Abstract
Background:
There is a paucity of prospectively collected data as they relate to nerve injuries after hip arthroscopic surgery. Studies describing the relationship of neurological injuries to portal placement and the duration and magnitude of traction force with regular and standardized patient follow-up protocols are limited.
Purpose/Hypothesis:
The purpose of this study was to characterize nerve deficits in a series of patients undergoing hip arthroscopic surgery as these deficits relate to axial traction and portal placement. It was hypothesized that in patients who presented without nerve deficits after surgery, the magnitudes of traction-related measurements would exceed previous recommendations based on expert opinion (<50 lb). Additionally, it was hypothesized that sensory disturbance would commonly be observed (≥16%) localized to the distal anterolateral thigh related to portal placement.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 45 patients scheduled to undergo hip arthroscopic surgery between July 2012 and February 2014 were included in this study. Traction force was measured and recorded every 5 minutes during surgery, and patients were assessed by a physical examination for deficits in light touch sensitivity at all lower extremity dermatomes preoperatively and at 3 weeks, 6 weeks, 3 months, and 1 year postoperatively. Patients were also tested for strength deficits and rated on the manual muscle testing grading scale. Patients reported modified Harris Hip Score (mHHS), Hip Outcome Score–Activities of Daily Living and –Sport subscales (HOS-ADL and HOS-Sport, respectively), Short Form–12 (SF-12) mental and physical component summaries, and international Hip Outcome Tool–12 (iHOT-12) scores preoperatively and at 1 year postoperatively.
Results:
Thresholds for maximum traction force, mean traction force, duration of traction, and traction impulse were 120 lb, 82 lb, 61 minutes, and 7109 lb·min, respectively, below which no patients presented with sensory or motor dysfunction thought to be related to traction. A minority (17.8%) of patients presented with highly localized, distal anterolateral sensory deficits suggestive of injuries related to portal placement, and 2.2% of patients presented with perineal numbness localized to the distribution of the pudendal nerve. All nerve deficits had resolved by 1-year follow-up.
Conclusion:
This study suggests that it may be possible to apply more axial traction force for a longer duration than expert opinion has previously suggested, without significant and, in the majority of cases (82.2%), any traction-related short-term complications. Transient traction- and portal placement–related nerve injuries after hip arthroscopic surgery may be more frequent (31.1% in this study) than have been reported historically.
Keywords: femoroacetabular impingement, labral tear, neurapraxia, postoperative complications
Hip arthroscopic surgery is a relatively new and rapidly expanding procedure.10 Axial traction has been used in hip surgery for many years with relatively low complication rates.4 Some suggest that injuries to the pudendal, sciatic, and perineal nerves occur because of prolonged distraction or compression of the nerves on the perineal post.19,26,31 It has been demonstrated that nerve stretching as little as 6% of the standard length can diminish the action potential amplitude and that nerve stretching as little as 8% of the standard length can lead to significant disturbance of blood flow to the affected nerve.37 Recommendations for decreasing the incidence of neurological injuries during hip arthroscopic surgery include proper patient positioning, minimization of traction duration (<120 minutes) and traction force (<50 lb), and a well-padded perineal post (≥9 cm).3,28,32 These recommendations are primarily based on expert opinion. Studies describing the relationship of neurological injuries to the duration and magnitude of traction force with regular and standardized patient follow-up protocols are limited.3,11,22,27,36
Nerve injuries as they relate to portal placement during hip arthroscopic surgery have been described with the sciatic, superior gluteal, and lateral femoral cutaneous nerves at risk. The posterolateral portal lies adjacent to the sciatic nerve at the level of the capsule at an average distance of 2.9 cm from the nerve, while the anterolateral portal (ALP) is placed in proximity to the superior gluteal nerve at an average distance of 4.4 cm inferior to the nerve.7,8 The lateral femoral cutaneous nerve is highly branched at the site of anterior portal placement, with this portal generally placed within millimeters of one of its branches. It is highly susceptible to damage on stab wounds and has been described as a frequent (80%) complication in anterior approaches for hip arthroplasty.17 Complication rates pertaining to lateral femoral cutaneous nerve injuries are less documented in the setting of hip arthroscopic surgery.
There is a paucity of prospectively collected data as they relate to nerve injuries and dysfunction after hip arthroscopic surgery. Studies characterized by a rigorous follow-up in the form of physical examinations and patient-reported outcome measures are needed. The purpose of this study was to characterize nerve deficits in a series of patients undergoing hip arthroscopic surgery as these deficits relate to traction and portal placement. In addition, this study looked to explore the amount and duration of force that can be applied in traction without persistent nerve deficits. It was hypothesized that in patients who presented without nerve deficits after surgery, the magnitudes of traction-related measurements would exceed previous recommendations (<50 lb).32 Additionally, we hypothesized that we would commonly observe (≥16%) sensory disturbance localized to the distal anterolateral thigh related to portal placement.11,20
Methods
Inclusion/Exclusion Criteria
This was a prospective study performed at a single institution. Institutional review board approval was obtained for this study. Patients who underwent hip arthroscopic surgery between July 2012 and February 2014 were eligible for study inclusion. Criteria for hip arthroscopic surgery candidacy included hip pain during a physical examination, persistent pain refractory to conservative treatment for at least 3 months, confirmed femoroacetabular impingement on plain radiographs or computed tomography, and confirmed labral tearing on magnetic resonance imaging.
Exclusion criteria were evidence of joint space narrowing on radiographs (<50% of contralateral joint space at any point on the medial, central, or lateral sourcil or <2 mm of joint space at the medial, central, or lateral sourcil), Tönnis grade ≥2, avascular necrosis, revision hip arthroscopic surgery, nerve deficits at lower extremity sensory points, low back pain, history of lumbar stenosis, lumbar radiculopathy, disc disease, herniations, erectile dysfunction, and any strength deficits (<5/5 on the Manual Muscle Testing [MMT] grading scale). Non-English–speaking patients, patients aged <18 years, and those with a history of and/or current hip dysplasia were also excluded. Patients who had psoas release performed at the time of arthroscopic surgery were excluded postoperatively. At the preoperative assessment, medical histories were collected by the lead author (D.S.C.), and patients were assessed by a physical examination for nerve deficits at lower extremity sensory points. Sensory and motor impairments for each patient were noted by extremity, with paralysis, neuritis, and neuralgia all grounds for study exclusion. Muscular atrophy and passive and active ranges of motion were assessed and pain with motion documented. Patients presenting with any lower extremity sensory or strength deficits were excluded from further study.
Surgical Procedure
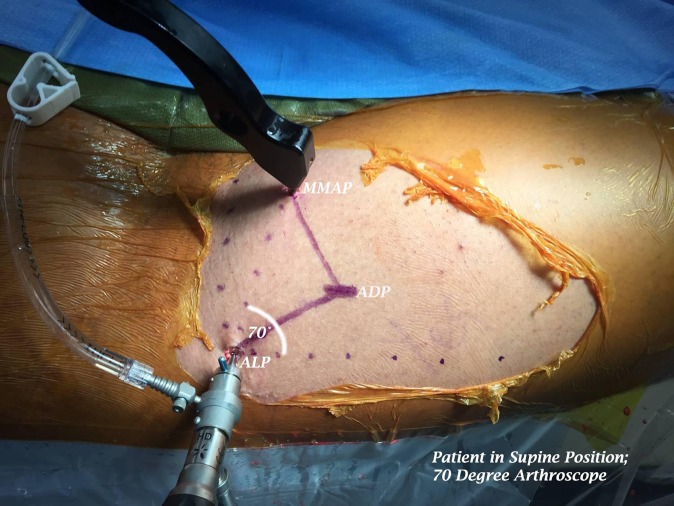
Patients underwent hip arthroscopic surgery performed in a modified supine position with a 70° arthroscope. One attending surgeon (D.S.C.) performed all surgical procedures. All patients underwent endotracheal intubation with the administration of general anesthesia with a muscle paralytic. No intra-articular injection was performed perioperatively. After intubation, all patients were placed on a fracture-type table with their legs in traction boots (Bledsoe Brace Systems). A 23 cm–wide well-padded perineal post (Bledsoe Brace Systems) was slightly lateralized against the medial thigh of the operative limb. Gentle countertraction was applied to the nonoperative leg. The operative leg was placed in traction (Figure 1), in 10° of hip flexion, at each patient’s maximum internal rotation but without exceeding 40°, and in 10° of abduction. The contralateral limb was placed in neutral flexion, neutral rotation, and 30° of abduction. Biplanar fluoroscopy was used to ensure adequate positioning and space for the insertion of arthroscopic instrumentation. An ALP, anterodistal portal (ADP), and modified midanterior portal (MMAP) were used in all cases. The ALP was placed first, located 1 cm proximal and 1 cm anterior to the tip of the greater trochanter. Subsequently, the MMAP was placed at an approximately 70° angle to the ALP, typically placed at a distance of 7 cm from the ALP, with minor adjustments made based on patient girth. Last, the ADP was placed, forming an isosceles triangle with the ALP and MMAP (Figure 2).
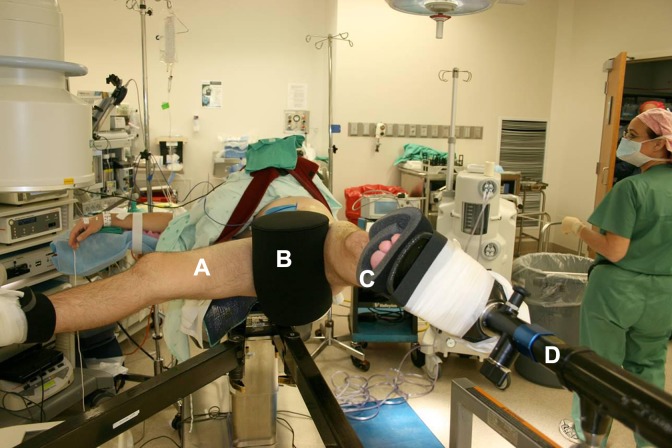
Figure 1.
(A) Hip arthroscopic surgery in the supine position with the contralateral leg in 70° abduction for demonstration purposes. (B) The patient is set against the 23 cm–wide well-padded perineal post (C) with traction boots. (D) Custom TenZor carbon fiber orthopaedic traction unit provides real-time readout of traction force.
Figure 2.
Right hip portal placement. ADP, anterodistal portal; ALP, anterolateral portal; MMAP, modified midanterior portal.
Initially, at the time of traction setup, the goal of the radiologically defined distraction was 1 cm. Force of traction was then adjusted to maintain a safe distance for arthroscopic visualization and treatment once the ALP was created. Traction force was measured and recorded every 5 minutes using a custom TenZor carbon fiber orthopaedic traction unit (Composite Manufacturing). The unit, tested for the validity and reliability of its measurements by its manufacturer, was calibrated using several weight stacks suspended from the distal end foot support connector (Figure 3).
Figure 3.

Custom TenZor carbon fiber orthopaedic traction unit undergoing calibration with weight stacks.
Maximum traction force was defined as the largest magnitude force recorded from each patient’s data points, mean traction force as the sum over the traction forces collected divided by the number of data points, and impulse of traction as the product of duration of traction and mean traction force. Duration and force of traction were recorded by an assistant in the operating room.
After surgery, all patients were placed in a hip abduction orthosis for 10 days, were enrolled in a physical therapy program starting between days 2 and 5 postoperatively, and began using a continuous passive motion machine starting on the surgery date. Patients were toe-touch weightbearing routinely for 2 weeks, unless microfracture was performed, in which case toe-touch weightbearing was extended to 6 weeks.
Postoperative Assessment
All patients were assessed via a physical examination. The examination was performed by the senior author for male patients and by a female athletic trainer for female patients. Patients were prompted to respond to queries about numbness or tingling, and a test of light touch sensation was performed at all lower sensory regions. Patients were tested regardless of whether they reported any nerve-related symptoms. Abnormalities were localized to specific dermatomes at lower body test points, with all abnormal dermatomes recorded for each patient. Abnormalities of the perineum were sublocalized to further nerve branches (genitofemoral, obturator, inferior cluneal, and pudendal nerves). The dermatological assignment of sensory deficits was conducted as a way to spatially map sensory abnormalities rather than its more traditional use as a means to discern the nature of spinal cord injuries. Patients with localized, abnormal light touch sensitivity at both L2 and L3 dermatomes at the distal anterolateral thigh were noted as presenting with lateral femoral cutaneous nerve injuries, given that these injuries did not follow the course of the dermatomal distribution but of specific nerve innervation. Likewise, S2 and S3 nerve injuries in the distribution of the pudendal nerve were deemed pudendal nerve injuries, given that localized pressure neurapraxia, rather than stretch-related neurapraxia, was likely at this level.
Strength testing was performed for hip flexion/extension, hip adduction/abduction, knee flexion/extension, and ankle dorsiflexion/plantar flexion, with findings rated on the MMT grading scale. The MMT grading scale is deemed a legitimate tool for the assessment of motor deficits, with demonstrated interobserver and intraobserver reliability across several muscle groups.1,14,15 MMT was performed at the 3-week and 6-week postoperative physical examinations, but patients were only deemed as presenting with motor deficits if these deficits persisted through the 3-month follow-up physical examination.
Patients who presented without sensory deficits at the 3-week postoperative physical examination and continued to present as symptom free at the 6-week and 3-month examinations (with a resolution of strength deficits by the 3-month examination) were deemed “asymptomatic.” On the other hand, patients presenting with sensory dysfunction at the 3-week postoperative physical examination were classified as having a “nerve deficit.” Additionally, patients with persistent strength deficits at 3 months were classified as having a “nerve deficit.” Patients who presented with persistent sensory and/or motor deficits at the 3-month postoperative physical examination continued follow-up physical examinations at 6 months and 12 months.
Patients completed the modified Harris Hip Score (mHHS), Hip Outcome Score–Activities of Daily Living and –Sport subscales (HOS-ADL and HOS-Sport, respectively), 12-Item Short Form Health Survey (SF-12) mental and physical component summaries, and international Hip Outcome Tool–12 (iHOT-12) preoperatively and at 1 year postoperatively. Patients were evaluated at 1 year for the completion of patient-reported outcome measures regardless of their status as “nerve deficit” or “asymptomatic.”
Statistical Analysis
Statistics were performed using SPSS (IBM). Traction-related measurements and continuous demographic variables were compared between subgroups using a 2-sample t test. The normality of traction-related variable distributions was confirmed with the Shapiro-Wilk test. Correlations were explored between continuous demographic variables and all traction-related measurements using a Pearson correlation coefficient. Changes in outcome scores within the nerve deficit and asymptomatic groups were evaluated using paired t tests. Differences in the frequency of procedure types and sex between the nerve deficit and asymptomatic groups were analyzed with a Fisher exact test. Values of P < .05 were considered significant.
Results
Overview
A total of 45 patients who underwent hip arthroscopic surgery were included in the study (Table 1). Forty-two (93.3%) patients underwent labral repair, 3 (6.7%) patients underwent labral debridement, 38 (84.4%) patients underwent femoral osteoplasty, 26 (57.8%) patients underwent acetabuloplasty, 14 (31.1%) patients underwent acetabular chondroplasty, and 10 (22.2%) patients underwent acetabular microfracture. Traction-related measurements for the study group as a whole (N = 45) are presented in Table 2. Five (11.1%) patients had traction interrupted for a mean time of 10.8 ± 5.9 minutes (range, 4-20 minutes). The mean time in traction before interruption was 110 minutes. Traction was interrupted in these 5 patients out of concern for possible traction-related injuries due to the prolonged duration of traction.
TABLE 1.
Demographics of the Study Groupa
| Value | |
|---|---|
| Age, y | 40.0 (16-69) |
| Height, cm | 171.2 (152-193) |
| Weight, kg | 71.3 (50-118) |
| Body mass index, kg/m2 | 24.3 (18.6-34.0) |
| Sex, n (%) | |
| Male | 16 (35.6) |
| Female | 29 (64.4) |
aData are shown as mean (range) unless otherwise specified.
TABLE 2.
Traction-Related Measurements by Sexa
| All Patients (N = 45) | Male Patients (n = 16) | Female Patients (n = 29) | P | |
|---|---|---|---|---|
| Maximum traction force, lb | 129.0 ± 32.0 (55-175) | 149.1 ± 22.3 (100-175) | 119.6 ± 32.7 (55-170) | .0025 |
| Mean traction force, lb | 109.6 ± 32.6 (42-165) | 131.8 ± 25.1 (86-165) | 97.3 ± 29.9 (42-162) | .0003 |
| Duration of traction, min | 100.2 ± 32.0 (40-203) | 113.0 ± 40.8 (40-203) | 92.5 ± 23.5 (50-130) | .0375 |
| Impulse, lb·min | 11,142.0 ± 4749.7 (3067-25,575) | 15,011.9 ± 4498.3 (7883-25,575) | 9007.6 ± 3366.8 (3067-15,048) | <.0001 |
aData are shown as mean ± SD (range). Bolded values indicate statistical significance (P < .05).
Sex Differences in Traction-Related Measurements
Male patients required significantly greater maximum traction force to adequately distract the hip joint than did female patients (P = .0025). Male patients also required significantly greater mean traction force (P = .0003), significantly greater duration of traction (P = .0375), and significantly greater traction impulse (P < .0001) (Table 2).
Incidence of Nerve Deficits After Hip Arthroscopic Surgery
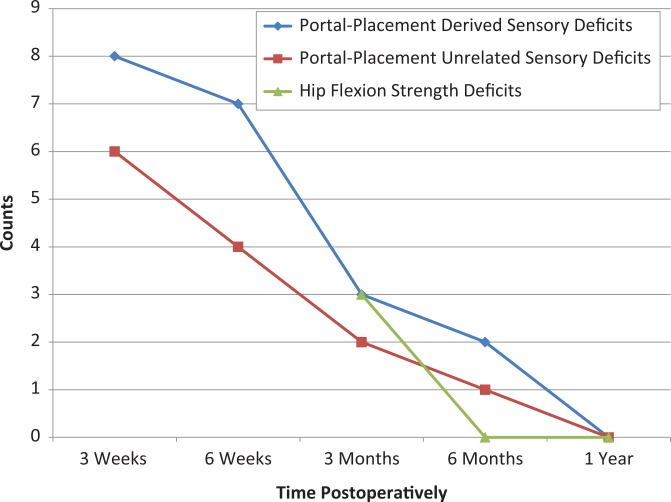
At the 3-week postoperative physical examination, 6 (13.3%) patients presented with localized, distal anterolateral thigh deficits thought to be related to portal placement (L2, L3) as their only sensory deficit (Table 3). Six (13.3%) patients presented with sensory deficits at other dermatomes (L1, T12, L5, S1, S2, S3) (Table 4). One (2.2%) patient presented with a sensory abnormality at the perineum. He demonstrated diminished light touch sensation at the S2 and S3 dermatomes in the perineal area as well as erectile dysfunction. Specifically, the diminished light touch sensitivity in the perineal area was sublocalized to the pudendal nerve’s distribution. This pudendal nerve injury was concomitant to diminished light touch sensation radiating along the S1 dermatome. Two (4.4%) of the 6 patients with sensory deficits at dermatomes unrelated to the lateral femoral cutaneous nerve distribution (L1, T12, L5, S1, S2, S3) also presented with sensory deficits at the anterolateral thigh (L2, L3) (Figure 4). At the 3-month physical examination, 3 (6.7%) patients presented with persistent strength deficits (4/5) with hip flexion in the absence of pain (Table 4 and Figure 4).
TABLE 3.
Patients Presenting With Localized, Distal Anterolateral Thigh Sensory Deficits as the Only Nerve Deficita
| Patient No. | Sex | Age, y | Height, cm | Weight, kg | Body Mass Index, kg/m2 | Duration of Traction, min | Time Out of Traction, min | Maximum Traction Force, lb | Mean Traction Force, lb | Traction Impulse, lb·min | Sensory Testing Findings and Resolution of Symptoms | Procedures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 22 | 172.7 | 63.6 | 21.3 | 115 | 0 | 125 | 95 | 10,899 | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area; resolved by 3 mo | Labral repair, rim trimming, femoral osteoplasty |
| 2 | Female | 24 | 170.1 | 63.6 | 21.9 | 107 | 0 | 150 | 114 | 12,232 | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area; resolved by 6 wk | Femoral osteoplasty, acetabular microfracture |
| 3 | Female | 34 | 167.6 | 71.8 | 25.6 | 125 | 0 | 155 | 120 | 15,048 | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area; resolved by 3 mo | Femoral osteoplasty, labral repair |
| 4 | Female | 43 | 172.7 | 62.3 | 20.9 | 118 | 0 | 100 | 81 | 9517 | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area; resolved by 3 mo | Femoral osteoplasty, labral repair, rim trimming |
| 5 | Female | 44 | 170.2 | 68.2 | 20.4 | 128 | 0 | 110 | 102 | 13,056 | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area; resolved by 1 y | Rim trimming, labral repair |
| 6 |
Male | 47 | 193.0 | 95.0 | 25.5 | 91 | 9 | 165 | 143 | 13,051 | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area; resolved by 3 mo | Labral repair, labral debridement, femoral osteoplasty |
aAll 6 patients had localized sensory deficits overlapping the L2/L3 dermatomes at the distal anterolateral thigh.
TABLE 4.
Patients With Sensory and Motor Deficits Beyond Isolated Anterolateral Thigh Sensory Deficitsa
| Patient No. | Sex | Age, y | Height, cm | Weight, kg | Body Mass Index, kg/m2 | Duration of Traction, min | Time Out of Traction, min | Maximum Traction Force, lb | Mean Traction Force, lb |
Traction Impulse, lb·min | Motor Muscle Testing Findings and Resolution of Symptoms | Sensory Testing Findings and Resolution of Symptoms | Procedures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 34 | 172.7 | 62.7 | 21.0 | 101 | 0 | 130 | 109 | 10,966 | No deficits | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area, L1, and T12; T12 resolved by 6 wk and L1 and lateral femoral cutaneous nerve resolved by 6 mo | Femoral osteoplasty, labral repair |
| 2 | Male | 38 | 193.0 | 109.1 | 29.3 | 155 | 20 | 165 | 165 | 25,575 | No deficits | Abnormal light touch sensitivity at lateral femoral cutaneous nerve distribution area, T12, L1, and L5; L1 resolved by 6 wk, T12 resolved by 3 mo, and L5 and lateral femoral cutaneous nerve resolved by 1 y | Labral repair, acetabuloplasty, femoral osteoplasty, acetabular chondroplasty, acetabular microfracture |
| 3 | Female | 44 | 175.2 | 81.2 | 26.4 | 61 | 0 | 130 | 117 | 7109 | No deficits | Abnormal light touch sensitivity at L5; resolved by 3 mo | Labral repair, acetabuloplasty, femoral osteoplasty |
| 4 | Female | 31 | 162.6 | 61.4 | 23.2 | 101 | 0 | 125 | 82 | 8319 | No deficits | Abnormal light touch sensitivity at L1 and T12; resolved by 3 mo | Labral repair, femoral osteoplasty |
| 5 | Female | 49 | 168.9 | 65.9 | 24.9 | 70 | 0 | 155 | 149 | 10,407 | No deficits | Abnormal light touch sensitivity at S1; resolved by 6 wk | Labral repair, acetabuloplasty, femoral osteoplasty, acetabular chondroplasty |
| 6 | Male | 34 | 182.9 | 87.3 | 26.1 | 117 | 0 | 165 | 135 | 15,746 | Strength deficit with hip flexion (4/5); full strength by 6 mo | Abnormal light touch sensitivity at S1, S2, and S3 and erectile dysfunction; resolved by 6 wk | Labral repair, acetabuloplasty, femoral osteoplasty |
| 7 | Male | 33 | 193.0 | 86.4 | 23.1 | 90 | 0 | 150 | 120 | 10,776 | Strength deficit with hip flexion (4/5); full strength by 6 mo | None | Labral repair, acetabuloplasty, femoral osteoplasty, acetabular chondroplasty |
| 8 | Male | 46 | 175.3 | 65.9 | 21.5 | 203 | 12 | 120 | 98 | 19,793 | Strength deficit with hip flexion (4/5); full strength by 6 mo | None | Labral repair, acetabuloplasty, femoral osteoplasty |
aPatients 1 and 2 presented with sensory deficits consistent with portal placement but additional deficits beyond disturbance at the portal site. All abnormalities localized to L5 and S1 were diffuse, radiating along the course of the dermatological distribution. T12 abnormalities radiated through the anterior aspect of distribution. S2 and S3 abnormalities in patient 6 were highly localized in the area of the perineum/penis, suggestive of pressure-related dysfunction.
Figure 4.
Number of cases of nerve dysfunction by case type and time after surgery. The count at each time point does not equal the number of patients with nerve dysfunction at each time point, as several patients presented with multiple classifications concomitantly (ie, lateral femoral cutaneous nerve sensory deficit and hip flexion strength deficit). Strength deficits were not deemed abnormal unless they persisted through 3-month follow-up.
Two (4.4%) patients presented with a nerve injury at 6 months: 1 (2.2%) strictly portal placement–related injury and 1 (2.2%) with portal placement–related and –unrelated sensory dysfunction still present concomitantly. All strength deficits with hip flexion had resolved by the 6-month follow-up. All patients fully recovered by 1 year (Figure 4).
Characterization of Nerve Dysfunction by Demographic and Surgical Risk Factors
A multimodal analysis of risk factors for nerve dysfunction after surgery was performed comparing demographics and procedures between the nerve deficit (n = 14) and asymptomatic groups (n = 31) (Table 5). The only significant difference between groups was height, with patients in the nerve deficit group being taller than asymptomatic patients (P = .0154).
TABLE 5.
Analysis of Risk Factors for Sensory/Motor Dysfunction After Hip Arthroscopic Surgerya
| Nerve Deficit (n = 14) | Asymptomatic (n = 31) | P Value | |
|---|---|---|---|
| Age, y | 37.99 ± 8.58 (22-49) | 40.88 ± 12.01 (16-69) | .4228 |
| Height, cm | 176.44 ± 10.07 (163-193) | 168.70 ± 9.18 (152-185) | .0154 |
| Weight, kg | 74.60 ± 14.82 (61-109) | 69.82 ± 15.68 (50-118) | .3455 |
| Body mass index, kg/m2 | 23.66 ± 2.67 (20-29) | 24.32 ± 3.85 (19-34) | .5652 |
| Sex, n (%) | >.9999 | ||
| Male | 5 (35.7) | 11 (35.5) | |
| Female | 9 (64.3) | 20 (64.5) | |
| Femoral osteoplasty, n (%) | 13 (92.9) | 25 (80.6) | .7460 |
| Acetabuloplasty, n (%) | 9 (64.3) | 17 (54.8) | .4069 |
| Acetabular chondroplasty, n (%) | 3 (21.4) | 11 (35.5) | .4921 |
| Acetabular microfracture, n (%) | 3 (21.4) | 7 (22.6) | >.9999 |
aData are shown as mean ± SD (range) unless otherwise specified. Bolded values indicate statistical significance (P < .05).
Traction-related measurements were compared between patients who presented with nerve deficits and those who were asymptomatic, with the caveat that patients with portal placement–related nerve dysfunction (n = 6) as their only diagnosed deficit were combined with asymptomatic patients (n = 31) for this analysis, as their injuries were not thought to be caused by the force or duration of traction . These measurements are presented by subgroup in Table 6.
TABLE 6.
Traction-Related Measurements by Patient Subgroupa
| All Patients (N = 45) | Asymptomatic and Isolated Portal Placement–Related Sensory Deficits (n = 37) | Sensory/Motor Deficits Unrelated to Portal Placement (n = 8) | P Value | |
|---|---|---|---|---|
| Maximum traction force, lb | 129.0 ± 32.0 (55-175) | 126.1 ± 33.7 (55-175) | 142.5 ± 18.3 (120-165) | .1913 |
| Mean traction force, lb | 109.6 ± 32.6 (42-165) | 106.9 ± 33.4 (42-165) | 121.9 ± 27.0 (82-165) | .2452 |
| Duration of traction, min | 100.2 ± 32.0 (40-203) | 97.6 ± 28.0 (40-150) | 112.3 ± 46.7 (61-203) | .2433 |
| Impulse, lb·min | 11,142.0 ± 4749.7 (3067-25,575) | 10,614 ± 4259 (3067-21,306) | 13,568 ± 6341 (7109-25,575) | .1093 |
aData are shown as mean ± SD (range).
Patient-Reported Outcomes
Forty patients (88.0%) completed outcome measures at 1 year postoperatively. The 5 patients lost to follow-up were all asymptomatic patients. Improvements in all outcome scores except the SF-12 mental component summary were statistically significant for both the nerve deficit and the asymptomatic groups (Table 7).
TABLE 7.
Patient-Reported Outcome Scores Preoperatively and 1 Year Postoperativelya
| Nerve Deficit (n = 14) | Asymptomatic (n = 26) | |
|---|---|---|
| mHHS | ||
| Preoperative | 66.2 ± 15.5 | 62.0 ± 14.3 |
| 1 year postoperative | 81.3 ± 15.5 | 83.0 ± 21.6 |
| P value | .0188 | .0022 |
| HOS-ADL | ||
| Preoperative | 71.3 ± 16.3 | 66.8 ± 15.9 |
| 1 year postoperative | 90.9 ± 10.8 | 86.1 ± 24.2 |
| P value | .0161 | .0255 |
| HOS-Sport | ||
| Preoperative | 46.3 ± 31.7 | 45.8 ± 16.5 |
| 1 year postoperative | 87.5 ± 14.5 | 81.7 ± 23.4 |
| P value | .0203 | .0338 |
| SF-12 mental component summary | ||
| Preoperative | 54.6 ± 10.1 | 48.8 ± 11.7 |
| 1 year postoperative | 56.0 ± 6.6 | 51.6 ± 9.9 |
| P value | .5728 | .2868 |
| SF-12 physical component summary | ||
| Preoperative | 38.2 ± 8.7 | 36.1 ± 8.7 |
| 1 year postoperative | 47.6 ± 10.9 | 47.2 ± 10.7 |
| P value | .0180 | .0009 |
| iHOT-12 | ||
| Preoperative | 37.2 ± 18.5 | 35.1 ± 14.2 |
| 1 year postoperative | 69.0 ± 23.4 | 70.5 ± 26.2 |
| P value | .0087 | .0005 |
aData are shown as mean ± SD. Five patients lost to follow-up at 1 year were all “asymptomatic” and were excluded from outcome score analysis. Bolded values indicate statistical significance (P < .05). HOS-ADL, Hip Outcome Score–Activities of Daily Living; HOS-Sport, Hip Outcome Score–Sport; iHOT-12, international Hip Outcome Tool–12; mHHS, modified Harris Hip Score; SF-12, 12-Item Short Form Health Survey.
Discussion
Neurological deficits relating to portal placement and traction after hip arthroscopic surgery were present with noteworthy frequency (31.1%) in this 45-patient series. Nerve dysfunction after hip arthroscopic surgery has been reported with a wide range of incidence, ranging from 0% to 74%.¶ We report on the higher range for the incidence of short-term neural complications compared with other studies that have quantified the incidence of neurapraxia after arthroscopic surgery. This may be because of careful physical examination testing, as these deficits were subtle. Many of the previous investigations have relied on patient-reported symptoms of nerve dysfunction and have lacked regular and standardized patient follow-up protocols through symptom resolution.
Moreover, 17.8% of patients presented with highly localized, distal anterolateral sensory deficits suggestive of injuries related to portal placement. The incidence of portal placement–related deficits in this study cohort is comparable with that reported by Dippmann et al11 in a 50-patient retrospective study in which neural dysfunction related to surgical incision and instrumentation was noted in 16% of the patients in the case series. Similarly, Larson et al20 reported lateral femoral cutaneous nerve disturbance in 16.5% of cases in 1615 consecutive hip arthroscopic procedures. While none of the nerve injuries were permanent in our case series, symptoms were persistent in 7 of 8 cases of lateral femoral cutaneous nerve injuries through 6-week follow-up. Patients should be informed of these risks preoperatively. While the sciatic nerve is a potential target for injuries with posterolateral incision and instrument mobilization in this posterolateral space, all procedures in our 45-patient series were performed with the patient in the supine position without posterolateral portal placement.7,8 Hence, the 2 patients who presented with diffuse, diminished light touch sensation radiating along the posterior aspect of the S1 dermatome were deemed to have some mechanism of injury unrelated to portal placement, with stretch/tension neurapraxia most likely at this level.
Further, 13.3% of patients in this case series presented with diffuse, diminished sensation at lower extremity dermatomes unrelated to the lateral femoral cutaneous nerve distribution and 6.7% with hip flexion strength deficits. One (2.2%) patient demonstrated diminished sensation highly localized in the pelvic region of the S2 and S3 dermatomes, consistent with compression-related disturbance of the pudendal nerve, a previously studied low-frequency (1%-2%) complication of hip arthroscopic surgery.27,34 This same patient presented with erectile dysfunction and diminished sensation radiating along the S1 dermatome that both resolved by the time of the 6-week physical examination concurrent with the return of perineal sensation. The duration of traction, mean traction force, maximum traction force, and traction impulse for this patient were 117 minutes, 135 lb, 165 lb, and 15,746 lb·min, respectively, with all measurements well exceeding study group means (Table 2). This low observed incidence (1/16 males, 6.3%) of erectile dysfunction after hip arthroscopic surgery is a finding consistent with the literature (0%-4.3%).11,25,30 At the same time, it is a difficult finding to place much weight on, considering the low number of male patients in this study. While a technique for performing hip arthroscopic surgery without the use of a perineal post has been described with 0 instances of perineal or pudendal neurapraxia in 2000 cases, the low incidence of pudendal nerve affection in this study and others suggests that the rate of complications may not necessarily warrant an adaptation of the existing and well-described procedure.24
Some authors have suggested that nerve compression is responsible for nerve injuries during hip arthroscopic surgery.3,36 Others have suggested that a “tourniquet-like,” time-dependent ischemic mechanism may lead to neurapraxia.4 Others believe that stretching of the nerve from distraction explains the injury. Most surgeons do not use a tensiometer to measure the force of traction during hip arthroscopic surgery, but guidelines recommend applying up to 50 lb of traction in most cases.32 Not using enough traction can lead to femoral head scuffing, inadequate intra-articular visualization, and iatrogenic cartilage damage. On the other hand, too much traction may lead to nerve damage and other complications. In this study, patients experienced a mean traction force of 109.6 lb, with it reaching a maximum of 175 lb. This amount of traction exceeds any previously reported measurements in anesthetized patients. Eriksson et al13 reported using 67 to 112 lb of force to maintain adequate distraction. Mason et al23 reportedly used 25 to 100 lb of traction force. Although the rate of sensory and motor deficits unrelated to portal placement in this study was relatively high (17.8%), patients reported full recovery in most cases in less than 3 months. Notably, in the combined asymptomatic and isolated portal placement injury subgroup (Table 6), traction force averaged 106.9 lb for a mean duration of 97.6 minutes.
We report a threshold of 61 minutes for traction time based on a previous description by Salas and O’Donnell.30 The threshold is defined as the time in traction, below which no patients suffered traction-related nerve dysfunction. This time of 61 minutes well exceeds the recent report of a threshold of 20 minutes from a 200-patient series.30 We also report thresholds for maximum traction force, mean traction force, and traction impulse of 120 lb, 82 lb, and 7109 lb·min, respectively. Given the transient nature of all traction-related deficits, the 17.8% incidence of traction-related deficits, and the fact that traction measurements in asymptomatic patients well exceeded previous recommendations, the suggestion here is that it may be possible to apply more traction force for longer periods of time than expert opinion has previously suggested. Yet, a 17.8% incidence of traction-related short-term deficits is not insignificant, and the risk of traction-related problems ought to be explained to all surgical candidates. Frandsen et al16 have challenged the historical reporting of this complication, noting a 74% incidence of transient traction-related nerve deficits after hip arthroscopic surgery. Even with this elevated incidence of complications, their traction times in both symptomatic and asymptomatic patients were less than in our study (39 and 36 minutes, respectively).16
The magnitudes of traction-related variables did not significantly differ between patients with injuries suspected to be related to traction and those who were asymptomatic or had portal placement injuries. This insignificance is consistent with previous attempts to explain the incidence of nerve dysfunction on the basis of duration of traction, in which traction times of small subgroups presenting with nerve deficits were compared with asymptomatic cohorts.11,30 As with this study, the low incidence of nerve dysfunction (n = 15,11 n = 430) unrelated to portal placement left these analyses underpowered. Larger studies need to be performed, characterized by rigorous and recurring physical examinations that evaluate the frequency of nerve injuries using the metrics of traction force and duration of traction, preferably including a measure such as impulse, which combines these factors. More robust analyses with heightened statistical power might shed further light on the risk factors for nerve injuries during hip arthroscopic surgery, with height being the only factor in this analysis differing between groups (Table 5). Whether height is an independent predictor of nerve injuries is unclear. By multivariate regression, male sex and height were found in combination to be stronger predictors of mean traction force than sex or height alone. While our analysis was underpowered to detect differences in traction force between the nerve deficit and asymptomatic groups, the associations both between increased height and nerve injuries and between increased height and increased traction force point to a possible association between nerve injuries and force of traction.
While not a directed objective of this study from its outset, significant differences were found between male and female patients for all traction-related variables (see Table 2). Differences in laxity across sex likely explain some of the differences in the force and duration of traction required to distract the hip joint for the mobilization of surgical instrumentation and adequate intra-articular visualization. This relationship of sex to joint laxity has been confirmed both in the cadaveric setting and with cross-sectional cohorts using the Beighton and Horan Joint Mobility Index.5,6,29
Last, it is possible that isolated anterolateral sensory deficits at the location of the lateral femoral cutaneous nerve distribution might have had their origin in a stretching- or compression-type event. However, supine patient positioning, minimal hip flexion, and recent cadaveric investigations demonstrating high rates of intersection of an anterior portal with the lateral femoral cutaneous nerve are all suggestive of portal placement as a cause of these deficits.2 A post hoc analysis was performed in which patients with isolated anterolateral thigh deficits were combined with patients with traction-related deficits. This group was then compared with asymptomatic patients to compare the magnitudes of traction-related variables. No significant differences were noted between groups with respect to any of the traction-related variables.
Limitations
The low incidence of nerve complications led to underpowered analyses of the differences in traction-related measurements between asymptomatic and affected patients. Additionally, nerve dysfunction was characterized by a physical examination beginning at the time of 3 weeks postoperatively. While patients were prompted to disclose the presence of any sensory or motor abnormality between surgery and first follow-up, and although such a disclosure failed to supplement any evidenced dysfunction at 3-week follow-up, it is possible that some subtle neural complication had resolved by the 3-week postoperative examination.16 In addition, it appears worthwhile to study the relationship between more frequent or longer pauses in traction and the incidence of neurological deficits. Given the small subgroup of patients in this study who had traction interrupted, such an analysis was underpowered. The knowledge that might proceed from such an analysis, used in conjunction with more clearly delineated guidelines pertaining to the force and duration of traction, would be valuable for decreasing the incidence of short-term nerve deficits after hip arthroscopic surgery.
While we found that 31.1% of patients presented with sensory and/or motor deficits after hip arthroscopic surgery, it is important to keep in mind that this number results from a small series of 45 patients. Finally, the use of a tensiometer is not widespread among surgeons performing hip arthroscopic surgery, and the value of a quantitative recommendation for traction force is unclear.
Conclusion
We report threshold values for maximum traction force, mean traction force, duration of traction, and traction impulse of 120 lb, 82 lb, 61 minutes, and 7109 lb·min, respectively, below which no patient suffered traction-related nerve deficits. Given the transient nature of all traction-related deficits, the 17.8% incidence of traction-related deficits, and the fact that traction measurements in asymptomatic patients well exceeded previous recommendations, the suggestion here is that it may be possible to apply more traction force for a longer duration than expert opinion has previously suggested.
Yet, transient nerve injuries after hip arthroscopic surgery may be more frequent (31.1%) than historical reports suggest. Also, 17.8% of patients presented with localized, distal anterolateral thigh sensory deficits suggestive of injuries related to portal placement, and 2.2% of patients presented with perineal numbness localized to the pudendal nerve’s distribution. All neural deficits resolved by 1 year postoperatively with good outcome scores, regardless of whether a neural deficit was observed postoperatively.
Acknowledgment
The authors thank Kelly Hearne for assisting in institutional review board approval and the study design.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: D.S.C. is a consultant for Zimmer Biomet, Linvatec, and DePuy Orthopaedics; is a paid speaker/presenter for Zimmer Biomet and Arthrex; has received hospitality payments from Zimmer Biomet, Linvatec, Arthrex, DePuy Orthopaedics, and Smith & Nephew; and has received educational support from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Broward Health Institutional Review Board.
References
- 1. Barr AE, Diamond BE, Wade CK, et al. Reliability of testing measures in Duchenne or Becker muscular dystrophy. Arch Phys Med Rehabil. 1991;72(5):315–319. [PubMed] [Google Scholar]
- 2. Bartlett JD, Lawrence JE, Khanduja V. What is the risk posed to the lateral femoral cutaneous nerve during the use of the anterior portal of supine hip arthroscopy and the minimally invasive anterior approach for total hip arthroplasty? Arthroscopy. 2018;34(6):1833–1840. [DOI] [PubMed] [Google Scholar]
- 3. Beutel BG, Collins JA, Garofolo G, Youm T. Hip arthroscopy outcomes, complications, and traction safety in patients with prior lower-extremity arthroplasty. Int Orthop. 2015;39(1):13–18. [DOI] [PubMed] [Google Scholar]
- 4. Birmingham P. Hip arthroscopy neurapraxia: is it only about weight of traction? J Bone Joint Surg Am. 2012;94(22):e169. [DOI] [PubMed] [Google Scholar]
- 5. Boguszewski DV, Cheung EC, Joshi NB, Markolf KL, McAllister DR. Male-female differences in knee laxity and stiffness. Am J Sports Med. 2015;43(12):2982–2987. [DOI] [PubMed] [Google Scholar]
- 6. Borsa PA, Sauers EL, Herling DE. Patterns of glenohumeral joint laxity and stiffness in healthy men and women. Med Sci Sports Exerc. 2000;32(10):1685–1690. [DOI] [PubMed] [Google Scholar]
- 7. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418–423. [DOI] [PubMed] [Google Scholar]
- 8. Byrd JWT. Position and setup: supine In: Hip and Pelvis Injuries in Sports Medicine. Philadelphia: Lippincott Williams & Wilkins; 2010:86–98. [Google Scholar]
- 9. Chan K, Farrokhyar F, Burrow S, Kowalczuk M, Bhandari M, Ayeni OR. Complications following hip arthroscopy: a retrospective review of the Mcmaster experience (2009-2012). Can J Surg. 2013;56(6):422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286–1292. [DOI] [PubMed] [Google Scholar]
- 11. Dippmann C, Thorborg K, Kraemer O, Winge S, Holmich P. Symptoms of nerve dysfunction after hip arthroscopy: an under-reported complication? Arthroscopy. 2014;30(2):202–207. [DOI] [PubMed] [Google Scholar]
- 12. Elsaidi GA, Ruch DS, Schaefer WD, Kuzma K, Smith BP. Complications associated with traction on the hip during arthroscopy. J Bone Joint Surg Br. 2004;86(6):793–796. [DOI] [PubMed] [Google Scholar]
- 13. Eriksson E, Arvidsson I, Advidsson H. Diagnostic and operative arthroscopy of the hip. Orthopedics. 1986;9(2):169–176. [DOI] [PubMed] [Google Scholar]
- 14. Florence JM, Pandya S, King WM, et al. Clinical trials in Duchenne dystrophy: standardization and reliability of evaluation procedures. Phys Ther. 1984;64:41–45. [DOI] [PubMed] [Google Scholar]
- 15. Florence JM, Pandya S, King WM, et al. Intrarater reliability of manual muscle test (medical research council scale) grades in Duchenne’s muscular dystrophy. Phys Ther. 1992;72:115–122. [DOI] [PubMed] [Google Scholar]
- 16. Frandsen L, Lund B, Grønbech Nielsen T, Lind M. Traction-related problems after hip arthroscopy. J Hip Preserv Surg. 2017;4(1):54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goulding K, Beaule PE, Kim PR, Fazekas A. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res. 2010;468(9):2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589–595. [DOI] [PubMed] [Google Scholar]
- 19. Kocaoglu H, Basarir K, Akmese R, et al. The effect of traction force and hip abduction angle on pudendal nerve compression in hip arthroscopy: a cadaveric model. Arthroscopy. 2015;31(10):1974–1980. [DOI] [PubMed] [Google Scholar]
- 20. Larson CM, Clohisy JC, Beaulé PE, et al. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. 2016;44(9):2292–2298. [DOI] [PubMed] [Google Scholar]
- 21. Lo Y-P, Chan Y-S, Lien L-C, Lee M S-S, Hsu K-Y, Shih C-H. Complications of hip arthroscopy: analysis of seventy three cases. Chang Gung Med J. 2006;29(1):86–92. [PubMed] [Google Scholar]
- 22. Martin HD, Palmer IJ, Champlin K, Kaiser B, Kelly B, Leunig M. Physiological changes as a result of hip arthroscopy performed with traction. Arthroscopy. 2012;28(10):1365–1372. [DOI] [PubMed] [Google Scholar]
- 23. Mason JB, McCarthy JC, O’Donnell J, et al. Hip arthroscopy: surgical approach, positioning, and distraction. Clin Orthop Relat Res. 2003;(406):29–37. [DOI] [PubMed] [Google Scholar]
- 24. Mei-Dan O, McConkey MO, Young DA. Hip arthroscopy distraction without the use of a perineal post: prospective study. Orthopedics. 2013;36(1):e1–e5. [DOI] [PubMed] [Google Scholar]
- 25. Moriya M, Fukushima K, Uchiyama K, et al. Clinical results of arthroscopic surgery in patients over 50 years of age: what viability does it have as a joint preservative surgery? J Orthop Surg Res. 2017;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ochs BC, Herzka A, Yaylali I. Intraoperative neurophysiological monitoring of somatosensory evoked potentials during hip arthroscopy surgery. Neurodiagn J. 2012;52(4):312–319. [PubMed] [Google Scholar]
- 27. Pailhé R, Chiron P, Reina N, Cavaignac E, Lafontan V, Laffosse JM. Pudendal nerve neuralgia after hip arthroscopy: retrospective study and literature review. Orthop Traumatol Surg Res. 2013;99(7):785–790. [DOI] [PubMed] [Google Scholar]
- 28. Papavasiliou AV, Bardakos NV. Complications of arthroscopic surgery of the hip. Bone Joint Res. 2012;1(7):131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quatman CE, Ford KR, Myer GD, Paterno MV, Hewett TE. The effects of gender and maturational status on generalized joint laxity in young athletes. J Sci Med Sport. 2008;11(3):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salas AP, O’Donnell JM. Prospective study of nerve injuries associated with hip arthroscopy in the lateral position using the modified portals. J Hip Preserv Surg. 2016;3(4):278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20(4):831–835. [DOI] [PubMed] [Google Scholar]
- 32. Sampson TG. Lateral approach to hip arthroscopy In: Sekiya JK, Safran MR, Ranawat AS, et al., eds. Techniques in Hip Arthroscopy and Joint Preservation Surgery. Philadelphia: WB Saunders; 2011:95–104. [Google Scholar]
- 33. Simpson J, Sadri H, Villar R. Hip arthroscopy technique and complications. Orthop Traumatol Surg Res. 2010;96(8):S68–S76. [DOI] [PubMed] [Google Scholar]
- 34. Soulié M, Vazzoler N, Seguin P, et al. Conséquences urologiques du traumatisme du nerf pudendal sur table orthopédique: mise au point et conseils pratiques. Prog Urol. 2002;12:504–509. [PubMed] [Google Scholar]
- 35. Souza BG, Dani WS, Honda EK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. 2010;26(8):1053–1057. [DOI] [PubMed] [Google Scholar]
- 36. Telleria JJM, Safran MR, Harris AHS, Gardi JN, Glick JM. Risk of sciatic nerve traction injury during hip arthroscopy: is it the amount or duration? An intraoperative nerve monitoring study. J Bone Joint Surg Am. 2012;94(22):2025–2032. [DOI] [PubMed] [Google Scholar]
- 37. Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86:92–109. [DOI] [PubMed] [Google Scholar]