The recent discovery that an osteosarcoma was found in a 1.7-million-year old bone from a hominin in South Africa (Odes et al. 2016) suggests that cancer has been a disease that has afflicted the human species for thousands of generations. This view is also supported by a number of other reports suggesting the presence of this disease including multiple myeloma, prostate and breast cancer in humans between 230 and 3000 years B.C. (Binder et al., 2014; Karpozilos and Pavlidis, 2004; Strouhal and Kritscher, 1990). The existence of cancer in very early human species and in humans before modernization and the introduction of synthetic contaminants into the environment and diets, could offer clues to the etiology of this disease. Discoveries made in the modern era of scientific investigations have led to a more complete understanding of the mechanisms of carcinogenesis.
The first documented study determining the cause of cancer was made in 1775 when Percivall Pott noted an association between exposure to soot in chimney sweeps with scrotal cancer (Pott, 1775). This report is often cited as the first description of an exposure to chemicals in the chimney soot as a cause of cancer, but it would be more than 100 years before experimental evidence would actually show more causally related evidence to support this hypothesis. For example, Boveri (1914) hypothesized that cancer is cellular in nature, and originates from a single cell, which developed a chromosomal abnormality that is passed on to cells when they divide, ultimately leading to rapid cell proliferation. That chemicals can directly cause cancer was demonstrated by Yamagiwa and Ichikawa who demonstrated that topical application of coal tar to rabbit ears produced carcinomas (Yamagiwa and Ichikawa, 1918). Because soot and coal tar are actually mixtures of chemicals, scientists began to identify specific compounds within different substances and found that specific chemicals such as polycyclic aromatic hydrocarbons, commonly found in coal tar, could cause cancer in animal models (Cook et al., 1933). Chemists went on to hypothesize that metabolism of chemicals to electrophilic derivatives capable of binding to cellular macromolecules was required for chemicals to cause cancer (Miller and Miller, 1947), and this was later confirmed by Conney et al. (1956) who were the first to identify hepatic enzymes induced by a variety of chemicals including polycyclic aromatic hydrocarbons that can metabolically activate chemicals, now referred to as chemical carcinogens. The main enzymes responsible for metabolism of chemical carcinogens are the monooxygenase cytochromes P450 (Omura and Sato, 1964a,b) or CYPs. CYPs essential nature in the process of chemical carcinogenesis is now firmly established, but CYPs also carryout the inactivation of chemicals and metabolize the majority of therapeutically used drugs. Collectively, CYPs are known as phase I xenobiotic metabolizing enzymes. However, it is important to note that it was later discovered that phase II xenobiotic metabolizing enzymes (eg, glutathione-S-transferases, Uridine diphosphate-glucuronosyltransferases, etc.) also exist and have critical roles in the metabolism and subsequent effects induced by chemical carcinogens (Omiecinski et al., 2011).
Having established that most chemical carcinogens require metabolic activation to produce cell transformation, the cellular targets that bind to cellular macromolecules were identified. The hallmark discovery of the structure of deoxyribose nucleic acid (DNA) by Watson and Crick (1953) was clearly pivotal in finding the cellular constituents that leads to cancer. Given the types of cellular macromolecules, an obvious choice to examine for potential interactions that may cause cancer by chemical metabolites was DNA. The metabolic activation of aflatoxin to reactive intermediates that bound to DNA was later established by Garner (1973) and others (reviewed in Rendic and Guengerich, 2012). Thus, it was not surprising that chemicals can selectively target regions of specific genes such as TP53 or APC leading to a direct link between chemical exposures and multiple cancers (Fearon and Vogelstein, 1990; Hollstein et al., 1991). It is particularly important to note that many of these discoveries were made because of numerous hallmark discoveries during the period of time when an explosion of molecular biology techniques were being developed, including polymerase chain reaction, DNA sequencing, and many, many others. It is also not surprising that the pace of discoveries linking not just chemical exposure, but also other exogenous and endogenous factors that contribute to the etiology of carcinogenesis, which coincided with this period of modern molecular biology and biochemistry.
It is now known that the etiology of carcinogenesis is extremely complicated and involves many different levels of regulation. In addition to chemical carcinogenesis, it is now known that endogenous molecular pathways can also cause mutations in critical genes through the production of reactive oxygen species that can damage cellular macromolecules including DNA. Moreover, it also known that environmental chemicals can interact with both genes and metabolic pathways, creating a scenario that leads to the complicated mechanisms that underlie carcinogenesis (Figs. 1 and 2). For example, environmental chemicals can cause mutations in critical genes after bioactivation to reactive intermediates, and these same chemicals (or others) can act as tumor promoters by enhancing the proliferation of cells with mutations in oncogenes. Moreover, polymorphisms in phase I and II xenobiotic metabolizing enzymes have also been linked to altered risk of developing cancers due to exposure to chemical carcinogens (Kiyohara et al., 2002; Tsuchiya et al., 2002; Zhang, 2010). Critical genes can also become mutated by endogenous pathways (eg, reactive oxygen species and/or lack of DNA repair), promoted by chemicals to become cancers due to mutations in genes that regulate the cell cycle due to exposure to environmental chemicals. Because there are multiple combinations of interactions between environmental chemicals, genes, and endogenous signaling that occurs in somatic, stem and immune cells that can collectively cause cancer, it has become increasingly difficult to conclusively determine the mechanisms that cause humans or other species to develop cancer.
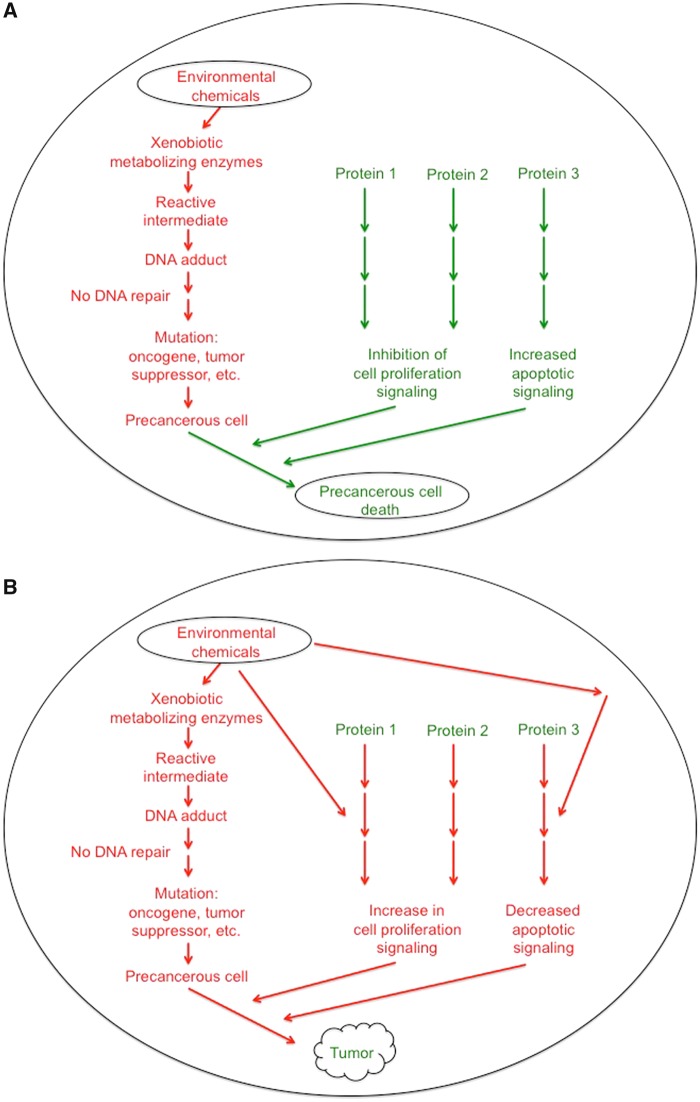
Figure 1.
Mechanisms by which environmental chemicals interact with chemical metabolism and endogenous signaling. A, Environmental chemical exposure can be metabolized by xenobiotic metabolizing enzymes to reactive intermediates that form adducts with DNA, and if not repaired by DNA repair enzymes, cause permanent mutations in critical genes that are known to be targeted by some chemicals. This results in the formation of precancerous cells. Under normal situations, there are molecular pathways that regulate cell proliferation and apoptosis that can cause these mutant cells to stop growing or undergo apoptosis, thereby preventing the formation of a tumor. B, Environmental chemical exposure can be metabolized by xenobiotic-metabolizing enzymes to reactive intermediates that form adducts with DNA, and if not repaired by DNA repair enzymes, cause permanent mutations in critical genes that are known to be targeted by some chemicals, such as tumor suppressors or proto-oncogenes. This metabolism can be influenced by polymorphisms in the xenobiotic metabolizing enzymes that catalyze these reactions. Collectively, the pathway outlined in (B) results in the formation of precancerous cells. If the environmental chemical(s) interfere with normal signaling such as those that prevent proliferation or induce apoptosis, and this can lead to the formation of tumors and cancer.
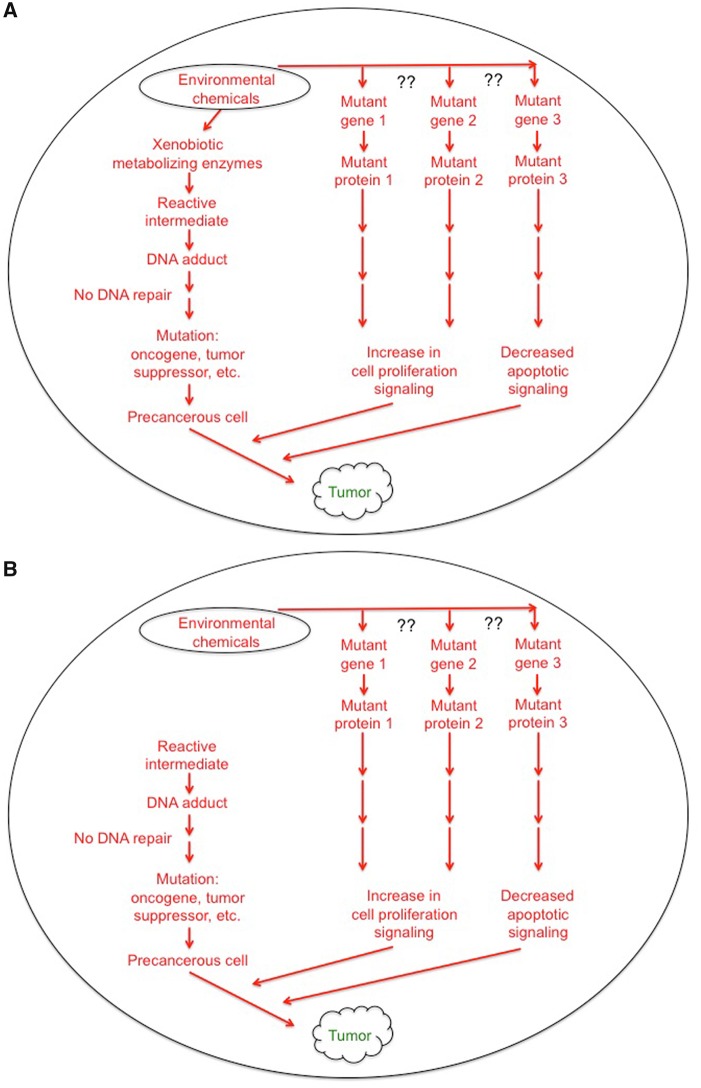
Figure 2.
Mechanisms by which environmental chemicals interact with genes and cause cancer. A, Environmental chemical exposure can be metabolized by xenobiotic metabolizing enzymes to reactive intermediates that form adducts with DNA, and if not repaired by DNA repair enzymes, cause permanent mutations in critical genes that are known to be targeted by some chemicals, such as tumor suppressors or proto-oncogenes. This metabolism can be influenced by polymorphisms in the xenobiotic metabolizing enzymes that catalyze these reactions. This results in the formation of precancerous cells. Environmental chemical(s) may also cause mutagenesis via xenobiotic metabolizing enzymes forming reactive intermediates that form adducts with genes encoding normal signaling that prevents proliferation or induction of apoptosis (??) and combined this can lead to the formation of tumors and cancer. B, Environmental chemical(s) may cause mutagenesis via xenobiotic metabolizing enzymes forming reactive intermediates that form adducts with genes encoding normal signaling proteins that prevents proliferation or induction of apoptosis (??). “Normal” endogenous signaling may result in the generation of reactive intermediates (or reactive oxygen species) that can cause mutations in genes that are critical for tumor suppression or oncogenes that combined with the former alterations, leads to formation of tumors and cancer. There are many combinations of the pathways outlined in Figures 1 and 2 that could theoretically participate in the etiology of how chemicals, genes and endogenous signaling interact to cause cancer. Moreover, the regulation of each of the critical steps in these pathways can also be central and possibly targeted to prevent or treat different cancers.
Despite the complexities of the mechanisms of carcinogenesis, considerable progress has been made in approaches to prevent and treat cancers due in large part to the extensive amount of mechanistic insight into various cancers. Decreased mortality in breast cancer patients can be attributed to the development of the estrogen receptor antagonist tamoxifen, and herceptin that attracts immune cells to breast cancer cells that express high levels of the HER2 gene, and aromatase inhibitors that inhibit the production of estrogen (Fisher et al., 1998; Goss et al., 2011; Narod et al., 2015). More recent discoveries in gene therapy and immunotherapy have led to the development of effective treatments for leukemia and lung cancer, through the application of genetically engineered CAR T cells and antiPD-1/antiPD-1L monoclonal antibodies that have led to remarkable increased survival for these diseases (Maude et al., 2014; Reck et al., 2016). However, this is not true for all types of cancer. For example, while significant decreases in breast cancer mortality have been observed in the past 40 years (Wingo et al., 1998), this is not true for pancreatic cancer where the 5 year survival rate remains approximately 5% (Ilic and Ilic, 2016).
There are many questions that remain about the causes of cancer, and how scientists can development new and effective preventive and therapeutic strategies to improve human lifespan of people affected by this disease. Why do some patients respond to immunotherapies while others do not? What combinations of preventive/therapeutic agents can be developed to prevent/treat different cancers? How significant is environmental chemical exposure interactions with endogenous signaling pathways in various cell types in the etiology of cancer? What is the relevance of preclinical evidence of cancers in nonhuman species to human cancers? Why do some people that use tobacco develops cancers but other do not? These are but a few of the challenging questions that remain to be answered. Given the marked progress in fighting this disease in the past 50 years, and the rapid pace observed in recent years, is it possible that cures for all forms of cancer is attainable in our lifetime? That question awaits the creative and novel discoveries that will certainly be developed in the future.
FUNDING
This work was supported in part by the National Institutes of Health (CA124533, CA140369 to J.M.P.).
REFERENCES
- Binder M., Roberts C., Spencer N., Antoine D., Cartwright C. (2014). On the antiquity of cancer: Evidence for metastatic carcinoma in a young man from ancient Nubia (c. 1200 BC). PLoS One 9, e90924.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. (1914). Zur Frage der Entstehung maligner Tumoren. G. Fischer, Jena.
- Conney A. H., Miller E. C., Miller J. A. (1956). The metabolism of methylated aminoazo dyes. V. Evidence for induction of enzyme synthesis in the rat by 3-methylcholanthrene. Cancer Res. 16, 450–459. [PubMed] [Google Scholar]
- Cook J. W., Hewett C. L., Hieger I. (1933). The isolation of a cancer-producing hydrocarbon from coal tar. Parts I, II, and III. J. Chem. Soc. (Resumed) 24, 395–405. [Google Scholar]
- Fearon E. R., Vogelstein B. (1990). A genetic model for colorectal tumorigenesis. Cell 61, 759–767. [DOI] [PubMed] [Google Scholar]
- Fisher B., Costantino J. P., Wickerham D. L., Redmond C. K., Kavanah M., Cronin W. M., Vogel V., Robidoux A., Dimitrov N., Atkins J., et al. (1998). Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 90, 1371–1388. [DOI] [PubMed] [Google Scholar]
- Garner R. C. (1973). Microsome-dependent binding of aflatoxin B1 to DNA, RNA, polyribonucleotides and protein in vitro. Chem. Biol. Interact. 6, 125–129. [DOI] [PubMed] [Google Scholar]
- Goss P. E., Ingle J. N., Ales-Martinez J. E., Cheung A. M., Chlebowski R. T., Wactawski-Wende J., McTiernan A., Robbins J., Johnson K. C., Martin L. W., et al. (2011). Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 364, 2381–2391. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. (1991). p53 mutations in human cancers. Science 253, 49–53. [DOI] [PubMed] [Google Scholar]
- Ilic M., Ilic I. (2016). Epidemiology of pancreatic cancer. World J. Gastroenterol. 22, 9694–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpozilos A., Pavlidis N. (2004). The treatment of cancer in Greek antiquity. Eur. J. Cancer 40, 2033–2040. [DOI] [PubMed] [Google Scholar]
- Kiyohara C., Otsu A., Shirakawa T., Fukuda S., Hopkin J. M. (2002). Genetic polymorphisms and lung cancer susceptibility: A review. Lung Cancer 37, 241–256. [DOI] [PubMed] [Google Scholar]
- Maude S. L., Frey N., Shaw P. A., Aplenc R., Barrett D. M., Bunin N. J., Chew A., Gonzalez V. E., Zheng Z., Lacey S. F., et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. (1947). The Presence and Significance of Bound Aminoazo Dyes in the Livers of Rats Fed p-dimethyl-aminoazobenzene. Cancer Res 7, 468–480. [Google Scholar]
- Narod S. A., Iqbal J., Miller A. B. (2015). Why have breast cancer mortality rates declined? J. Cancer Policy 5, 8–17. [Google Scholar]
- Odes E. J., Randolph-Quinney P. S., Steyn M., Throckmorton Z., Smilg J. S., Zipfel B., Augustine T. N., de Beer F., Hoffman J. W., Franklin R. D., et al. (2016). Earliest hominin cancer: 1.7-million-year-old osteosarcoma from Swartkrans Cave, South Africa S. Afr. J. Sci. 112, 1–5. [Google Scholar]
- Omiecinski C. J., Vanden Heuvel J. P., Perdew G. H., Peters J. M. (2011). Xenobiotic metabolism, disposition, and regulation by receptors: From biochemical phenomenon to predictors of major toxicities. Toxicol. Sci. 120, S49–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T., Sato R. (1964a). The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 239, 2370–2378. [PubMed] [Google Scholar]
- Omura T., Sato R. (1964b). The Carbon Monoxide-Binding Pigment of Liver Microsomes. II. Solubilization, Purification, and Properties. J. Biol. Chem. 239, 2379–2385. [PubMed] [Google Scholar]
- Pott P. (1775). The Chirurgical Works. In Chirurgical Observations Relative to the Cataract, The Polypus of the Nose, The Cancer of the Scrotum, The Differenent Kinds of Ruptures, and The Mortification of the Toes and Feet (Hawes, W. Clarke and R. Collins, Eds.), Vol. III, pp. 60–68. Printed by T.J. Carnegy for L. Hawes, W. Clarke and R. Collin, London.
- Reck M., Rodríguez-Abreu D., Robinson A. G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. (2016). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Rendic S., Guengerich F. P. (2012). Contributions of human enzymes in carcinogen metabolism. Chem. Res. Toxicol. 25, 1316–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouhal E., Kritscher H. (1990). Neolithic case of a multiple myeloma from Mauer (Vienna, Austria). Anthropologie 28, 79–87. [Google Scholar]
- Tsuchiya Y., Sato T., Kiyohara C., Yoshida K., Ogoshi K., Nakamura K., Yamamoto M. (2002). Genetic polymorphisms of cytochrome P450 1A1 and risk of gallbladder cancer. J. Exp. Clin. Cancer Res. 21, 119–124. [PubMed] [Google Scholar]
- Watson J. D., Crick F. H. (1953). Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171, 737–738. [DOI] [PubMed] [Google Scholar]
- Wingo P. A., Ries L. A., Rosenberg H. M., Miller D. S., Edwards B. K. (1998). Cancer incidence and mortality, 1973-1995: A report card for the U.S. Cancer 82, 1197–1207. [DOI] [PubMed] [Google Scholar]
- Yamagiwa K., Ichikawa K. (1918). Experimental study of the pathogenesis of carcinoma. J. Cancer Res. 3, 1–29. [DOI] [PubMed] [Google Scholar]
- Zhang Y. J. (2010). Interactions of chemical carcinogens and genetic variation in hepatocellular carcinoma. World J. Hepatol. 2, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]