Abstract
Objective:
The objective was to investigate the relationship of body mass index (BMI) to differing glycemic responses to psychological stress in patients with type 1 diabetes.
Methods:
Continuous blood glucose monitor (CGM) data were collected for 1 week from a total of 37 patients with BMI ranging from 21.5-39.4 kg/m2 (mean = 28.2 ± 4.9). Patients reported daily stress levels (5-point Likert-type scale, 0 = none, 4 = extreme), physical activity, carbohydrate intake, insulin boluses and basal rates. Daily reported carbohydrates, total insulin bolus, and average blood glucose (BG from CGM) were compared among patients based on their BMI levels on days with different stress levels. In addition, daily averages of a model-based “effectiveness index” (quantifying the combined impact of insulin and carbohydrate on glucose levels) were defined and compared across stress levels to capture meal and insulin independent glycemic changes.
Results:
Analyses showed that patient BMI likely moderated stress related glycemic changes. Linear mixed effect model results were significant for the stress-BMI interaction on both behavioral and behavior-independent glycemic changes. Across participants, under stress, an increase was observed in daily carbohydrate intake and effectiveness index at higher BMI. There was no significant interactive effect on daily insulin or average BG.
Conclusion:
Findings suggest that (1) stress has both behavioral and nonbehavioral glycemic effects on T1D patients and (2) the direction and magnitude of these effects are potentially influenced by level of stress and patient BMI. Possibly responsible for these observed effects are T1D/BMI related alterations in endocrine response.
Keywords: body mass index, effectiveness index, glycemic variability, stress, type 1 diabetes
In patients with type 1 diabetes (T1D), optimal management of the disease requires accounting for as many factors as possible that can affect daily and nocturnal blood glucose (BG). While there is no particular suggestion for adjusting treatment according to daily psychological stress, the American Diabetes Association states that it is a factor that potentially affects diabetes management due to both physiological and behavioral changes.1 Automatic detection of stress and corresponding adjustment of BG control decisions have been suggested as elements of the future artificial pancreas.2 Although stress has well-known effects on the endocrine system by changes in glucocorticoids, catecholamines, growth hormone, and prolactin,3-9 studies that investigated its impact on BG in T1D have reported diverse results.
In a review published in 1985, Carter et al integrated the results from three of their previous studies to evaluate the reaction of BG levels to increased stress in patients with T1D. The results showed that it was not possible to infer psychological stress induced hyperglycemia in these patients. Therefore, they concluded that the assumption of hyperglycemia occurrence in the presence of stress needs to be avoided in T1D treatment planning.10 In 1990, Halford et al reported a significant patient-specific stress effect for 7 of the 15 patients in the study who showed an increase in BG levels. Authors inferred that stress was influential on BG levels in at least some patients with diabetes.11 In another study published in 1990, a significant BG response to psychological stress was found and the type and magnitude of the response were affected by unknown personal factors.12 Later, in 2000, Kramer et al published a study where the results supported the existence of a metabolic disturbance by stress with idiosyncratic variability between T1D patients.13 The diversity of stress response in direction and magnitude was recently bolstered by Gonder-Frederick et al’s study using the same data set as that used in the current study.14 In that study, which was the precursor to this current study, they grouped patients according to variability of their self-reported stress levels during the study period, and then analyzed the relationship between daily stress and BG variables derived from continuous glucose monitoring (CGM) data. They found that increased stress was associated with (1) higher BG variability, (2) higher percentage of time spent in hypoglycemic BG range, (3) reduced carbohydrate intake, and (4) no change in total daily bolus insulin, though this varied by individual participant.14 As a follow up, the purpose of the current study is to explore whether body mass index (BMI) is a factor in consistently observed glycemic response differences.
BMI has long been recognized to influence human endocrine system.15,16 Osuna et al showed in their 2006 study that sex steroids, leptin, insulin, and insulin resistance were affected by BMI in men.15 Another study, in 2004, investigated the effect of BMI on sex steroids and insulin growth factor related hormones in women, and found that BMI was also significantly related to these hormones.16
Given that both stress and BMI affect endocrine systems, their interaction is suspected to have a part in the observed glycemic variability in the previous study14 and other previous literature.10,12,13 Analyses in this study focused on the BMI-stress interaction and its effects on glycemia in T1D.
Methods
Participants
A total of 38 participants with T1D, HbA1c <10%, and insulin pump use for at least 6 months were recruited as part of a larger artificial pancreas study.17 Exclusion criteria included pregnancy, diabetic ketoacidosis or severe hypoglycemia in the 12 months prior to enrollment, history of a seizure disorder, medical conditions, and drug use that might interfere with the completion of study. Informed consent was obtained from all patients. All participants completed the study; however, one participant did not complete the daily stress questionnaire and was excluded from subsequent data analysis. After outlier removal based on the effectiveness index (6 days in total), the remaining data set consisted of 188 days from 37 participants with age range 25-62 years (mean = 46.8 ± 10.8), HbA1c range 5.7-9.9% (mean = 7.4 ± 0.98) and BMI range 21.5-39.4 kg/m² (mean = 28.2 ± 4.9).
Procedure
The principal study was conducted to assess the performance of a zone model-predictive controller implementation in an artificial pancreas device used at home. It was carried out at three different sites (William Sansum Diabetes Center, University of Virginia, and Mayo Clinic, Rochester, MN) and required one week of open-loop outpatient data.17 These outpatient data were used in Gonder-Frederick et al14 as well as in this present follow-up study.
During the outpatient period, for each participant, blinded continuous BG monitor (Dexcom G4 Platinum, Dexcom, San Diego, CA) readings were collected for 7 consecutive days during which participants followed their daily routines at their homes. Insulin records were obtained from patients’ insulin pumps. Carbohydrate intake, daily stress level (5-point Likert-type scale, 0 = none, 4 = extreme stress), and physical activity were self-reported by patients using a structured paper diary. Further details of the procedure are available in Gonder-Frederick et al.14
Data Analysis
Stress may complicate the glycemic control in T1D via both behavioral and physiological changes.1,11,14 Statistical analyses evaluated (1) stress effects on eating and insulin injection behavior of subjects that may interfere with their regular treatments and (2) stress effects on human physiology that leads to a significant change in BG. For accuracy purposes, days with CGM gaps with more than 3 consecutive hours or more than sixty missing CGM values were excluded from analyses.
Behavioral Changes
Studies conducted on human eating behavior in presence of psychological stress showed alteration in food intake, with the direction influenced by type and intensity of the stressor.18 However, these studies were not T1D specific. The first part of this study analyzes whether stress affects meal intake and insulin injection behaviors in T1D.
Physiological Changes
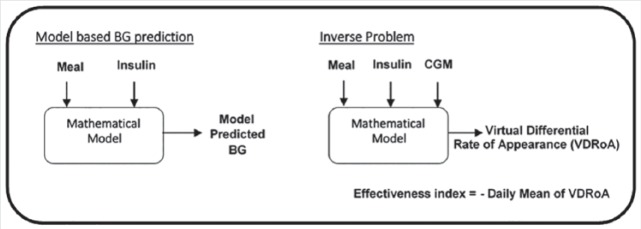
To capture the effect of stress on glycemia due to factors beyond and other than behavioral changes, we developed an “effectiveness index” that quantifies unexplained variability in BG after accounting for modeled effects of meals and insulin. The effectiveness index, evaluated at the end of each day, is computed as the negative of the daylong average of virtual differential rate of appearance “net effect,” which, following Patek et al,19 accounts for reported meals and insulin delivery using a compartmental model that is adapted to the patient through knowledge of body weight, total daily insulin, and total daily basal (see Figure 1).
Figure 1.
Effectiveness index.
The main idea of the effectiveness index is that descriptive statistics based on BG alone do not provide enough information to infer whether variability is due to a change in eating/bolus behavior or other factors like psychological stress. Here, a change in the effectiveness index implies presence of a factor that affects BG and that this factor is different from the modeled effects of meal intake and injected insulin. Therefore, we investigated effectiveness index changes in the presence of stress to infer its glycemic effect in T1D patients. The effectiveness index can take a positive or negative value and has a nominal value of zero when the patient’s BG returns to his or her reference BG under quiescent conditions (between meals and boluses), where the reference BG is computed from lab reported HbA1c and Nathan’s formula.20 The higher the absolute effectiveness index is, the higher the deviation of BG is from its estimated value by the inputs (ie, meal intake and insulin bolus). More specifically, a negative effectiveness index represents a higher BG level than the estimated value, while a positive effectiveness index value represents a lower BG level than the estimate.
Considering possible inaccuracy that might stem from subjectivity in self-evaluation of stress level, the first set of models (analysis 1) compares no stress (stress level 0) versus some stress (stress levels 1-4 combined) before exploring whether the effect differs by graduated stress levels with another set of models (analysis 2).
Both analyses consist of four separate linear mixed effects models that were designed to explore the BMI-stress interaction effect on (A) daily carbohydrate intake (gr), (B) total daily insulin (units), (C) daily average BG (mg/dl), and (D) daily average of effectiveness index. Patient effect is modeled as a random factor along with fixed factors of stress level and BMI. The effectiveness index concept allows analyses to show whether stress significantly accounts for glycemic changes beyond what would be expected from meals and boluses alone.
Factors with a P value less than .05 were considered significant. We concentrate on the results of the interactions between stress and BMI, as main effects of stress match those reported elsewhere.14
Results
No Stress Versus Some Stress
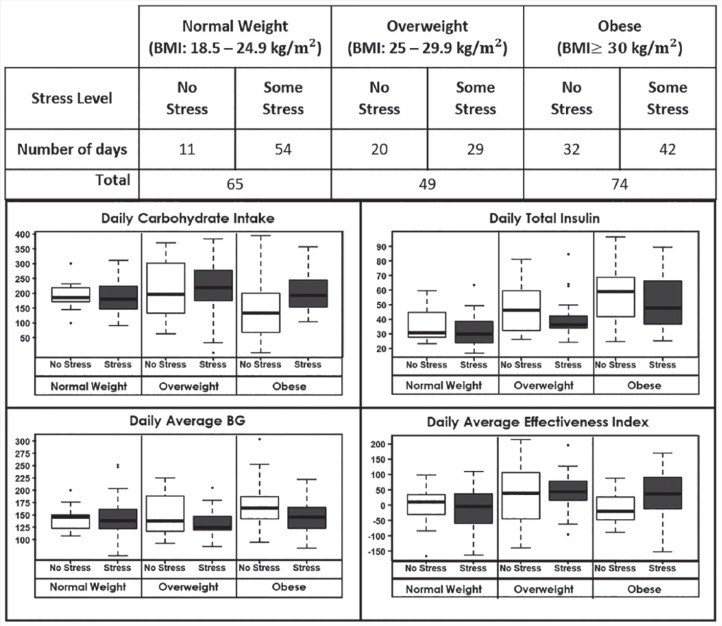
Analysis 1 examines whether patient behavior and glycemia are affected by patient BMI in the presence of any stress (Likert-type scale: 1-4) versus no stress (0) (see Table 1). Although BMI is treated as a continuous variable, for purposes of data visualization, Figure 2 shows boxplots of glycemic and nonglycemic dependent variables based on participant clinical BMI category21 and stress level. There was a significant interaction between stress and BMI for (A) carbohydrate intake (P = .0496) and (D) effectiveness index (P = .010). Participants with higher BMI increased carbohydrate intake more on days with stress compared to those with lower BMI, while concordantly showing a diminished effect of stress on BG as measured by the effectiveness index. However, BMI did not significantly interact with stress when examining effects on (B) total daily insulin (P = .133) or (C) average BG (P = .837).
Table 1.
Analysis 1: Changes in Carbohydrate Intake (A), Total Daily Insulin (B), Average BG (C), and Effectiveness Index (D) in the Presence of Some Stress.
| Analysis 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response variable |
(A) Carbohydrate intake |
(B) Total daily insulin |
(C) Average BG |
(D) Effectiveness index |
||||
| Parameter | Coefficient ± SE | P value | Coefficient ± SE | P value | Coefficient ± SE | P value | Coefficient ± SE | P value |
| Intercept | 312.3 ± 76.4 | <.05* | −25.3 ± 15.1 | .1 | 85.2 ± 37 | <.05* | 66.1 ± 72 | .36 |
| Stress | −135.1 ± 79.4 | .09 | 15.4 ± 10.6 | .15 | 0.5 ± 35.3 | .99 | −183.2 ± 77.9 | <.05* |
| BMI | −4.7 ± 2.6 | .08 | 2.5 ± 0.5 | <.01* | 2.4 ± 1.3 | .07 | −2.1 ± 2.4 | .4 |
| Stress × BMI | 5.5 ± 2.8 | <.05* | −0.6 ± 0.4 | .13 | −0.3 ± 1.2 | .84 | 7.0 ± 2.7 | <.05* |
p < .05.
Figure 2.
Stress-related changes in behavior, glycemia, and average effectiveness index by BMI categories.
No Stress Versus Graduated Stress Levels
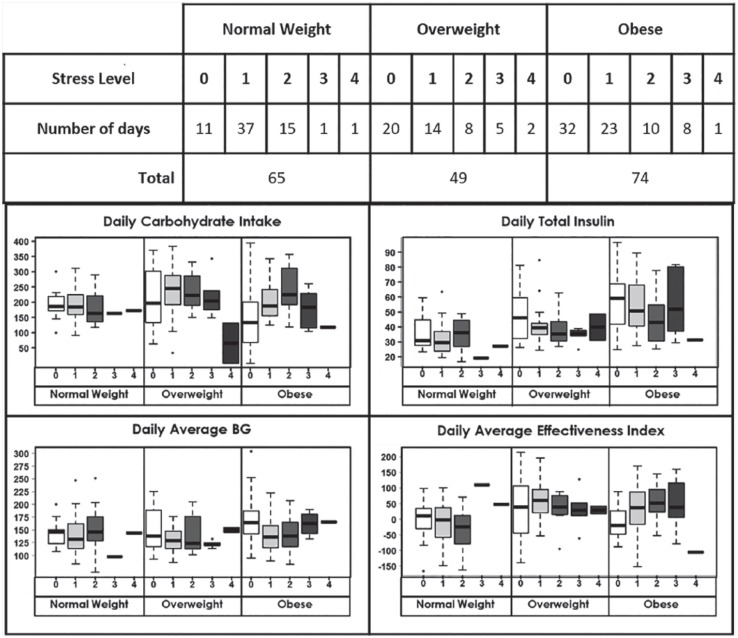
Analysis 2 is designed to explore whether the BMI dependent stress effect on behavior and glycemia that analysis 1 showed differs from no stress (0) based on graduated stress levels (1, 2, 3, or 4) (see Table 2). For stress levels 3 and 4, the BMI interaction does not significantly influence any of the dependent variables, though limited data at all BMI levels (n = 14 for stress level 3, n = 4 for stress level 4) may have limited the ability to conduct any statistical inference. On the other hand, results are concordant with the results of analysis 1 for stress levels 1 and 2. For stress level 1, a slight decrease in total daily insulin (P = .057) with no significant change in carbohydrate intake are observed for participants with increased BMI. Conversely, at stress level 2, higher BMI corresponded with an increase in daily carbohydrate intake (P = .002) with no significant change in total daily insulin. Compatible with these findings, there is evidence for an increase in effectiveness index at both stress level 1 (P = .011) and stress level 2 (P = .007) as BMI increases, suggesting a diminished role of stress on BG level. Visualization of data for different BMI categories also supports that the effect of stress on effectiveness index changes based on BMI (see Figure 3). This is to say that stress had an effect on BG independent from food intake and insulin injection related changes and this effect is BMI dependent.
Table 2.
Analysis 2: Changes in Carbohydrate Intake (A), Total Daily Insulin (B), Average BG (C), and Effectiveness Index (D) by Graduated Stress.
| Analysis 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response variable |
(A) Carbohydrate intake |
(B) Total daily insulin |
(C) Average BG |
(D) Effectiveness index |
||||
| Parameter | Coefficient ± SE | P value | Coefficient ± SE | P value | Coefficient ± SE | P value | Coefficient ± SE | P value |
| Intercept | 300.02 ± 75.80 | <.05* | −26.35 ± 15.36 | .09 | 79.80 ± 37.34 | .03 | 61.44 ± 71.66 | .39 |
| Stress level 1 | −91.03 ± 87.46 | .3 | 22.11 ± 12.08 | .07 | 37.19 ± 39.72 | .35 | −204.03 ± 86.80 | <.05* |
| Stress level 2 | −275.13 ± 95.97 | <.05* | 14.16 ± 12.56 | .26 | −25.92 ± 42.66 | .54 | −253.31 ± 97.23 | <.05* |
| Stress level 3 | −35.14 ± 139.95 | .8 | −10.06 ± 18.40 | .59 | −72.51 ± 62.33 | .25 | 90.28 ± 141.50 | .52 |
| Stress level 4 | 153.01 ± 249.53 | .54 | 16.83 ± 31.86 | .6 | −38.27 ± 109.86 | .73 | 302.79 ± 255.05 | .24 |
| BMI | −4.25 ± 2.57 | .11 | 2.51 ± 0.53 | <.05* | 2.59 ± 1.28 | .05 | −1.92 ± 2.42 | .43 |
| Stress level 1 × BMI | 4.11 ± 3.08 | .18 | −0.82 ± 0.43 | .06 | −1.75 ± 1.40 | .21 | 7.86 ± 3.05 | <.05* |
| Stress level 2 × BMI | 10.65 ± 3.42 | <.05* | −0.47 ± 0.45 | .29 | 0.92 ± 1.52 | .55 | 9.43 ± 3.47 | <.05* |
| Stress level 3 × BMI | 1.46 ± 4.71 | .76 | 0.28 ± 0.62 | .65 | 2.25 ± 2.10 | .29 | −1.97 ± 4.76 | .68 |
| Stress level 4 × BMI | −7.76 ± 8.98 | .39 | −0.52 ± 1.44 | .65 | 1.78 ± 3.95 | .65 | −10.78 ± 9.18 | .24 |
p < .05.
Figure 3.
Changes in behavior, glycemia, and average effectiveness index with graduated stress by BMI categories.
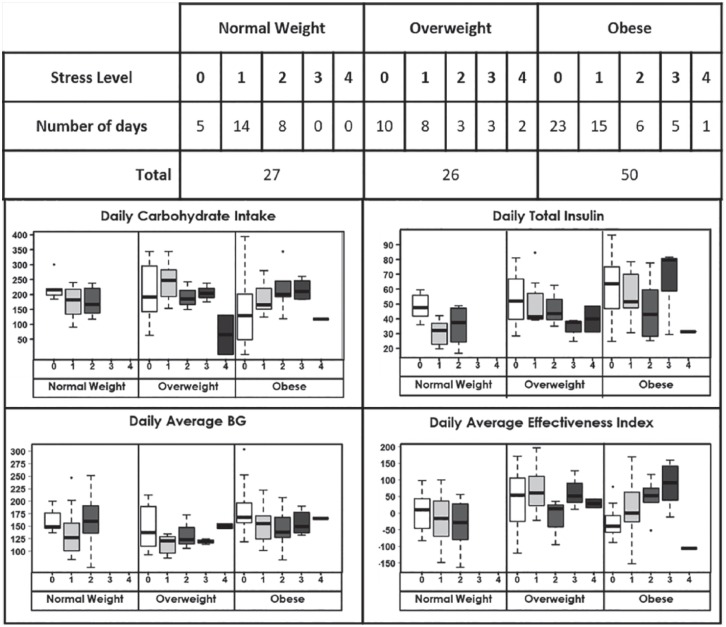
Finally, to eliminate possible confounding effects of exercise, analyses were conducted only for the days when patients did not report any exercise (n = 103 days). Although statistical significance for stress-BMI effect on response variables in LME models was obtained only for daily average effectiveness index (P = .008 for stress level 1 and P = .004 for stress level 2), the patterns of the effect were sustained at stress levels 1 and 2 for all response variables (see Figure 4).
Figure 4.
Changes in behavior, glycemia, and average effectiveness index with graduated stress by BMI categories on days with no exercise.
Although this study concentrates on the results of the interactions between stress and BMI, univariate analyses on associations between the explanatory variables (ie, stress and BMI) and the aforementioned behavioral and glycemic response variables were also conducted. Increased BMI was associated with greater total daily insulin (P = .0001) and higher daily average BG (P = .024). There were no statistically significant associations between reported stress by itself and any of the response variables (all Ps > .05). Finally, no statistically significant correlation was observed between the average reported stress and patient BMI (P > .05).
Discussion
In this study, we analyzed whether daily stress had an effect on eating and bolusing behavior of T1D patients as well as a glycemic effect independent from meal intake and insulin bolusing related changes. “Effectiveness index” was used to control for meal and bolus related BG disturbances. When stress was considered by itself without an interaction with BMI, we did not observe a common statistically significant influence on effectiveness index: neither for some stress versus no stress (P = .26) nor for any of the graduated stress levels (P > .2). However, results suggested a change in average effective index by stress with the magnitude and direction moderated by patient BMI. Specifically, while heightened daily stress typically increased BG over predicted values from carbohydrate intake and insulin administration, this effect was diminished (or reversed) with higher BMI.
In another study, Touma et al showed that mild mental stress resulted in increased insulin sensitivity in healthy young men who were tested by mental arithmetic.22 We also observed results consistent with an increase in glucose uptake for mild stress (ie, stress levels 1 and 2) associated with higher BMI.
Stress is mostly expected to cause a decrease in insulin sensitivity due to increases in glucocorticoids and catecholamines.23 Yet, we observed a reverse effect for high BMI participants, suggesting that there are additional factors affecting glucose dynamics when patients experience stress.
Given the results of our study, BMI may be one of the factors that led to the different observations and conclusion of idiosyncratic responses to mental stress for patients with T1D reported by prior studies. Also, altered glucoregulatory responses in T1D24 may generate differences in response to psychological stress. Nonetheless, since we did not test for changes in glucocorticoids and catecholamines during the study, it is not possible to give a definite clinical explanation to the physiological factors behind the observed effect.
Though the results of this study are potentially valuable to the study of glucose dynamics, they should be considered in light of methodological limitations. Participants of this study were current pump users and agreed to take part in an artificial pancreas study. This may indicate a higher level of self-treatment and engagement in diabetes care by the patient than in the general population because of the rigorous behavioral requirements of the trial (frequent finger-stick BG measurements, meal recording, pump and CGM use). As for stress reporting, collecting only a one-time global stress rating by the patient at the end of the day might cause a bias toward under or overrating of the stress that occurred later in the day. In addition, we did not measure cortisol levels. Thus, self-reported stress data are highly subjective, with participants potentially evaluating the same stressor differently. Future studies may benefit from ecological momentary assessment (EMA),25,26 which would provide multiple measures of stress throughout the day with the exact time of the assessments. As mentioned by Gonder-Frederick et al, this would potentially provide a more accurate assessment of glucose dynamics by being more responsive to fluctuations in stress throughout the day.14
Also, the results may differ for different types of stress (eg, acute and chronic stress) that were not assessed by the present study. For instance, Figures 3 and 4 show lower daily carbohydrate intake for overweight and obese group at high stress levels. Although this may suggest a different behavioral and physiological response to high stress, scarce data for stress levels 3 and 4 did not allow any inference about high stress related behavioral and glycemic changes.
Another limitation is that improperly reported carbohydrates would affect the effectiveness index. However, it is assumed that a patient’s bias toward under- or overestimating carbohydrate intake would be the case for all days and so a relative difference would still be representative of stress effect.
Despite these limitations, preliminary results of this study suggest that there is need for further investigation of BMI’s influence on the behavioral and glycemic effects of psychological stress. These could be highly controlled laboratory studies exposing different weight groups to the same psychological stressors and/or naturalistic studies using EMA to capture multiple measures of daily stress levels.
Conclusion
The novelty of this study lies in (1) inclusion of BMI as a factor that may explain a part of the idiosyncratic pattern of stress effect and (2) the use of effectiveness index to examine stress effects on BG by controlling for carbohydrate and insulin intake. The results show that stress may influence BG dynamics in T1D and the effect is not identical for all patients. BMI appears to significantly influence the direction and magnitude of this glycemic stress response.
Acknowledgments
We would like to show our gratitude to Boris Kovatchev, PhD (University of Virginia Center for Diabetes Technology) who provided insight and expertise during the course of this research, to Joe Clayton Ford, MS (University of Virginia, StatLab) for his knowledge and guidance on the statistical analyses conducted in this study, and to Chiara Fabris, PhD (University of Virginia Center for Diabetes Technology) for support and insightful discussions.
Footnotes
Abbreviations: BG, blood glucose; BMI, body mass index; CGM, continuous glucose monitor; EMA, ecological momentary assessment; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SDP acknowledges equity interest in and active participation with TypeZero Technologies, Inc. SAB reports materials support managed by the University of Virginia from Roche Diagnostics Inc, Dexcom, Tandem Diabetes Care, and LifeScan and grant support from Animas and Medtronic.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institutes of Health Grant Numbers DP3DK094331, R01DK085628 to UCSB, DK85516 (Mayo) and Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS) and the Urdang Family Foundation to Mayo Clinic. Product support was provided by Animas Corp (insulin pumps in-kind), Dexcom Inc (research discount on CGM supplies), and the Investigator-Initiated Study Program of LifeScan, Inc (glucometer test strips in-kind).
ORCID iD: Jordan E. Pinsker  https://orcid.org/0000-0003-4080-9034
https://orcid.org/0000-0003-4080-9034
References
- 1. American Diabetes Association. Foundations of care and comprehensive medical evaluation. Diabetes Care. 2016;39(suppl 1):s23-s35. [DOI] [PubMed] [Google Scholar]
- 2. Harvey RA, Wang Y, Grosman B, et al. Quest for the artificial pancreas: combining technology with treatment. IEEE Eng Med Biol Mag. 2010;29(2):53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ranabir S, Reetu K. Stress and hormones. Indian J Endocrinol Metab. 2011;15(1):18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanson SL, Pichert JW. Perceived stress and diabetes control in adolescents. Health Psychol. 1986;5(5):439-452. [DOI] [PubMed] [Google Scholar]
- 5. Frenzel MP, McCaul KD, Glasgow RE, Schafer LC. The relationship of stress and coping to regimen adherence and glycemic control of diabetes. J Soc Clin Psychol. 1988;6(1):77-87. [Google Scholar]
- 6. Surwit RS, Schneider MS. Role of stress in the etiology and treatment of diabetes mellitus. Psychosom Med. 1993;55(4):380-393. [DOI] [PubMed] [Google Scholar]
- 7. Moberg E, Kollind M, Lins PE, Adamson U. Acute mental stress impairs insulin sensitivity in IDDM patients. Diabetologia. 1994;37(3):247-251. [DOI] [PubMed] [Google Scholar]
- 8. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kemmer FW, Bisping R, Steingrüber HJ, et al. Psychological stress and metabolic control in patients with type I diabetes mellitus. N Engl J Med. 1986;314(17):1078-1084. [DOI] [PubMed] [Google Scholar]
- 10. Carter WR, Gonder-Frederick LA, Cox DJ, Clarke WL, Scott DR. Effect of stress on blood glucose in IDDM. Diabetes Care. 1985;8(4):411-412. [DOI] [PubMed] [Google Scholar]
- 11. Halford WK, Cuddihy S, Mortimer RH. Psychological stress and blood glucose regulation in type I diabetic patients. Health Psychol. 1990;9(5):516-528. [DOI] [PubMed] [Google Scholar]
- 12. Gonder-Frederick LA, Carter WR, Cox DJ, Clarke WL. Environmental stress and blood glucose change in insulin-dependent diabetes mellitus. Health Psychol. 1990;9(5):503-515. [DOI] [PubMed] [Google Scholar]
- 13. Kramer JR, Ledolter J, Manos GN, Bayless ML. Stress and metabolic control in diabetes mellitus: methodological issues and an illustrative analysis. Ann Behav Med. 2000;22(1):17-28. [DOI] [PubMed] [Google Scholar]
- 14. Gonder-Frederick LA, Grabman JH, Kovatchev B, et al. Is psychological stress a factor for incorporation into future closed-loop systems? J Diabetes Sci Technol. 2016;10(3):640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52(5):355-361. [DOI] [PubMed] [Google Scholar]
- 16. Lukanova A, Lundin E, Zeleniuch-Jacquotte A, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150(2):161-171. [DOI] [PubMed] [Google Scholar]
- 17. Dassau E, Brown SA, Basu A, et al. Adjustment of open-loop settings to improve closed-loop results in type 1 diabetes: a multicenter randomized trial. J Clin Endocrinol Metab. 2015;100(10):3878-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11):887-894. [DOI] [PubMed] [Google Scholar]
- 19. Patek SD, Lv D, Ortiz EA, et al. Empirical representation of blood glucose variability in a compartmental model. In: Kirchsteiger H, Jørgensen JB, Renard E, del Re E.eds. Prediction Methods for Blood Glucose Concentration. Cham, Switzerland: Springer; 2016:133-157. [Google Scholar]
- 20. Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310(6):341-346. [DOI] [PubMed] [Google Scholar]
- 21. National Institutes of Health and National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):s51-s209. [PubMed] [Google Scholar]
- 22. Touma T, Takishita S, Kimura Y, Muratani H, Fukiyama K. Mild mental stress increases insulin sensitivity in healthy young men. Clin Exp Hypertens. 1996;18(8):1105-1114. [DOI] [PubMed] [Google Scholar]
- 23. Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9(6):787-793. [DOI] [PubMed] [Google Scholar]
- 24. Vranic M. Banting lecture: glucose turnover. A key to understanding the pathogenesis of diabetes (indirect effects of insulin). Diabetes. 1992;41(9):1188-1206. [DOI] [PubMed] [Google Scholar]
- 25. Moskowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. J Psychiatry Neurosci. 2006;31(1):13-20. [PMC free article] [PubMed] [Google Scholar]
- 26. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1-32. [DOI] [PubMed] [Google Scholar]