data capture (including; falling off, battery, data upload issue).
Conclusions
In conclusion, research-grade wearable technology, that differentiates PA intensity and, appear to be accurate tools for estimates of EE, respiratory rate, and HR. Although the results for EE and HR were, at times, statistically different, the values were still well within the generally acceptable <10% margin of error. To enhance accuracy, HR should be used in conjunction with EE, to refine the differentiation of PA intensity that could impact glucose control in persons with T1D who are doing non-steady-state activties.29 Single variables alone, such as breathing rate, EE or HR may misclassify the PA intensity which could pose potential threats to clinical populations who may require more precise activity monitoring for clinical decision making and for the development of the “exercise-smart” artificial pancreas.11 The results of this study build on previously published studies showing considerable error in estimating EE in consumer-based products.8-10,24,25,29 Based on the findings of this investigation, it appears that, the research-grade PA tracking devices that track more physiological variables present an enhanced ability to differentiate PA intensities.
Footnotes
Abbreviations: AP, artificial pancreas; CGM, continuous glucose-monitoring device; EE, energy expenditure; HR, heart rate; L-MI, light-moderate intensity; METs, metabolic equivalents; M-VI, moderate-vigorous intensity; ND, persons without diabetes;PA, physical activity; T1D, type 1 diabetes; V-MI, vigorous-maximal intensity; VO2, oxygen consumption/aerobic power; VO2max, maximal oxygen uptake/maximal aerobic power.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institutes of Health (NIH/NIDDK 1DP3 DK101077).
ORCID iD: Ali Cinar  https://orcid.org/0000-0002-1607-9943
https://orcid.org/0000-0002-1607-9943
References
- 1. Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet. 1953;265(6796):1111-1120. [DOI] [PubMed] [Google Scholar]
- 2. Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med. 1953;10(4):245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paffenbarger RS, Jr, Brand RJ, Sholtz RI, Jung DL. Energy expenditure, cigarette smoking, and blood pressure level as related to death from specific diseases. Am J Epidemiol. 1978;108(1):12-18. [PubMed] [Google Scholar]
- 4. Paffenbarger RS, Hale WE. Work activity and coronary heart mortality. N Engl J Med. 1975;292(11):545-550. [DOI] [PubMed] [Google Scholar]
- 5. Bouchard C, Shephard RJ, Stephens T, Sutton JR, McPherson BD. Exercise, fitness, and health: the consensus statement. In: Exercise, Fitness, and Health: A Consensus of Current Knowledge: Proceedings of the International Conference on Exercise, Fitness and Health Champaign, IL: Human Kinetics Publishers; 1990:3-28. [Google Scholar]
- 6. Bouchard CE, Shephard RJ, Stephens TE. Physical activity, fitness, and health: International proceedings and consensus statement. In: International Consensus Symposium on Physical Activity, Fitness, and Health Champaign, IL: Human Kinetics Publishers; 1994:160-269. [Google Scholar]
- 7. Kesaniemi YK, Danforth E, Jr, Jensen MD, Kopelman PG, Lefèbvre P, Reeder BA. Dose-response issues concerning physical activity and health: an evidence-based symposium. Med Sci Sports Exerc. 2001;33(suppl 6):S351-S358. [DOI] [PubMed] [Google Scholar]
- 8. Gillinov S, Etiwy M, Wang R, et al. Variable accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sports Exerc. 2017;49(8):1697-1703. [DOI] [PubMed] [Google Scholar]
- 9. Shcherbina A, Mattsson CM, Waggott D, et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med. 2017;7(2):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hargens TA, Deyarmin KN, Snyder KM, Mihalik AG, Sharpe LE. Comparison of wrist-worn and hip-worn activity monitors under free living conditions. J Med Eng Technol. 2017;41(3):200-207. [DOI] [PubMed] [Google Scholar]
- 11. Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles. J Diabetes Sci Technol. 2015;9(6):1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turksoy K, Paulino TML, Zaharieva DP, et al. Classification of physical activity: information to artificial pancreas control systems in real time. J Diabetes Sci Technol. 2015;9(6):1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breton MD. Handling exercise during closed loop control. Diabetes Technol Ther. 2017;19(6):328-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163(12):1440-1447. [DOI] [PubMed] [Google Scholar]
- 15. Jamnik V, Gledhill N. Physical Activity and Lifestyle “R” Medicine: A Health-Related Physical Activity, Physical plus Physiological Fitness and Lifestyle, Toronto, Canada: Northview Print; 2015. [Google Scholar]
- 16. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377-390. [DOI] [PubMed] [Google Scholar]
- 17. Franklin BA, Swain DP, Shephard RJ. New insights in the prescription of exercise for coronary patients. J Cardiovasc Nurs. 2003;18(2):116-123. [DOI] [PubMed] [Google Scholar]
- 18. McInnes N, Smith A, Otto R, et al. Piloting a remission strategy in type 2 diabetes: results of a randomized controlled trial. J Clin Endocrinol Metab. 2017;102(5):1596-1605. [DOI] [PubMed] [Google Scholar]
- 19. National Institutes of Health. The Practical Guide to the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; 2000. [Google Scholar]
- 20. Gledhill N, Cox D, Jamnik R. Endurance athlete’s stroke volume does not plateau: major advantage in diastolic function. Med Sci Sports Exerc. 1994;29(9):1116-1121. [PubMed] [Google Scholar]
- 21. Howley ET, Bassett DR, Jr, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;14(5):1292-1301. [PubMed] [Google Scholar]
- 22. Hess D. Respiratory Care: Principles and Practice. Burlington, MA: Jones & Bartlett Learning; 2011. [Google Scholar]
- 23. Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16(1):23-29. [PubMed] [Google Scholar]
- 24. Lee JM, Kim Y, Welk GJ. Validity of consumer-based physical activity monitors. Med Sci Sports Exerc. 2014;46(9):1840-1848. [DOI] [PubMed] [Google Scholar]
- 25. Chowdhury EA, Western MJ, Nightingale TE, Peacock OJ, Thompson D. Assessment of laboratory and daily energy expenditure estimates from consumer multi-sensor physical activity monitors. PLOS ONE. 2017;12(2):e0171720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobs I, Tesch PA, Bar-Or O, Karlsson J, Dotan R. Lactate in human skeletal muscle after 10 and 30 s of supramaximal exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):365-367. [DOI] [PubMed] [Google Scholar]
- 27. Gollnick PD, Bayly WM, Hodgson DR. Exercise intensity, training, diet, and lactate concentration in muscle and blood. Med Sci Sports Exerc. 1986;18(3):334-340. [DOI] [PubMed] [Google Scholar]
- 28. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381. [PubMed] [Google Scholar]
- 29. Huyett LM, Ly TT, Forlenza GP, et al. Outpatient closed-loop control with unannounced moderate exercise in adolescents using zone model predictive control. Diabetes Technol Ther. 2017;19(6):331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]


