Abstract
As the older class of brominated flame retardants (BFRs) are phased out of commercial use because of findings of neurotoxicity with developmental exposure, a newer class of flame retardants have been introduced, the organophosphate flame retardants (OPFRs). Presently, little is known about the potential for developmental neurotoxicity or the behavioral consequences of OPFR exposure. Our aim was to characterize the life-long neurobehavioral effects of 4 widely used OPFRs using the zebrafish model. Zebrafish embryos were exposed to 0.1% DMSO (vehicle control); or one of the following treatments; isopropylated phenyl phosphate (IPP) (0.01, 0.03, 0.1, 0.3 µM); butylphenyl diphenyl phosphate (BPDP) (0.003, 0.03, 0.3, 3 µM); 2-ethylhexyl diphenyl phosphate (EHDP) (0.03, 0.3, 1 µM); isodecyl diphenyl phosphate (IDDP) (0.1, 0.3, 1, 10 µM) from 0- to 5-days postfertilization. On Day 6, the larvae were tested for motility under alternating dark and light conditions. Finally, at 5–7 months of age the exposed fish and controls were tested on a battery of behavioral tests to assess emotional function, sensorimotor response, social interaction and predator evasion. These tests showed chemical-specific short-term effects of altered motility in larvae in all of the tested compounds, and long-term impairment of anxiety-related behavior in adults following IPP, BPDP, or EHDP exposures. Our results show that OPFRs may not be a safe alternative to the phased-out BFRs and may cause behavioral impacts throughout the lifespan. Further research should evaluate the risk to mammalian experimental models and humans.
Keywords: zebrafish, organophosphate flame retardants, development, neurobehavioral toxicology, anxiety-related behavior
Developmental neurotoxicity caused by exposure to environmental contaminants has been a growing concern for several decades (Grandjean and Landrigan, 2006, 2014). It has been shown that many anthropogenically released chemicals can cause disruption of normal neurodevelopment even at very low levels of exposure, far below those causing overt toxicity in adults. Flame retardants (FRs) are a diverse group of chemicals that are added to many consumer products to reduce their flammability. The use of FRs has increased markedly since the introduction of strict flammability regulations in the United States during the 1970s. However, additive FRs are not chemically bound to the materials to which they are inserted and thus gradually escape into the surrounding environment, resulting in their almost ubiquitous presence in indoor air and dust (Fromme et al., 2014; Phillips et al., 2017; Stapleton et al., 2009). In the past few decades, various chemicals have been used as FRs and later banned or phased out due to evidence of toxicity, most notably carcinogenicity and developmental neurotoxicity. These include polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs), that were replaced with other brominated FRs such as tetrabromobisphenol A (TBBPA) and polybrominated diphenyl ethers (PBDEs).
PBDEs were widely used until the early 2000s, when they were phased out due to reports of neurotoxicity, especially following developmental exposure. Recent human cohort studies found multiple correlations between elevated levels of PBDEs in breast milk, cord-blood, and serum, and altered neurobehavioral measures such as anxiety, attention, social performance, executive function, and motor skills in children (Adgent et al., 2014; Cowell et al., 2015; Ding et al., 2015; Hendriks and Westerink, 2015; Herbstman and Mall, 2014; Linares et al., 2015; Sagiv et al., 2015). These findings are further supported by animal studies showing alterations in spontaneous locomotor activity and habituation following pre- and postnatal exposures to various PBDE congeners (Costa and Giordano, 2007; Eriksson and Fredriksson, 1998; Eriksson et al., 2001, 2002; Hendriks and Westerink, 2015; Kuriyama et al., 2005; Viberg et al., 2003, 2004).
To replace the phased out PBDEs, several groups of organophosphate flame retardants (OPFRs) have been introduced into products, including halogenated and nonhalogenated variants. Accordingly, in the past decade, indoor and outdoor exposure levels to OPFRs have gone up, and these compounds and their metabolites can be detected in house dust samples and samples from human populations (Butt et al., 2014; Fromme et al., 2014; Kim et al., 2014; Phillips et al., 2017; Stapleton et al., 2009). Some OPFRs are present in consumer products in other capacities, such as plasticizers, thus potentially increasing their availability for environmental exposure. Of the nonhalogenated OPFRs, phenylphosphates comprise a large group of compounds with very little information available, from either epidemiological studies or experimental rodent models, on their exposure, bioaccumulation and potential health risks (Behl et al., 2015; Hendriks and Westerink, 2015). However, they have structural similarity to OP pesticides, some of which have been shown to cause developmental neurotoxicity and behavioral alterations (Gonzalez-Alzaga et al., 2014).
The zebrafish model has been used widely in studies of developmental neurobehavioral toxicology (Bailey et al., 2013; Guo, 2009; Joseph et al., 2012; Levin et al., 2015; McCollum et al., 2011; Nishimura et al., 2015). In particular, the zebrafish model has been shown to be sensitive to the short- and long-term behavioral impacts of flame retardant chemicals and mixtures (Bailey and Levin, 2015; Cheng et al., 2017; Glazer et al., 2018; Jarema et al., 2015; Noyes et al., 2015; Oliveri et al., 2015; Usenko et al., 2011; Zhao et al., 2014). These studies have shown that early life exposures to FRs can cause alterations in larval activity and response to light and dark stimuli, as well as changes in anxiety-related behavior and social affiliation in adults. These data support the use of zebrafish as an intermediate model for medium-to-large scale screens of suspected neurotoxins, providing valuable information such as relative potency within chemical groups, and potentially affected behavioral domains.
Here, we present novel data on the behavioral effects of zebrafish developmental exposure to 4 OPFRs; isopropylated phenyl phosphate (IPP), t-butylphenyl diphenyl phosphate (BPDP), 2-ethylhexyl diphenyl phosphate (EHDP), and isodecyl diphenyl phosphate (IDDP). These results are part of a larger study in which we are screening for potential early- and later-life neurobehavioral effects of low level exposure to several classes of FRs. In our first publication from this series of studies, we showed both short-term and persisting effects of exposure to the brominated FRs, BDE-47, and BDE-99 (Glazer et al., 2018). Here, we investigated the behavioral effects in a larval motility assay for response to light/dark changes, as well as an adult battery of tests measuring emotional function, sensorimotor response, social affiliation, and boldness.
MATERIALS AND METHODS
Fish housing and husbandry
All the experiments were conducted using a local colony of AB* wild-type strain of zebrafish, maintained, and bred in the Levin Lab at Duke University. The experimental procedures were approved by the Duke University Institutional Animal Care and Use Committee. Adult zebrafish were held in mixed (females and males) groups at a density of ≤5 fish/l in 3- or 10-l tanks kept on a recirculating flowing water system (Aquatic Habitats, Inc., Apopka, Florida; Aquatic Enterprises, Inc., Bridgewater, Massachusetts). System water was a mixture of sea salt (Instant Ocean, 0.5 parts per thousand) and buffer (Seachem Neutral Regulator, 125 mg/l) in deionized water. Water chemistry, salinity, and temperature were monitored weekly. Illumination was set to 14:10 h light:dark cycle and water temperature was kept at 28 ± 1°C. The fish were fed 3 times daily; morning and afternoon with brine shrimp (Artemia salina) hatched in-house over 24 h (eggs from Brine Shrimp Direct, Ogden, Utah); and noon feeding with a mixture of solid pellet food containing; TetraMIN Tropical Flakes (Blacksburg, Virginia); GEMMA Micro 300 micropellets (Skretting USA, Tooele, Utah); Zebrafish Complete Diet (Ziegler Bros., Inc., Gardners, Pennsylvania).
Embryo collection, and embryo and larval rearing were conducted as described in Glazer et al. (2018). Briefly, fertilized eggs were obtained by group breeding using in-tank inserts. Immediately after collection, the embryos were quickly bleached and transferred to large plastic Petri dishes for sorting, then glass Petri dishes for rearing and chemical exposures. At 0- to 6-day postfertilization (dpf), the embryos were kept in an incubator at a temperature range of 28–29°C, and a 14:10 h light:dark cycle. At 6 dpf, larvae were transferred to flow-through recirculating-system tanks, and the water level and diet were gradually adjusted to the age and size of the fish.
Chemicals
Dimethyl Sulfoxide ReagentPlus, ≥99.5% (DMSO; CAS No. 67-68-5, Lot No. SHBG9650V) was purchased from Sigma-Aldrich (St Louis, Missouri). Isopropylated phenyl phosphate (IPP), t-butylphenyl diphenyl phosphate (BPDP), 2-ethylhexyl diphenyl phosphate (EHDP), and isodecyl diphenyl phosphate (IDDP) were provided by the National Toxicology Program (NTP), at a volume of 5 ml and a concentration of 20 mM each. Supplementary Table 1 presents supplier specifications and purity analysis for the NTP-provided chemicals. According to this analysis, the IPP and BPDP solutions were found to contain high percentages of impurity; 54.8% and 35.5%, respectively. These impurities are largely due to the presence of the base compound triphenylphosphate (TPHP/TPP) from which both chemicals are synthesized. Further internal testing conducted by Dr. Heather Stapleton (Nicholas School of the Environment, Duke University) validated the TPHP levels reported for the IPP solution in the supplier specifications, whereas the TPHP levels in the BPDP solution were measured at 33.3%. Both mixtures also contain smaller quantities of various IPP and BPDP isoforms. We chose to continue our work with the mixture solutions for 2 reasons; (1) in our larger project, we are also testing the effects of TPHP alone, and therefore this data will also be made available in future; (2) commercial solutions labeled IPP or BPDP are also mixtures containing large fractions of TPHP and other impurities, thus, we think it is worthwhile testing the product as it is used by the industry.
Developmental exposure
Zebrafish embryos were exposed from approximately 5-h postfertilization (hpf) until 5 dpf to either 0.1% DMSO alone or one of the following individual chemicals and concentrations in 0.1% DMSO: IPP (0.01, 0.03, 0.1, 0.3 µM); BPDP (0.003, 0.03, 0.3, 3 µM); EHDP (0.03, 0.3, 1 µM); IDDP (0.1, 0.3, 1, 10 µM). Stock solutions were 20 mM, and working solutions were prepared at 1000× final concentration in 100% DMSO by conducting serial dilutions from the stock solutions. Before the start of exposure, a lab member who was not involved in the study would replace the solution names with letters, thus blinding the experimenters to the identity of the treatments for the full duration of the exposure and behavioral testing. Two separate sets of exposure were conducted; Set 1 consisted of IPP with a DMSO control; Set 2 consisted of BPDP, EHDP, and IDDP with a DMSO control. At 5 hpf, embryos were sorted under a dissecting microscope, discarding unfertilized or abnormally developing embryos, then randomly and evenly distributed into glass Petri dishes (inner diameter 9 cm; depth 2 cm) at a density of up to 50 embryos per dish with 1 ml system water per embryo, and immediately exposed to the above detailed treatments. Every 24 h, the embryos were examined and dead or malformed individuals were recorded and removed, and the exposure solution was renewed. If a certain dose appeared to cause wide-spread death or dysmorphogenesis, it was excluded from behavioral testing, and the dose range for subsequent exposures was adjusted. At 5 dpf, the embryos were rinsed twice with nondosed system water, transferred to clean glass Petri dishes with nondosed system water, and placed in the incubator until larval activity testing at 6 dpf.
Larval locomotor activity testing
At 6 dpf, larvae were placed into 96-well plates with 0.5 ml glass well inserts filled with system water and tested for locomotor activity in response to alternating light conditions. Supplementary Table 2 lists the cohorts and fish numbers in each treatment group. Exposure conditions were all represented within each plate and across multiple plates. Plates were then returned to the incubator for an hour before being placed into a DanioVision lightbox controlled by the EthoVision XT tracking software (version 11.5, Noldus, Wageningen, The Netherlands). Locomotor activity was tracked during a paradigm in which an initial 10-min acclimation period in the dark (0% illumination) was followed by 2 cycles of 10 min at 100% illumination (5000 lx) and 10 min at 0% illumination. An infrared camera tracked larval locomotion across the 50-min trial. All larval testing was conducted between 11 am and 5 pm. Locomotor activity was recorded at a rate of 30 frames per second and a track smoothing protocol was applied based on 10 samples before and after every sample point in order to exclude slight movements that might introduce noise to the calculations. Total distance moved is reported in cm per minute or cm per 10 min. After testing, the larvae were placed in flow-through tanks and reared as described earlier (Fish housing and husbandry).
Adult behavioral test battery
Developmentally exposed adult zebrafish were tested at the age of 5–7 months in a series of 4 behavioral assays; novel tank dive test; tap test; shoaling test; and predator avoidance test, to evaluate several emotional, social, and cognitive functions. Each assay was conducted on a separate day. All testing was conducted between 10 am and 5 pm, and testing times were counterbalanced across all experimental groups. Each testing day began after the routine morning brine shrimp feeding. Fish tanks designated for testing were transferred to the behavior testing room and let acclimate for 30–60 min. Freshly made system water was used in all testing apparatuses. An HD camcorder (VIXIA HFR700; Canon Inc., Tokyo, Japan) was used for video recording in all assays, and the videos were fed to the EthoVision XT software (Noldus) for fish tracking and activity analysis. Supplementary Table 3 summarizes the exposure cohorts and fish numbers used in adult behavior testing. Differences in numbers of fish tested and exposure cohorts per treatment are a result of our range seeking process in which certain concentrations were present in most or all exposure cohorts while other, lower or higher concentrations, were added in subsequent exposures. See Results section on Survival and Dysmorphogenesis at 6 dpf (Supplementary Table 4) for details on all exposure cohorts and concentrations. All adult behavioral assays were conducted as described in Glazer et al. (2018). The following are brief descriptions of the testing procedures.
Novel tank dive test
Adult zebrafish were tested for novel environment response based on the method developed in our laboratory (Levin et al., 2007) with modifications. At the beginning of each trial, 2 fish were individually placed in an experimental set-up consisting of 2 adjacent trapezoid 1.5-l plastic tanks (Aquatic Habitats) filled with 10 cm of system water. The tanks were video recorded from the side for 5 min. Measurements extracted were total distance traveled in cm for each min of testing and the mean distance from the tank floor in cm per min.
Startle tap test
Sensorimotor startle response and habituation were tested using a custom-built apparatus and a protocol developed in our laboratory (Crosby et al., 2015; Eddins et al., 2010), with modifications described in Glazer et al. (2018). The apparatus consisted of 8 clear cylindrical arenas, which were arranged in a 2 × 4 setup, and were video recorded from above. At the beginning of each trial, 8 fish were individually placed in the testing arenas and the testing sequence was initiated. The testing sequence consisted of a 30-s acclimation period followed by 10 consecutive taps at 1-min intervals. The taps were generated by 24-volt DC push solenoids located under each arena and activated by the EthoVision XT software. Measurements extracted were total distance traveled in cm during the 5-s period immediately before (pre) and after (post) each tap.
Shoaling test
Individual social interaction was tested using the Multiple Use Partitioned Experimental Tank (MUPET), a custom-built adult behavior testing tank. The MUPET was situated on 2 metal bars and a light box (Huion Technology, Shenzhen, China) was placed underneath the tank bottom providing even light throughout the tank. Black acrylic partitions were used to create 2 adjacent lanes across the tank width. Two 19.5-inch LCD monitors flanked the narrow ends of the 2 lanes. A digital video camcorder was placed above the tank.
The test procedure was based on a protocol developed in our laboratory (Oliveri et al., 2015), with modifications described in Glazer et al. (2018). Briefly, adult fish were singly isolated for 30 min before being netted into the MUPET lanes described earlier. Behavior was recorded for a 7-min session consisting of a 2-min habituation period followed by 5 min in which a video recording of a zebrafish shoal was played on one of the monitors. Measurements extracted were total distance traveled in cm per min and the mean distance in cm per min to the side of the tank on which the video was displayed. A pre-post video difference value was calculated for each treatment group by subtracting the average distance from the tank side in the 2 min after the video started playing from the average distance in the 2 min before the video began.
Predator avoidance test
Threat recognition and evasion behavior were tested using the same testing apparatus and set-up described in the previous section (Shoaling test). The test procedure was based on a protocol developed in our laboratory (Oliveri et al., 2015), with modifications described in Glazer et al. (2018). Briefly, individual fish were placed in the MUPET lanes and recorded for 9-min consisting of 1-min acclimation followed by 8-min of alternating minute-long stimulus/no stimulus (NS) events. The stimulus was a power point presentation showing either a blue slow-growing dot (4-s) or a red fast-growing dot (1-s) appearing repeatedly on one of the screens. These stimuli are 2-dimentional representations of a large entity, such as predator, approaching the fish at either slow (blue) or fast (red) speeds. The blue dot appeared in the first 2 stimulus events and the red dot appeared in the last 2 stimulus events. Measurements extracted were total distance traveled in cm per min, and the mean distance in cm per min to the side of the tank on which the stimulus was displayed. A flee response value was calculated for each stimulus by subtracting the average distance from the tank side in trial minutes in which there was no stimulus from minutes in which the dot stimulus was presented.
Statistical analysis
All statistical analyses were performed in GraphPad Prism (GraphPad Software, Inc., version 7.01). Significance was set at p < .05 for all analysis of variance (ANOVA) and Dunnett’s post hoc comparisons, and for linear regression comparisons.
Larval locomotor activity at 6 dpf was analyzed with two-way ANOVA, with treatment as the between subjects factor and illumination phase as the repeated measure. Distance moved (cm per 10-min illumination phase) was the dependent variable.
Two-way ANOVA was used to analyze novel tank total distance traveled and mean distance from the tank floor; tap test total distance traveled pre- and posttap; shoaling test and predator avoidance test total distance traveled and mean distance from tank side. Treatment was defined as the between subjects factor, time (min), or tap as the repeated measure, and distance (cm per min) as the dependent variable. Two-way ANOVA was also used to analyze shoaling test before and after video 2-min intervals. Treatment was defined as the between subjects factor, the 2-min intervals as the repeated measure, and average distance from tank side as the dependent variable. One-way ANOVA was used to analyze shoaling test before and after video difference; predator avoidance test blue or red flee response. Treatment was defined as the between subjects factor, and the above described calculated values were defined as dependent variables.
Linear regression was used to analyze pre- and posttap activity patterns. Each replicate Y value (distance traveled per tap per fish) was considered as an individual point and used to generate linear functions of activity across the session. Significance was calculated for the difference in slopes and in intercepts between treatment groups.
All data are presented as mean ± SEM.
RESULTS
Survival and Dysmorphogenesis at 6 dpf
On each day of exposure until 6 dpf, dead individuals or embryos that developed deformities such as spinal curvature, short-body, craniofacial malformations or edema were noted and removed, and their numbers were summed across the entire period. Supplementary Table 4 summarizes the percent of survival (including dead and deformed individuals) and highlights incidents of increased dysmorphogenesis at 6 dpf in each cohort and treatment. Exposure to 0.1% DMSO alone (vehicle control group) resulted in an average survival percentage of 82.8% for Set 1 (range across cohorts 75–92.5%), and 75.8% for Set 2 (range across cohorts 60–92%). With control treatments, the number of dead embryos or individuals with any specific malformation did not exceed 10% overall. In Set 1, treatment with 0.3 µM IPP resulted in wide spread spinal curvature in over 50% of individuals by 6 dpf, exposure to 3 µM IPP resulted in pericardial edema that was observed in all individuals at 4–5 dpf. Therefore, the highest exposure for behavioral analysis was set at 0.1 µM IPP. Treatment with 3 µM BPDP caused spinal curvature in over 50% of individuals at 4–5 dpf, and thus the highest exposure concentration for behavior analysis was set at 0.3 µM. Treatment with 1 µM EHDP caused pericardial edema in most individuals by 4 dpf, setting an exposure limit of 0.3 µM. IDDP exposure did not cause elevation in mortality or deformities in any concentration up to 10 µM.
Larval Locomotor Activity
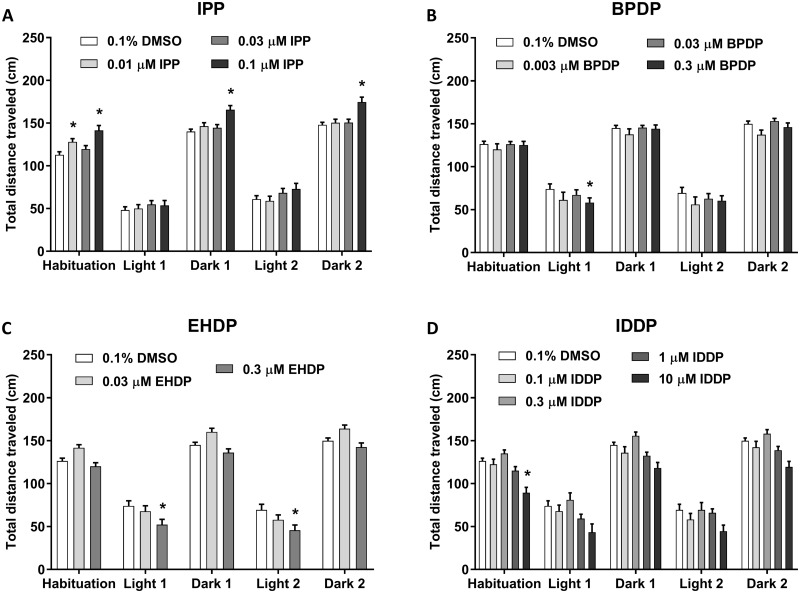
Developmental exposure to IPP altered locomotor activity of 6 dpf larvae at specific doses (Figure 1A). During the first dark phase of habituation, Dunnett’s comparisons revealed that 0.01 µM, the lowest IPP dose used in this study, caused an increase in activity relative to control. After the initial habituation phase, during the repeating light-dark sequence, two-way ANOVA analysis revealed overall effects of treatment (F(3, 1495) = 16.5, p < .0001) and illumination phase (F(4, 1495) = 457.9, p < .0001) on larval activity, but no interaction between the 2 parameters. Exposure to 0.1 µM, the highest dose used for behavioral testing, significantly increased activity during all dark phases. Exposure to 0.03 µM, IPP was not found to alter larval locomotor activity.
Figure 1.
Six dpf larval locomotor activity in response to alternating light/dark conditions following developmental exposure to IPP (A), BPDP (B), EHDP (C), and IDDP (D). The fish were recorded for 50 min, starting with 10 min in the dark (Habituation), followed by 2 cycles of 10 min in the light and 10 min in the dark. Asterisks indicate significant difference from the 0.1% DMSO control group. Sample sizes: 0.1% DMSO—Set 1 n = 92, Set 2 n = 63; IPP—0.01 µM n = 62, 0.03 µM n = 88, 0.1 µM n = 81; BPDP—0.003 µM n = 18, 0.03 µM n = 67, 0.3 µM n = 67; EHDP—0.03 µM n = 64, 0.3 µM n = 57; IDDP—0.1 µM n = 63, 0.3 µM n = 49, 1 µM n = 65, 10 µM n = 15.
Treatment with BPDP (Figure 1B) caused only a mild effect on larval locomotor activity (F(3, 1060) = 2.624, p < .05), whereas the effect of illumination phase was strong as is commonly observed in this assay (F(4, 1060) = 191.3, p < .0001). There was no significant interaction between illumination phase and treatment. Posthoc analysis revealed a reduction in activity in the highest exposure dose of 0.3 µM during the first light phase.
In EHDP-exposed fish, there were pronounced effects of both treatment (F(2, 905) = 18.83, p < .0001) and illumination phase (F(4, 905) = 254, p < .0001) without interaction (Figure 1C). Interestingly, treatment with the higher concentration of 0.3 µM caused hypoactivity in both light phases compared with the DMSO control.
Exposure to IDDP reduced activity compared with the DMSO during the habituation phase. In the following phases of alternating light and dark conditions, there were significant main effects of treatment (F(4, 1240) = 13.42, p < .0001) and illumination phase (F(4, 1240) = 148.2, p < .0001) but no interaction between the factors (Figure 1D). Larvae treated with 10 µM IDDP displayed a reduction in activity regardless of illumination phases.
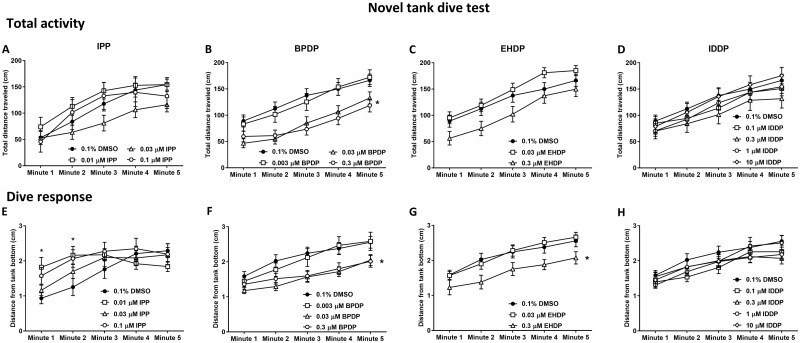
Adult Novel Tank Dive Test
Two parameters were measured in the novel tank test; the distance traveled by the fish was used as a measure of total activity (Figure 2A–D), and the distance of the fish from the bottom of the tank was used as a measure of the dive response (Figure 2E-H), both presented in 1-min time bins (Figure 2E–H). Across both Set 1 and Set 2, there was a gradual increase in values of both the total activity (F(4, 2072) = 158, p < .0001) and the dive response (F(4, 2056) = 84.52, p < .0001) over the duration of the test. This pattern is observed normally in control fish. Treatment of zebrafish embryos with IPP, BPDP, and EHDP, resulted in significant effects on distance from bottom of the tank, whereas BPDP and EHDP also caused changes in total activity of the fish. No effects were observed following IDDP treatment in any of the doses.
Figure 2.
Novel tank dive test. Adult zebrafish that were developmentally exposed to IPP (A, E), BPDP (B, F), EHDP (C, G), and IDDP (D, H) were individually placed in the testing tank (novel environment) and recorded for 5 min. Total activity (A–D) was calculated as the total distance traveled by the fish in each minute of the trial. Dive response (E–H) was calculated as the average distance of the fish from the bottom of the tank in each minute of the trial. Asterisks indicate significant difference from the 0.1% DMSO control group. Sample sizes: 0.1% DMSO—Set 1 n = 20, Set 2 n = 59; IPP—0.01 µM n = 18, 0.03 µM n = 26, 0.1 µM n = 9; BPDP—0.003 µM n = 36, 0.03 µM n = 71, 0.3 µM n = 55; EHDP—0.03 µM n = 58, 0.3 µM n = 40; IDDP—0.1 µM n = 46, 0.3 µM n = 28, 1 µM n = 60, 10 µM n = 31. The same sample sizes were used in all adult behavior assays.
IPP exposure caused a significant interaction of time x treatment (F(12, 244) = 3.613, p < .0001) in the distance from bottom, although there was no overall significant effect of treatment alone. Specifically, there was an increase in distance from the bottom in the lowest exposure concentration of 0.01 µM in minutes 1 and 2 (Figure 2E). Similarly, regression analysis of the linear slopes revealed a significant difference from control only in the lower dose (Supplementary Table 5). The total activity of IPP exposed fish was not affected by treatment nor was there a time x treatment interaction.
Exposure to BPDP resulted in significant treatment effects of reduction in both total activity (F(3, 217) = 6.564, p < .001) and distance from bottom (F(3, 213) = 4.699, p < .01) compared with controls, without time x treatment interaction. Posthoc analysis revealed that the effect was specific to the 2 higher exposure concentrations of 0.03 and 0.3 µM. Total activity of the 0.03 µM exposed fish was significantly reduced in minutes 1–4, whereas 0.3 µM treated fish were hypoactive in minutes 2–5. Distance from bottom was reduced following 0.03 µM treatment from minute 2 to minute 5, and following 0.3 µM treatment in minutes 3–5.
Treatment effects were also identified following EHDP exposure in both total activity (F(2, 154) = 3.6, p < .05) and distance from bottom of the tank (F(2, 154) = 3.947, p < .05), without time x treatment interaction. Total activity was not significantly altered in any specific time point, however distance from bottom of 0.3 µM treated fish was significantly reduced in minute 2.
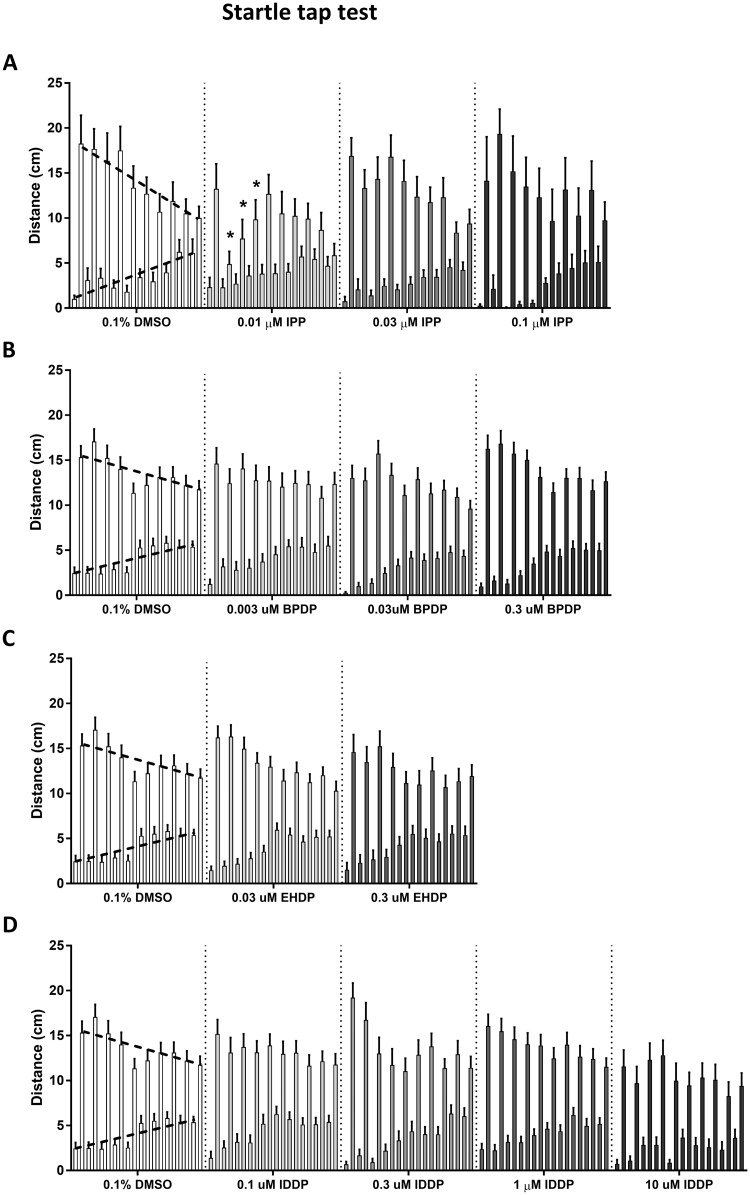
Adult Startle Tap Test
Pretap activity, referred to as the baseline activity level, is the distance traveled by the fish during the 5 s before each tap (Figure 3, bottom dashed line on DMSO activity); and posttap activity, referred to as the tap response, is the distance traveled in the 5 s after each tap (Figure 3, top dashed line). Baseline activity shows a significant main effect of tap, whereby it follows an upward slope across the session (F(9, 4725) = 48.39, p < .0001). Tap response is overall higher than the baseline activity throughout the trial, and shows a gradual decrease across the 10 taps (F(9, 4725) = 13.91, p < .0001).
Figure 3.
Startle tap test. Adult zebrafish were individually placed in cylindrical arenas, allowed a short 30-s acclimation period and subjected to a sequence of 10 taps, 1 tap per min. Average activity of IPP (A), BPDP (B), EHDP (C), and IDDP (D) exposed fish during 5 s before and after each tap. The lower, gradually rising bars (upward dashed line on 0.1% DMSO) show the pretap activity, and the higher, gradually decreasing bars (downward dashed line on 0.1% DMSO) show the posttap activity. Asterisks indicate significant difference from the 0.1% DMSO control group.
Comparison of the baseline activity in each treatment to the controls using ANOVA did not find any significant overall effect of the treatment. Tap response as well, was not found to be overall significantly affected by any of the treatments. However, a significant reduction in tap response was found in the lowest IPP exposure dose of 0.01 µM in taps 2–4 (Figure 3A). Linear regression analysis revealed that the tap response slope for this IPP dose was also significantly lower than control (Supplementary Table 5). Linear regression was not found to be altered in any of the other treatments (data not shown).
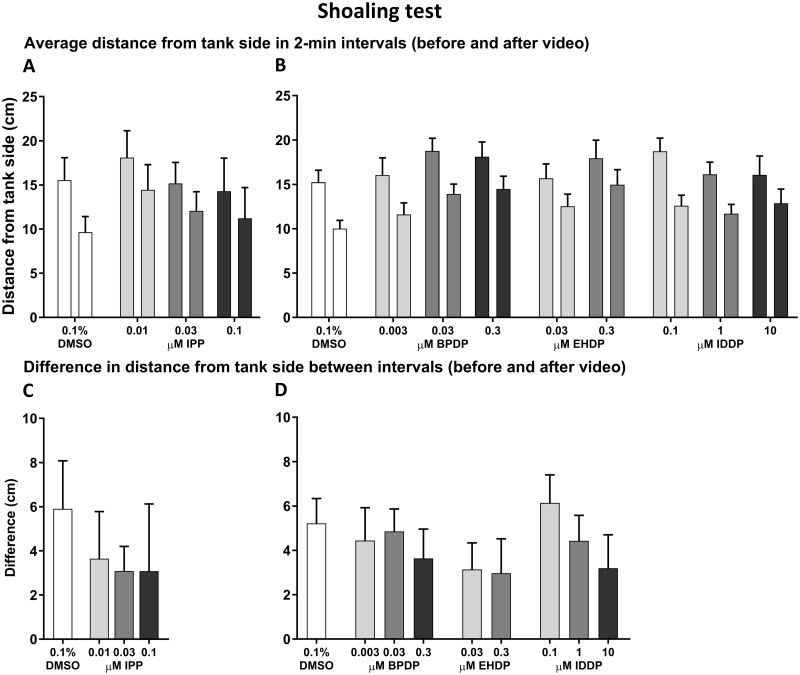
Adult Shoaling Test
Social affiliation was evaluated by measuring the average distance of the fish from the side of the tank on which the shoaling video played in the 2 min right before and right after it started playing. These data were analyzed as distance from the side during the 2-min intervals (Figure 4A and 4B), and as the difference between the 2 time intervals (Figure 4C and 4D). There was an effect of time interval on the distance from tank side across all treatments (F(1, 525) = 95.08, p < .0001), with a reduction in distance after initiation of the shoaling video. However, there was no effect of treatment nor an interval x treatment interaction. A difference in distance from tank side was calculated for each treatment by subtracting the distance after video initiation from the distance before the video started playing, however there was no significant effect on difference in any of the treatments. Total activity of the fish (Supplementary Figure 1A–D) and distance from the video-screening side of the tank (shoal response; Supplementary Figure 1E–H) were compared between the control and treated groups over time (min). In Set 1 (IPP), there was an effect of time on both total activity (F(6, 390) = 32.02, p < .0001) and shoal response (F(6, 348) = 6.626, p < .0001), whereas treatment was not found to have an effect on either parameter nor was there a time x treatment interaction. Similarly, in Set 2, ANOVA analysis detected an effect of time on total activity (F(6, 2760) = 209.7, p < .0001) and on shoal response (F(6, 2760) = 53.68, p < .0001), but no effect of treatment or interaction between the 2 parameters. However, in the BPDP-treated groups, posthoc comparisons revealed significantly higher distances from tank side in the 0.03 µM treated fish in the 3rd minute of the trial, and in the 0.3 µM treated group in the 4th minute, compared with the control. In the EHDP-treated groups, there was lower total activity in the 0.3 µM treated fish in minute 2, and higher distance from tank side in the same group in minutes 4–5. In the 0.1 µM IDDP exposed group, there was a significantly higher distance from side in minute 2.
Figure 4.
Shoaling test. Adult zebrafish that were developmentally exposed to IPP (A, E), BPDP (B, F), EHDP (C, G), and IDDP (D, H) were individually placed in the testing tank (MUPET) and recorded for 7 min. After the first 2 min, a video of a zebrafish shoal was played on one of the 2 flanking monitors for the remaining 5 min of the trial. Average distance from the tank side on which the video was played was calculated in the 2-min before (left bar) and the 2-min after (right bar) the video began playing for Set 1 (A; IPP) and Set 2 (B; BPDP, EHDP, IDDP). Asterisk indicates significant difference between the 2 time intervals. Differences were calculated between the 2 intervals described earlier in the Set 1 (C) and Set 2 (D) treatments.
Adult Predator Avoidance Test
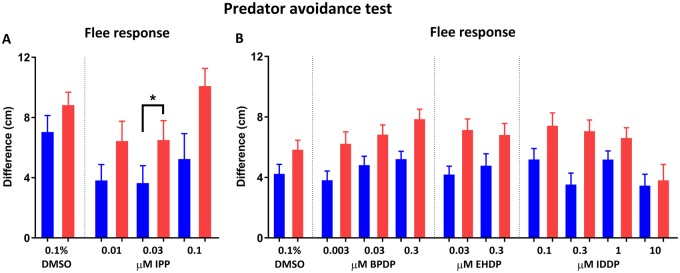
Flee response was overall greater during the red predator-simulating stimulus compared with the blue stimulus, as identified in the ANOVA analysis for Set 1 (F(1, 128) = 10.71, p < .01; Figure 5A) and Set 2 (F(1, 918) = 42.24, p < .0001; Figure 5B). In Set 1, treatment with 0.03 µM IPP caused a significant reduction in flee response across both blue and red stimuli compared with the control, however in Set 2 no differences were found in any of the treatments. We also compared total activity of the fish (Supplementary Figure 2A–D) and distance from the video-screening side of the tank (predator response; Supplementary Figure 2E–H) between the control and treated groups over time (min). Total activity increased over time in all treatment groups as evidenced by ANOVA analysis of Set 1 (F(8, 512) = 29.58, p < .0001) and Set 2 (F(8, 3680) = 150.5, p < .0001) groups. In both Set 1 and Set 2, there was no overall effect of treatment on total activity, nor a time x treatment interaction. Predator response was also affected by time in both Set 1 (F(8, 512) = 28.33, p < .0001) and Set 2 (F(8, 3680) = 157.8, p < .0001). In addition, in Set 2 there was an overall effect of treatment (F(9, 460) = 2.201, p < .05) that can be traced to IDDP exposures, where 0.1 or 10 µM exposed fish were at a higher distance from the tank side in minute 1, and 0.3 µM exposed fish were at a higher distance from side in minute 3.
Figure 5.
Predator avoidance test. Developmentally exposed adult zebrafish were individually placed in the testing tank (MUPET) and recorded for 9 min consisting of 1-min acclimation followed by alternating minute-long stimulus/no stimulus events. The stimulus was either a blue slow-growing dot or a red fast-growing dot appearing repeatedly on one of the screens. Flee response was calculated for Set 1 (A) and Set 2 (B) treatments as the difference in average distance from the tank side between trial minutes in which the dot stimulus was presented and minutes in which there was no stimulus. Dark bars represent blue dot flee response and light bars represent red dot flee response. Asterisk indicates significant difference between blue and red stimulus response in IPP-exposed fish.
DISCUSSION
To our knowledge, this is the first study conducted in zebrafish in which the life-long behavioral effects of developmental exposure to nonhalogenated phenyl phosphate OPFRs were tested. We found that the newer class of OPFRs caused both short and longer term behavioral alterations (Table 1). The observed effects were chemical-specific in the type of behavioral alteration they caused and life-stage specific in the timing in which they appeared. Of the 4 OPFRs tested, IPP was the most active in causing behavioral alterations in both larvae and adult fish, including hyperlocomotion in larvae and reduced response to anxiety-promoting situations in adults. Surprisingly, the adult behavioral effects of IPP occurred in the lowest exposure concentration but not in the 2 higher doses. The lack of effect in high exposure doses could indicate survival bias caused by long-term toxicity that lead to reduction in survival of the more highly affected individuals, thus only the least affected fish were tested at adulthood. Detailed analysis of toxicological endpoints and survival of each individual during and after exposure could help determine whether a survival bias is occurring at doses >0.01 µM. BPDP appeared to be more potent than EHDP in producing behavioral effects in adults, because they were observed at an order of magnitude lower concentration. Interestingly, the opposite was observed in the larval activity testing, as 0.3 µM BPDP exposure produced a limited effect on activity in the first light phase, whereas 0.3 µM EHDP exposure caused reduction in activity in both light phases. Both larval and adult behaviors were not found to be altered following exposure to IDDP in any of our assays. Behl et al. (2015) conducted a thorough study in which several OPFRs, including IPP, BPDP, EHDP, IDDP, and TPHP were evaluated for their overall toxicity as well as their neurotoxicity and developmental neurotoxicity. The authors used a range of in vitro and in vivo assays including mouse embryonic stem cells, human neural stem cells, rat primary mixed cortical culture, Caenorhabditis elegans and zebrafish embryos. In these assays, IPP and BPDP came out as highly active chemicals in their general toxicity and as neurotoxicants, followed closely by EHDP, whereas IDDP triggered the least toxicity of the 4. These results are strengthened by our behavioral data indicating similar potencies for developmental neurotoxicity of these 4 chemicals using the zebrafish model. IPP (also referred to as isopropylated triarylphosphate esters [ITPs]) and BPDP are found to be common components of currently used FR products and are widely detected in house dust samples (Phillips et al., 2017), however information on their long-term neurotoxicity and developmental neurotoxicity as well as on their potential additive effects is critically lacking from both epidemiological and animal model-based research (Hendriks and Westerink, 2015). In light of our current results on the strong larval and adult behavioral effects of developmental exposure to IPP, BPDP, and EHDP, we call for additional investigation into the potential neurobehavioral harm that these chemicals may be causing.
Table 1.
Summary of Behavioral Findings
| Life Stage | Test | Treatment |
IPP |
BPDP |
EHDP |
IDDP |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0.01 µM | 0.03 µM | 0.1 µM | 0.003 µM | 0.03 µM | 0.3 µM | 0.03 µM | 0.3 µM | 0.1 µM | 0.3 µM | 1 µM | 10 µM | ||
| 6 dpf larvae | Light/dark locomotor activity | Distance traveled per light phase | – | – | ↑ Dark | – | – | ↓ Light | – | ↓ Light | – | – | – | ↓ Dark |
| Adults | Novel tank | Total activity | – | – | – | – | ↓ | ↓ | – | ↓ | – | – | – | – |
| Dive response | ↑ | – | – | – | ↓ | ↓ | – | ↓ | – | – | – | – | ||
| Tap test | Pretap activity | – | – | – | – | – | – | – | – | – | – | – | – | |
| Posttap activity | ↓ | – | – | – | – | – | – | – | – | – | – | – | ||
| Shoaling | Total activity | – | – | – | – | – | – | – | – | – | – | – | – | |
| Shoal response | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Predator avoidance | Total activity | – | – | – | – | – | – | – | – | – | – | – | – | |
| Predator response | – | – | – | – | – | – | – | – | – | – | – | – | ||
In contrast to IPP, the main effect observed for both BPDP and EHDP was increased anxiety-related response to the novel tank test. In both treatments, the pattern of gradual increase in distance from the tank bottom was similar to that of the control group, however this distance was smaller throughout the trial indicating that the fish were able to recover and habituate to some extent. A large-scale study by Rihel et al. (2010) used the zebrafish larval locomotor activity assay to classify a large number of psychoactive small molecules into functional categories based on the behavioral phenotypes, and used these classifications to predict mechanisms of action for poorly characterized chemicals. However, the results from our screen so far suggest that such functional classifications may need to be revised when incorporating adult behavior data. For example, IPP and BPDP are both nonhalogenated triphenyl phosphates yet they caused very different larval and adult behavioral effects, indicating somewhat different pathways of toxicity. In contrast, in our previous work, we found that the brominated diphenyl ether BDE-47 caused reduction in larval locomotor activity (Glazer et al., 2018), which would place it in a different classification than IPP, yet in adults both chemicals caused reduction in anxiety-related response to the novel tank and tap tests. Another example can be found in BPDP and EHDP which are different in their chemical structure because EHDP is a diphenyl phosphate with a long alkyl chain, but the behavioral alterations caused by the 2 chemicals are similar.
Our larval results can be compared with 2 other recent studies that conducted similar protocols of embryonic exposure to various FRs, including the 4 used in the current study, followed by light/dark larval activity testing (Jarema et al., 2015; Noyes et al., 2015). Jarema et al. (2015) found that IPP exposure caused hyperlocomotion during dark phases that was similar to the effect observed in the current study. In contrast, Noyes et al. (2015) found effects that were quite different from our observations. The authors conducted exposures to 3 different IPP formulations from 3 different manufacturers, finding clear hyperlocomotion in the light but varying locomotion alterations in the dark that were concentration and formulation-dependent. As with IPP, our findings on BPDP, EHDP, and IDDP are mostly aligned with the data presented by Jarema et al. (2015) but differ from the effects observed by Noyes et al. (2015). Some of the differences in observed effects may be due to differences in the sources and purities of the chemicals. For example, IPP was purchased from 3 different suppliers for the study by Noyes et al. (2015) all with unknown purity, whereas Jarema et al. (2015) purchased the chemical from a 4th supplier at a purity of 72.9%, and in our current work it was provided by a 5th company at a purity of 45.2%.
Both IPP and BPDP were supplied to us as mixture solutions containing a large fraction of TPHP (up to approximately 40%) and minor isomeric fractions (see Materials and Methods section on Chemicals). A similar large fraction of TPHP was reported by Phillips et al. (2017) in an ITP mixture that was obtained from a different supplier than the one used in the current study. Since it appears that there is no single formulation for IPP or BPDP, the effects of single components including TPHP and others need to be further investigated. In our larger project with the NTP, we are also investigating the effects of exposure to TPHP alone, however these experiments are yet to be completed. In a similar study, Oliveri et al. (2015) exposed zebrafish embryos to either 0.03 or 0.3 µM TPHP in 0.03% DMSO throughout embryodevelopment (5–120 hpf) and tested for behavioral effects at 6 dpf using the light/dark locomotor activity assay and in the adults using the 4-test behavioral battery. No effects were found on larval locomotor activity in either exposure concentration. In adults, there was attenuation of exploration in the novel tank test, but no other observed effect. In the study by Behl et al. (2015), TPHP displayed marked developmental toxicity at concentrations lower than 1 µM, however only mild developmental neurotoxicity affecting human stem cell neurite outgrowth at 15.9 µM. Despite the large fraction of TPHP in both IPP and BPDP solutions, in the current study, they caused distinctly different behavioral effects in both larvae and adult zebrafish, indicating differences in their mechanisms of neurotoxicity.
To our knowledge, there is no experimental data available yet on the specific pathways activated by the nonhalogenated OPFRs tested in the current study. Recent studies using zebrafish embryos and larvae to look at pathways potentially involved in TPHP toxicity have indicated disruption of thyroid regulation at the hormone expression and receptor levels (Kim et al., 2015; Liu et al., 2013). Furthermore, in a study examining the relationship between urine levels of diphenyl phosphate (DPHP), a TPHP metabolite, and thyroid hormones in humans it was found that high levels of DPHP were associated with an increase in mean thyroid hormone levels (Preston et al., 2017). However, the link between thyroid disruption and the toxicological effects observed, including the behavioral alterations is still unknown.
The long-term behavioral alterations caused by developmental exposure to IPP and BPDP occurred at doses that were not found to significantly alter larval locomotor activity at 6 dpf, suggesting a delayed effect for these chemicals. Similarly, we previously observed that developmental exposure of zebrafish to concentrations <0.1 µM of the brominated FRs BDE-47 and BDE-99 did not cause altered larval locomotor activity, but resulted in reduced anxiety-related response in both the novel tank test and the tap test (Glazer et al., 2018). In another recent study, zebrafish embryonic exposure to the dioxin-like PCB 126 did not change larval activity in response to illumination changes, however it caused a lack of long- and short-term habituation to a novel environment in adults (Glazer et al., 2016). Larval behavior testing is a popular endpoint when using the zebrafish model for developmental neurotoxicity studies, because it provides a large amount of information in a relatively short time frame of days from exposure to testing. Furthermore, the light/dark locomotor activity assay is the most common larval behavioral test, because in contrast to most other larval assays it can be conducted in 96-well plates, thus allowing for high-throughput screening of a large number of chemicals. However, the neurobehavioral interpretations that can be done from this assay are limited because it relies on the swimming capacity and visual health of the larvae. It is possible that some neurotoxic effects resulting from low-level exposure can be detected at the larval stage using other behavior assays. For example, the acoustic startle response assay (Burgess and Granato, 2007) is used to measure sensorimotor gating and habituation to an anxiety-promoting stimulus in larvae as early as 5 dpf. In contrast to the larval testing, adult behavior testing is much less common because it is conducted several months after exposure and is done in much lower throughput, however it may be that using larval behavioral testing alone is leading to underestimation of the neurotoxicity of some chemicals, and there is justification for later-life behavioral testing when low-level exposure has no observed effects at early life stages.
In conclusion, the earlier class of FRs, the brominated diphenyl ethers, has been phased out because they produced developmental neurotoxicity. Here, we show that the newer class of FRs based on the organophosphate chemical structure also have neurotoxic effects after developmental exposure. These effects may be similar or different in character from those caused by the brominated FRs, but like brominated FR exposures to low doses during early development they can result in behavioral impacts that last into adulthood.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by the National Toxicology Program is an NIH division grant HHSN273201500214P and the Duke University Superfund Research Program (ES010356). L.G. was supported by the Leon Golberg Postdoctral Fellowship.
Supplementary Material
REFERENCES
- Adgent M. A., Hoffman K., Goldman B. D., Sjodin A., Daniels J. L. (2014). Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr. Perinat. Epidemiol. 28, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J., Levin E. D. (2015). The neurobehavioral toxicity of FireMaster 550 (R) in zebrafish (Danio rerio): chronic developmental and acute adolescent exposures. Neurotoxicol. Teratol. 49, 118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J., Oliveri A., Levin E. D. (2013). Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. C Embryo Today 99, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M., Hsieh J. H., Shafer T. J., Mundy W. R., Rice J. R., Boyd W. A., Freedman J. H., Hunter E. S., Jarema K. A., Padilla S., et al. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52, 181–193. [DOI] [PubMed] [Google Scholar]
- Burgess H. A., Granato M. (2007). Sensorimotor gating in larval zebrafish. J. Neurosci. 2718, 4984–4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt C. M., Congleton J., Hoffman K., Fang M., Stapleton H. M. (2014). Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 48, 10432–10438. [DOI] [PubMed] [Google Scholar]
- Cheng R., Jia Y. L., Dai L. L., Liu C. S., Wang J. H., Li G. Y., Yu L. Q. (2017). Tris(1, 3-dichloro-2-propyl) phosphate disrupts axonal growth, cholinergic system and motor behavior in early life zebrafish. Aquat. Toxicol. 192, 7–15. [DOI] [PubMed] [Google Scholar]
- Costa L. G., Giordano G. (2007). Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28, 1047–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell W. J., Lederman S. A., Sjodin A., Jones R., Wang S., Perera F. P., Wang R., Rauh V. A., Herbstman J. B. (2015). Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3-7 years. Neurotoxicol. Teratol. 52, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby E. B., Bailey J. M., Oliveri A. N., Levin E. D. (2015). Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol. Teratol. 49, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G. D., Yu J., Cui C., Chen L. M., Gao Y., Wang C. F., Zhou Y. J., Tian Y. (2015). Association between prenatal exposure to polybrominated diphenyl ethers and young children’s neurodevelopment in China. Environ. Res. 142, 104–111. [DOI] [PubMed] [Google Scholar]
- Eddins D., Cerutti D., Williams P., Linney E., Levin E. D. (2010). Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 32, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P., Fredriksson A. (1998). Neurotoxic effects in adult mice neonatally exposed to 3, 3’4, 4’5-pentachlorobiphenyl or 2, 3, 3’4, 4’-pentachlorobiphenyl. Changes in brain nicotinic receptors and behaviour. Environ. Toxicol. Pharmacol. 5, 17–27. [DOI] [PubMed] [Google Scholar]
- Eriksson P., Jakobsson E., Fredriksson A. (2001). Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 109, 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P., Viberg H., Jakobsson E., Orn U., Fredriksson A. (2002). A brominated flame retardant, 2, 2’, 4, 4’, 5-pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol. Sci. 67, 98–103. [DOI] [PubMed] [Google Scholar]
- Fromme H., Lahrz T., Kraft M., Fembacher L., Mach C., Dietrich S., Burkardt R., Volkel W., Goen T. (2014). Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ. Int. 71, 158–163. [DOI] [PubMed] [Google Scholar]
- Glazer L., Hahn M. E., Aluru N. (2016). Delayed effects of developmental exposure to low levels of the aryl hydrocarbon receptor agonist 3, 3’, 4, 4’, 5-pentachlorobiphenyl (PCB126) on adult zebrafish behavior. NeuroToxicology 52, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer L., Wells C. N., Drastal M., Odamah K. A., Galat R. E., Behl M., Levin E. D. (2018). Developmental exposure to low concentrations of two brominated flame retardants, BDE-47 and BDE-99, causes life-long behavioral alterations in zebrafish. Neurotoxicology 66, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alzaga B., Lacasana M., Aguilar-Garduno C., Rodriguez-Barranco M., Ballester F., Rebagliato M., Hernandez A. F. (2014). A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol. Lett. 230, 104–121. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Landrigan P. J. (2006). Developmental neurotoxicity of industrial chemicals. Lancet 368, 2167–2178. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Landrigan P. J. (2014). Neurobehavioural effects of developmental toxicity. Lancet Neurol. 13, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. (2009). Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin. Drug Dis. 4, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks H. S., Westerink R. H. S. (2015). Neurotoxicity and risk assessment of brominated and alternative flame retardants. Neurotoxicol. Teratol. 52, 248–269. [DOI] [PubMed] [Google Scholar]
- Herbstman J. B., Mall J. K. (2014). Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr. Environ. Health Rep. 1, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarema K. A., Hunter D. L., Shaffer R. M., Behl M., Padilla S. (2015). Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 52, 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Guanalan B., Jyothi S. (2012). Modelling toxicity induced neurological disorderes in zebrafish. Int. J. Drug Dev. Res. 4, 291–294. [Google Scholar]
- Kim J. W., Isobe T., Muto M., Tue N. M., Katsura K., Malarvannan G., Sudaryanto A., Chang K. H., Prudente M., Viet P. H., et al. (2014). Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 116, 91–97. [DOI] [PubMed] [Google Scholar]
- Kim S., Jung J., Lee I., Jung D., Youn H., Choi K. (2015). Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat. Toxicol. 160, 188–196. [DOI] [PubMed] [Google Scholar]
- Kuriyama S. N., Talsness C. E., Grote K., Chahoud I. (2005). Developmental exposure to low-dose PBDE-99: effects on male fertility and neurobehavior in rat offspring. Environ. Health Perspect. 113, 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. D., Bencan Z., Cerutti D. T. (2007). Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 90, 54–58. [DOI] [PubMed] [Google Scholar]
- Levin E. D., Kalueff A. V., Gerlai R. T. (2015). Perspectives on zebrafish neurobehavioral pharmacology. Pharmacol. Biochem. Behav. 139, 93.. [DOI] [PubMed] [Google Scholar]
- Linares V., Belles M., Domingo J. L. (2015). Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 89, 335–356. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang Q., Liang K., Liu J., Zhou B., Zhang X., Liu H., Giesy J. P., Yu H. (2013). Effects of tris(1, 3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat. Toxicol. 128–129, 147–157. [DOI] [PubMed] [Google Scholar]
- McCollum C. W., Ducharme N. A., Bondesson M., Gustafsson J. A. (2011). Developmental toxicity screening in zebrafish. Birth Defects Res. C Embryo Today 93, 67–114. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Murakami S., Ashikawa Y., Sasagawa S., Umemoto N., Shimada Y., Tanaka T. (2015). Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. 55, 1–16. [DOI] [PubMed] [Google Scholar]
- Noyes P. D., Haggard D. E., Gonnerman G. D., Tanguay R. L. (2015). Advanced morphological – behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 145, 177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri A. N., Bailey J. M., Levin E. D. (2015). Developmental exposure to organophosphate flame retardants causes behavioral effects in larval and adult zebrafish. Neurotoxicol. Teratol. 52, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A., Hammel S., Konstantinov A., Stapleton H. M. (2017). Characterization of individual isopropylated and tert-butylated triarylphosphate (ITP & TBPP) isomers in several commercial flame retardant mixtures and house dust standard reference material SRM 2585. Environ. Sci. Technol. 5122, 13443–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston E. V., McClean M. D., Henn B. C., Stapleton H. M., Braverman L. E., Pearce E. N., Makey C. M., Webster T. F. (2017). Associations between urinary diphenyl phosphate and thyroid function. Environ. Int. 101, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihel J., Prober D. A., Arvanites A., Lam K., Zimmerman S., Jang S., Haggarty S. J., Kokel D., Rubin L. L., Peterson R. T., et al. (2010). Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 327, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv S. K., Kogut K., Gaspar F. W., Gunier R. B., Harley K. G., Parra K., Villasenor D., Bradman A., Holland N., Eskenazi B. (2015). Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9-12 years of age. Neurotoxicol. Teratol. 52, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Klosterhaus S., Eagle S., Fuh J., Meeker J. D., Blum A., Webster T. F. (2009). Detection of organophosphate flame retardants in furniture foam and US house dust. Environ. Sci. Technol. 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko C. Y., Robinson E. M., Usenko S., Brooks B. W., Bruce E. D. (2011). PBDE developmental effects on embryonic zebrafish. Environ. Toxicol. Chem. 30, 1865–1872. [DOI] [PubMed] [Google Scholar]
- Viberg H., Fredriksson A., Eriksson P. (2003). Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 192, 95–106. [DOI] [PubMed] [Google Scholar]
- Viberg H., Fredriksson A., Eriksson P. (2004). Neonatal exposure to the brominated flame-retardant, 2, 2’, 4, 4’, 5-pentabromodiphenyl ether, decreases cholinergic nicotinic receptors in hippocampus and affects spontaneous behaviour in the adult mouse. Environ. Toxicol. Pharmacol. 17, 61–65. [DOI] [PubMed] [Google Scholar]
- Zhao J., Xu T., Yin D. Q. (2014). Locomotor activity changes on zebrafish larvae with different 2, 2’, 4, 4’-tetrabromodiphenyl ether (PBDE-47) embryonic exposure modes. Chemosphere 94, 53–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.