Abstract
Background
Resistance to cisplatin results in recurrence or relapse of cervical cancer in women. An understanding of the mechanisms of cisplatin resistance will be important to improve the efficacy of cisplatin treatment. The aim of this study was to investigate the role of microRNA-7-5p (mir-7-5p) in cisplatin-resistant cervical cancer cells in vitro.
Material/Methods
The expression levels of miR-7-5p were detected in cisplatin-resistant cervical cancer cells, HeLa, and SiHa cells (HPV16-positive), and in clinical tissue samples, using miR-7-5p inhibition and a luciferase reporter assay. Fifteen paired cervical cancer tissue samples and adjacent normal cervical tissues were obtained from 15 patients who underwent surgery for cervical cancer. Western blot and flow cytometry were used to investigate cell apoptosis. The expression of mir-7-5p was detected by quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR).
Results
The level of miR-7-5p was increased in cisplatin-resistant HeLa and SiHa cervical cancer cells. Increased expression of miR-7-5p inhibited DNA repair by modulating the expression of poly (ADP-ribose) polymerase 1 (PARP-1), reducing energy consumption, and promoting autophagy via suppression of the expression of Bcl-2. These findings supported that increasing energy generation and reducing energy consumption, resulted in miR-7-5p maintaining energy homeostasis during cisplatin treatment.
Conclusions
The findings of this study showed that there was a protective role of miR-7-5p in cervical cancer cells treated with cisplatin and that miR-7-5p expression maintained energy homeostasis in cisplatin-resistant cervical cancer cells. However, miR-7-5p reduced energy consumption via inhibiting PARP-1 expression, and miR-7-5p increased energy generation by suppressing the expression of Bcl-2.
MeSH Keywords: Autophagy, Cisplatin, MicroRNAs, Poly(ADP-ribose) Polymerases
Background
Worldwide, cervical cancer is one of the most prevalent gynecological cancers and is one of the leading causes of cancer-related death among women [1]. The International Federation of Gynecology and Obstetrics (FIGO) classifies early-stage cervical cancer as Stage IA-IB1 and recommends that the standard management includes radical hysterectomy and lymph node dissection and/or radiation, with or without chemotherapy. For women with locally-advanced cervical cancer, external beam radiotherapy with concurrent cisplatin-based chemotherapy is recommended [2,3]. Cisplatin is the first-line chemotherapy agent for advanced cervical cancer, but intrinsic or acquired resistance to cisplatin in cervical cancer may develop and lead to treatment failure [4,5]. Therefore, further understanding is required on the mechanisms of cisplatin resistance in cervical cancer, with the aim of overcoming treatment resistance.
MicroRNAs (miRNAs) are a class of endogenous conserved small RNAs, which are involved in many physiologic and pathologic processes via regulating target gene expression. MicroRNA-7-5p (mir-7-5p) is a microRNA that is highly conserved among vertebrates, and has been demonstrated to be involved in tumorigenesis, including cell proliferation and apoptosis, in animal models and humans, including breast cancer [6], in angiogenesis in glioblastoma [7], and in tumor invasion in melanoma [8]. Previous studies have also shown that miR-7-5p may also be used as a biomarker for glioblastoma [7]. Although miR-7-5p has previously been shown to be involved in the growth and apoptosis of cervical cells [9], its role in the response of cervical cancer to cisplatin remains poorly understood.
Therefore, the aim of this study was to investigate the role of mir-7-5p in cisplatin-resistant cervical cancer cells in vitro, including its role energy homeostasis, autophagy, DNA repair, and expression of Bcl-2 and poly (ADP-ribose) polymerase 1 (PARP-1), using cisplatin-resistant HeLa and SiHa cervical cancer cells.
Material and Methods
Cisplatin-resistant cervical cancer cells
The cell lines used in the study included cisplatin-resistant cervical cancer cells, HeLa and SiHa, which were purchased from American Type Culture Collection (ATCC). The cell lines were maintained in liquid nitrogen until needed. The cells were thawed and passaged in RPMI 1640 containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cells were maintained at 37°C and 5% CO2. Transfections were conducted with Lipofectamine™ 2000 reagent (Invitrogen, Carlsbad, CA, USA) following treatments. The cisplatin-resistant cervical cancer cells, HeLa and SiHa, were screened by passage with increasing concentrations of cisplatin for ten generations in culture. Where indicated, endogenous miR-7-5p was blocked with antisense oligonucleotides (ASO)-miR-7-5p, and ASO-NC was used as a control. Endogenous PARP1 and BCL2 were knocked down with small interfering (si)-PARP1 or si-BCL2, respectively, and si-NC was used as a control.
Clinical samples of cervical cancer tissue and normal cervical tissue
Fifteen paired cervical cancer tissue samples, and adjacent normal cervical tissues were obtained from 15 patients who underwent surgery for histologically confirmed cervical cancer. All patients provided informed consent. The cervical cancer tissues and non-cancerous cervical tissues were harvested with histological confirmation of the presence or absence of tumor. Total RNAs were extracted with Trizol reagent and stored at −80°C. All the procedures were performed in accordance with local guidelines and with local ethical approval.
Western blot
Cells were lysed prior to Western blot using lysis buffer containing 0.1% sodium dodecyl sulfate (SDS), 1% NP-40, 1 mM MgCl2 and 10 mM Tris at pH 8.0. A proteinase inhibitor was added directly to the cells with each treatment. The cell lysis supernatant was separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to methanol-treated polyvinylidene difluoride (PVDF) membranes. After blocking with 5% non-fat dried milk powder in TBST, the membranes were treated with primary mouse polyclonal IgG antibodies to PARP-1, Bcl-2, or rabbit polyclonal antibodies to GAPDH (all at a dilution of 1: 5000) (Saier Bio., Tianjin, China), according to the manufacturer’s instructions. Horseradish peroxidase (HRP)-conjugated secondary antibodies were used (Saier Bio., Tianjin, China). The bands were visualized using a chemiluminescence method.
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNAs from cultured cells or cervical cancer samples, following the manufacturer’s instructions. Moloney murine leukemia virus (M-MLV) reverse transcriptase (TAKARA, Dalian, China) was used to synthesize the first-strand cDNA, according to the manufacturer’s instructions.
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was performed with SYBR Green Supermix and the iCycler real-time PCR detection system (Bio-Rad), and the relative expression level of targeted RNA was calculated using the 2−(ΔCt) method, where ΔCt=Cttarget–CtU6/actin, and Δ(ΔCt)=ΔCt sample–ΔCt control.
The following primers were used:
miR-7-5p RT: TCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG ACAA;
miR-7-5p Fr: ACACTCCAGCTGGGTGGACTAGTGATTT;
miR-7-5p Rv: TGGTGTCGTGGAGTCG;
PARP1 Fr: GAGTCGGCGATCTTGGACC;
PARP-1 Rv: TGACCCGAGCATTCCTCG; BCL2 Fr: TCGCCCTGTGGATG ACTG;
BCL2 Rv: GCT TGGCAATTAGTGGTC.
Autophagy and mitophagy fluorescence
The cultured cells were co-treated with pcDNA3/GFP-LC3 and plasmids or oligonucleotides, as indicated in the Figures. Cisplatin was added to a final concentration of 50 μM. Eight hours later, the cells were treated with paraformaldehyde. The blue fluorescent marker 4′,6-diamidino-2-phenylindole (DAPI) was used for nuclear staining. The formation of autophagosomes was viewed with fluorescence microscopy.
Flow cytometry
The cultured cells were treated with respective plasmids or oligonucleotides and then treated with cisplatin at a final concentration of 50 μM for 8 hours. After harvesting and the cells were washed twice with PBS. The cells were resuspended in fluorescein isothiocyanate (FITC) -conjugated Annexin-V binding buffer, and 5 ul FITC-conjugated Annexin-V (Invitrogen, Carlsbad, CA, USA) was added to the cell suspension, avoiding light exposure, and maintained at room temperature for 15 mins before adding 5 ul of propidium iodide (PI), and after 10 mins, the cells were analyzed by flow cytometry.
ATP and ADP measurements
Measurements of ATP and ADP were performed according to the colorimetric method described in a previous study [10]. The absorbance spectrum of ATP and ADP was examined at 254 nm. The concentrations of these energy substances were calculated compared with the standard curve, as previously constructed [10]. To investigate the role of autophagy in energy regulation of cisplatin-resistant cells, 3-methyladenine (3-MA) or chloroquine (CQ) were used to inhibit autophagy, at a final concentration of 50 μM.
Results
MicroRNA-7-5p (mir-7-5p) was upregulated in cisplatin-resistant cervical cancer cells and associated with cell survival on cisplatin exposure
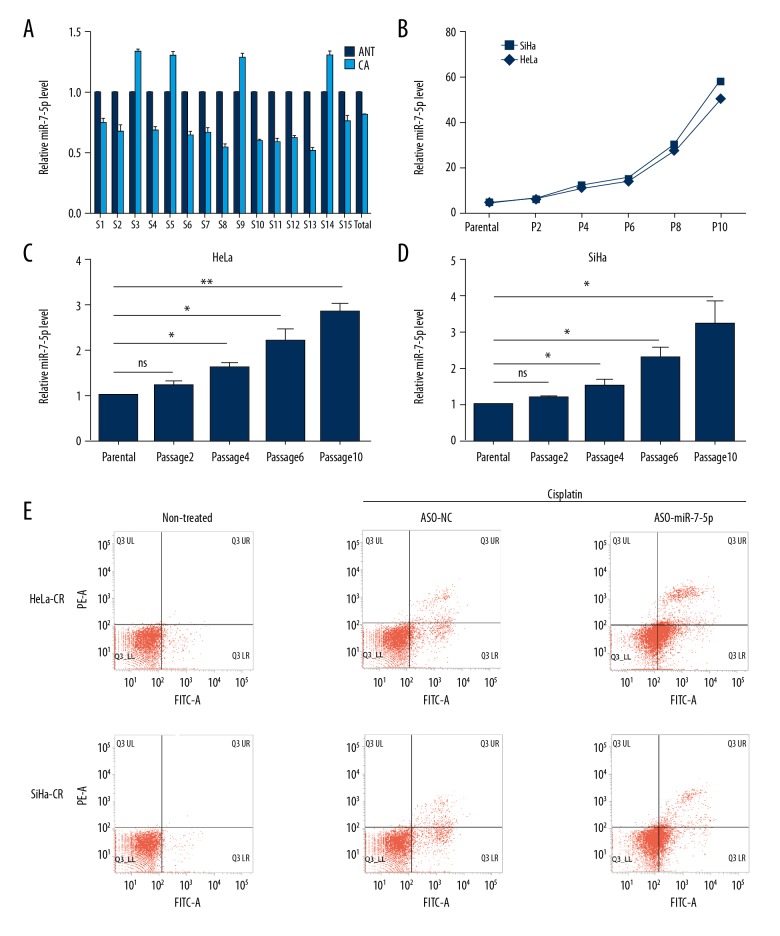
MicroRNA-7-5p (mir-7-5p) has been demonstrated to be involved in the pathogenesis of human cancer, but the role of miR-7-5p in the regulation of the response to treatment with cisplatin in cervical cancer remains unclear. The expression levels of miR-7-5p in clinical tissue samples showed that miR-7-5p was under-expressed when compared with adjacent non-cancerous tissues (Figure 1A). In four cases of cervical cancer, the expression of miR-7-5p was upregulated in cancer tissue (Figure 1A), and these four cases were found to be taken from cisplatin treatment unresponsive patients.
Figure 1.
MicroRNA-7-5p (mir-7-5p) is upregulated in cisplatin-resistant cervical cancer cells and is associated with increased cell survival. (A) The expression of microRNA-7-5p (mir-7-5p) in cervical cancer tissues and adjacent non-cancerous tissues was detected by quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR). (B) The IC50 of cisplatin of cervical cell treated with increasing concentration of cisplatin. (C, D) The expression of miR-7-5p in cisplatin-resistant cervical cancer cells was detected by qRT-PCR. (E) The apoptosis rate of the cisplatin-resistant cells with indicated treatment were measured by a flow cytometry assay. * p<0.05, ** p<0.01, ns – no significance.
To determine whether miR-7-5p was overexpressed in cisplatin-resistant cervical cancer tissues or cells, cisplatin-resistant HeLa or SiHa cells (HeLa-CR, SiHa-CR which were constructed by the authors) were studied. The IC50 of the HeLa-CR or SiHa-CR was significantly increased compared with controls (parental, or cisplatin-sensitive HeLa or SiHa cells) (Figure 1B). The expression of miR-7-5p was significantly increased in both HeLa-CR and SiHa-CR compared with respective control (parental) cells (Figure 1C, 1D). Blocking endogenous miR-7-5P in HeLa-CR or SiHa-CR cells increased cell apoptosis following cisplatin treatment (Figure 1E) indicating a protective role of miR-7-5p in cisplatin-resistant cervical cancer cells.
miR-7-5p inhibited DNA repair in cisplatin-resistant cells and maintained energy homeostasis
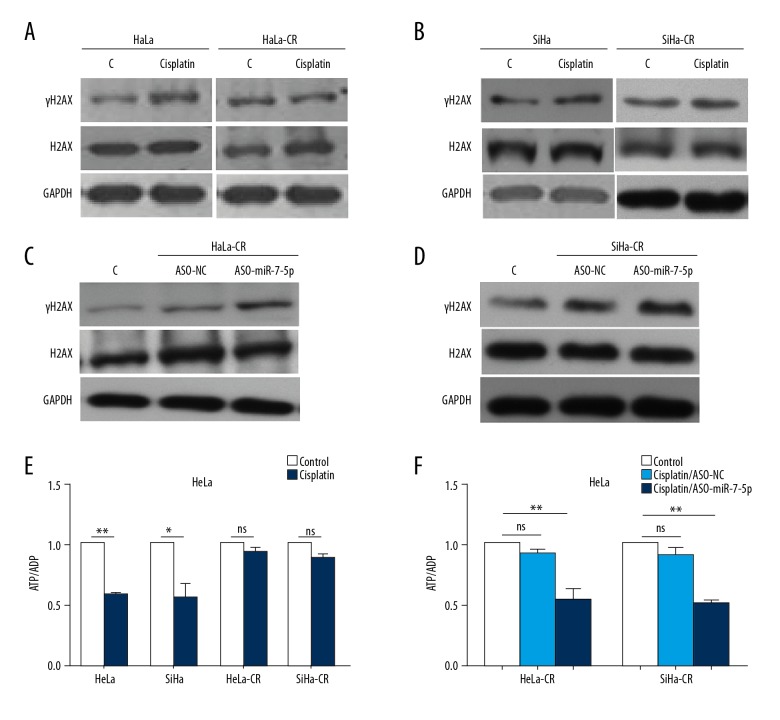
DNA repair activity was detected in both the control (parental) and cisplatin-resistant cells by measuring the protein level of γH2AX, a marker of DNA repair. Following cisplatin treatment, the protein level of γH2AX was significantly increased in control (parental) cells, but only slightly increased in cisplatin-resistant cells (Figure 2A, 2B). The level of H2AX was consistent in all the cases, indicating that the DNA repair activity of cisplatin-resistant cells was reduced. Blocking endogenous miR-7-5p with ASO-miR-7-5p significantly increased the protein level of γH2AX in both HeLa-CR and SiHa-CR cells (Figure 2C, 2D), which supported the involvement of miR-7-5p in the DNA repair process during cisplatin treatment.
Figure 2.
MicroRNA-7-5p (mir-7-5p) inhibits DNA repair in cisplatin-resistant cells and maintains energy homeostasis. (A, B) The protein level of γH2AX and H2AX in cisplatin-resistant cervical cancer cells or respective control (parental) cells, measured by Western blot. (C, D) The protein level of γH2AX and H2AX in cisplatin-resistant cervical cancer cells were blocked endogenous miR-7-5p with ASO- microRNA-7-5p (mir-7-5p), measured with Western blot following cisplatin treatment. (E) The ratio of ATP/ADP in cisplatin-resistant cervical cancer cells and control (parental) cells with indicated treatments were measured. (F) The ratio of ATP/ADP in cisplatin-resistant cervical cancer cells transfected with ASO-miR-7-5P following cisplatin treatment was measured. * p<0.05, ** p<0.01, ns – no significance.
The DNA repair process is an energy demanding process, and excessive DNA repair might result in cell death. The energy state of the control (parental) or cisplatin-resistant cells was evaluated by measuring the ATP/ADP ratio, which showed that following cisplatin treatment, the energy in cisplatin-resistant cells remained constant, while the ATP/ADP ratio was significantly reduced in control (parental) cells (Figure 2E). Blocking endogenous miR-7-5p with antisense oligonucleotides (ASO)-miR-7-5p reduced the ATP/ADP ratio in HeLa-CR and SiHa-CR cells (Figure 2F). These results supported that miR-7-5-p inhibited DNA repair activity in HeLa-CR and SiHa-CR cells, maintaining energy under treatment stress.
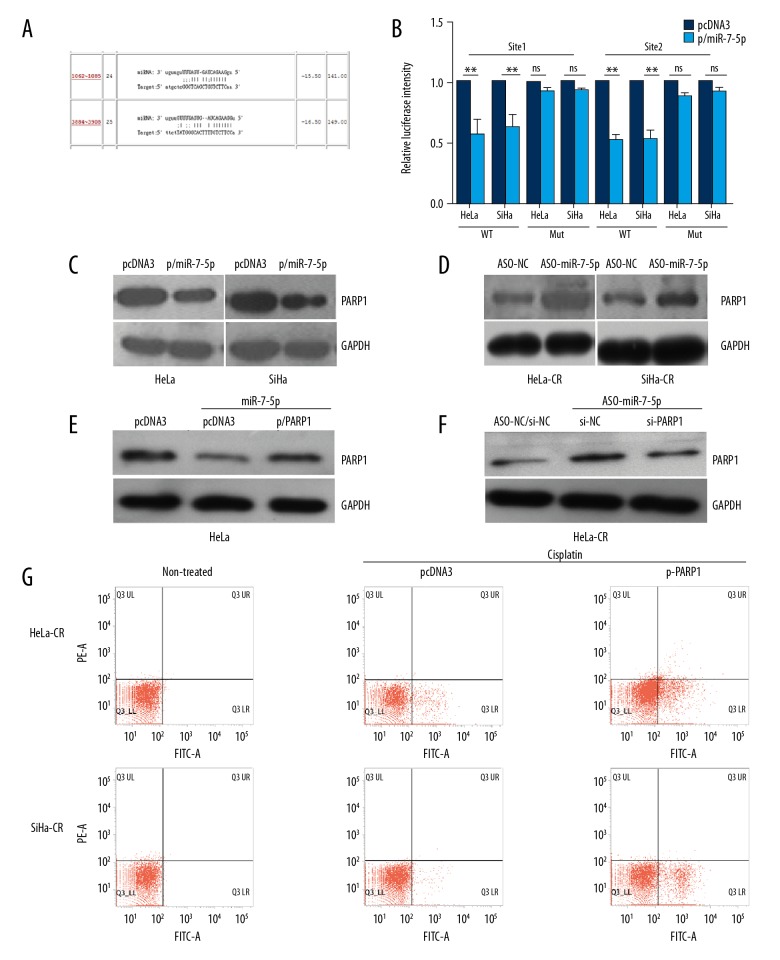
miR-7-5P down-regulated the expression of poly (ADP-ribose) polymerase 1 (PARP-1), which is involved in the regulation of apoptosis
Two target sites of miR-7-5p were found in the 3 UTR of PARP-1 mRNA (Figure 3A). The results of the luciferase reporter assay demonstrated that both sites were involved in the regulation of reporter gene expression by mIR-7-5p (Figure 3B). Overexpression of miR-7-5p in control (parental) cells led to a decrease in the levels of PARP-1 protein while blocking endogenous miR-7-5p with ASO-miR-7-5p led to an increase in the levels of PARP-1 protein (Figure 3C, 3D). The use of the PARP-1 expression plasmid or si-PARP-1 could reverse the impact of miR-7-5p or ASO-miR-7-5p in control (parental) or cisplatin-resistant cells, respectively, indicating the direct regulation of PARP-1 by miR-7-5p. Re-expression of PARP-1 in cisplatin-resistant cells increased the apoptosis rate of HeLa-CR or SiHa-CR cells (Figure 3G). These findings support that miR-7-5p down-regulated the expression of PARP-1, which is involved in the process of apoptosis under cisplatin treatment.
Figure 3.
MicroRNA-7-5p (mir-7-5p) negatively regulated the expression poly (ADP-ribose) polymerase 1 (PARP-1), which was involved in the regulation of apoptosis in cisplatin-resistant cervical cancer cells. (A) The target sites of microRNA-7-5p (mir-7-5p) in 3′UTR of poly (ADP-ribose) polymerase 1 (PARP-1) mRNA predicted by ReRNA2.0. (B) Luciferase reporter assay was conducted with the indicated reporter plasmid. (C, D) The protein levels of PARP-1 in cisplatin-resistant cervical cancer cells and control (parental) cells with indicated treatment were measured by Western blot. (E, F) The protein levels of PARP-1 in the control (parental) cells simultaneously transfected with miR-7-5p and pcDNA3/PARP-1 were measured by Western blot. (E) Apoptosis of the cisplatin-resistant cervical cancer cells, transfected with indicated plasmid, following cisplatin treatment was measured by flow cytometry. ** p<0.01, ns – no significance.
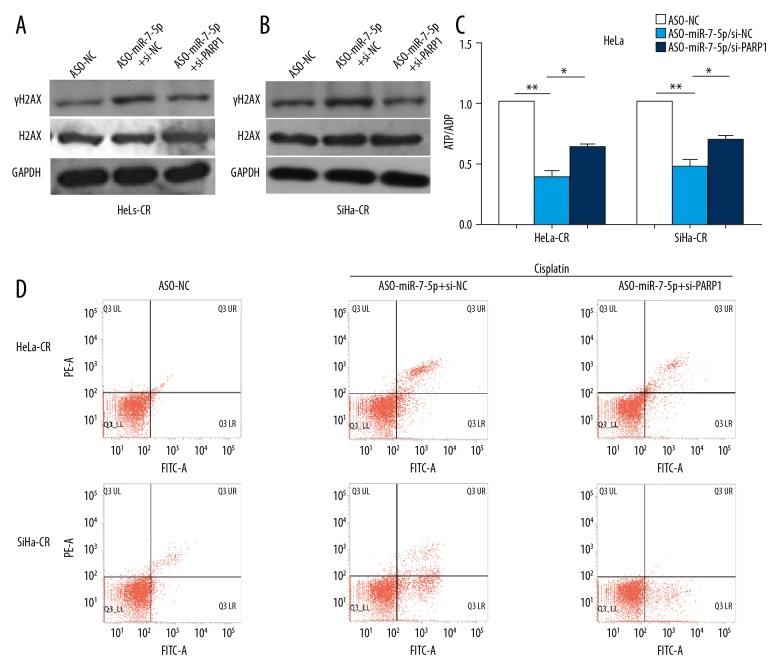
PARP-1 was the functional target of miR-7-5p in cisplatin-resistant cervical cancer cells
Knockdown of endogenous PARP-1 with si-PARP-1 reversed the increase in levels of γH2AX by ASO-miR-7-5p following cisplatin treatment in both HeLa-CR and SiHa-CR cells (Figure 4A, 4B). The reduced ATP/ADP ratio in cisplatin-resistant cells induced by ASO-miR-7-5P could also be partially reversed by si-PARP-1 (Figure 4C). Blocking endogenous miR-7-5p with ASO-miR-7-5p significantly increased apoptosis of HeLa-CR or SiHa-CR cells, and this could also be reduced by si-PARP-1 (Figure 4D). The above results demonstrated that PARP-1 was a functional target of miR-7-5p and was involved in the regulation of energy as well as apoptosis by miR-7-5p.
Figure 4.
Poly (ADP-ribose) polymerase 1 (PARP-1) is the functional target of microRNA-7-5p (mir-7-5p) in cisplatin-resistant cervical cancer cells. (A, B) The protein levels of γH2AX and H2AX in cisplatin-resistant cervical cancer cells with indicated treatment were measured by Western blot. (C) The ratio of ATP/ADP in cisplatin-resistant cervical cancer cells with indicated treatment was measured. (D) Apoptosis of cisplatin-resistant cervical cancer cells with indicated treatment was measured by flow cytometry. * p<0.05, ** p<0.01.
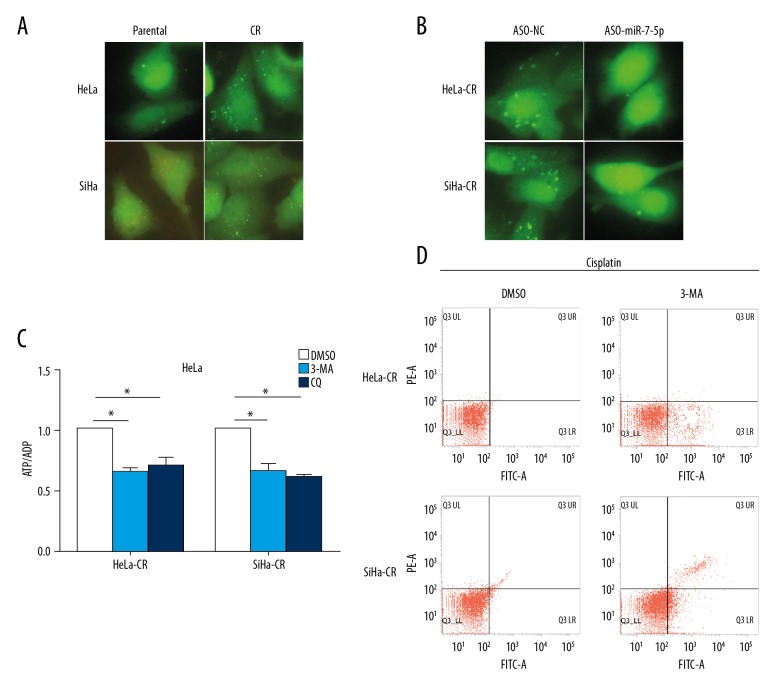
miR-7-5p promoted autophagy in chemotherapy-resistant cervical cancer cells
In cisplatin-resistant cervical cancer cells, the activity of autophagy was significantly increased compared with the control (parental) cells (Figure 5A). Blocking endogenous miR-7-5p with antisense oligonucleotides (ASO)-miR-7-5p significantly inhibited autophagy in both HeLa-CR and SiHa-CR cells (Figure 5B), indicating the involvement of miR-7-5p in autophagy in cisplatin-resistant cervical cancer cells. Inhibition of autophagy with both 3-methyladenine (3-MA) or chloroquine (CQ) led to a significant decrease of ATP/ADP levels in both HeLa-CR and SiHa-CR cells (Figure 5C), demonstrating that autophagy provided energy for cisplatin-resistant cells. Blocking autophagy with 3-MA significantly increased apoptosis in cisplatin-resistant cells (Figure 5D). These results indicated that autophagy involved in cisplatin resistance of cervical cancer cells might provide alternative energy for the cells and that miR-7-5p is involved in the regulation of autophagy in cisplatin-resistant cervical cancer cells.
Figure 5.
MicroRNA-7-5p (mir-7-5p) promotes autophagy in cisplatin-resistant cervical cancer cells, which is essential for cell survival. (A) Control (parental) or cisplatin-resistant cervical cancer cells were transfected with the GFP/LC3 expression plasmid and then treated with cisplatin. Formation of autophagosomes were visualized with fluorescence microscopy. (B) Cisplatin-resistant cervical cancer cells were transfected with GFP/LC3 expression plasmid and ASO-miR-7-5p, and the formation of autophagosomes were visualized with fluorescence microscopy. (C) The ratio of ATP/ADP cisplatin-resistant cervical cancer cells with indicated treatments were measured. (D) Apoptosis of the cisplatin-resistant cervical cancer cells with indicated treatment was measured by flow cytometry. * p<0.05.
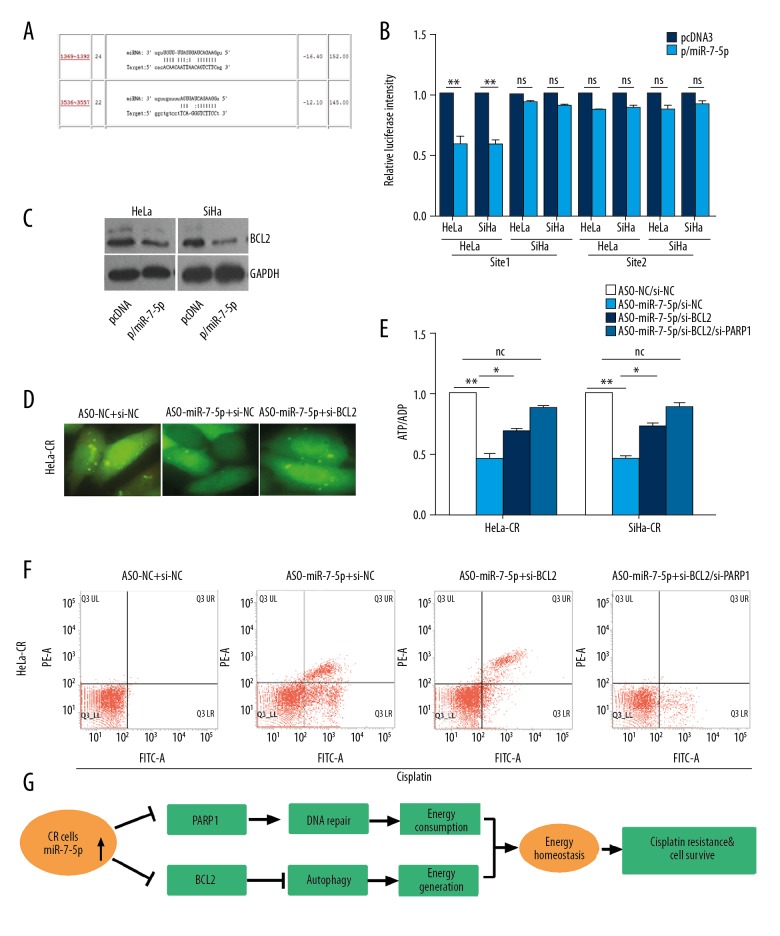
miR-7-5p modulated autophagy in chemotherapy-resistant cervical cancer cells by regulating the expression of BLC2 and PARP-1 to modulate energy homeostasis
Two target sites of miR-7-5p were found in the 3 UTR of the BCL2 gene (Figure 6A), a gene that has been demonstrated to be involving in the regulation of autophagy. The results of luciferase reporter assay demonstrated that only one site was involved in the regulation of reporter gene expression by miR-7-5p (Figure 6B), and the overexpression of miR-7-5p in the control (parental) cells reduced the protein level of Bcl-2 (Figure 6C). Blocking miR-7-5p with ASO-miR-7-5p reduced autophagy in HeLa-CR and SiHa-CR cells, but simultaneous knockdown of endogenous BCL2 with si-BCL2 could rescue the autophagy activity (Figure 6D). Knockdown of endogenous BCL2 with si-BCL2 could also rescue the ATP/ADP level in ASO-miR-7-5p-treated cells (Figure 6E). Simultaneously knocked down endogenous BCL2 and PARP-1 in ASO-miR-7-5p treated cells could further rescue the ATP/ADP ratio (Figure 6E). The increase of apoptosis in ASO-miR-7-5p treated cisplatin-resistant cervical cancer cells was also reduced by BCL2 and PARP-1 gene knockdown (Figure 6F).
Figure 6.
MicroRNA-7-5p (mir-7-5p) modulates autophagy in cisplatin-resistant cervical cancer cells via regulating the expression of BLC2, which in combination with PARP-1, modulates the energy homeostasis. (A) The target sites of miR-7-5p in 3′UTR of BCL2 mRNA predicted by ReRNA2.0. (B) Luciferase reporter assay was conducted with the indicated reporter plasmid. (C) The protein levels of PARP-1 in cisplatin-resistant cervical cancer cells with indicated treatment were measured by Western blot. (D) Cisplatin-resistant cervical cancer cells were transfected with the GFP/LC3 expression plasmid simultaneously transfected with ASO-miR-7-5p and si-BCL2 and then treated with cisplatin. Formation of the autophagosomes were visualized with fluorescence microscopy. (E) The ratio of ATP/ADP in cisplatin-resistant cervical cancer cells with indicated treatment were measured. (F) Apoptosis of cisplatin-resistant cervical cancer cells transfected with the indicated plasmids under cisplatin treatment was measured by flow cytometry. (G) A diagrammatic summary of the findings of the study.
These results demonstrated that in cisplatin-resistant cervical cancer cells, the expression of miR-7-5P was increased, which suppressed the expression PARP-1 resulting in reduced DNA repair activity and reducing cell energy consumption. The expression of miR-7-5P in cisplatin-resistant cervical cancer cells suppressed the expression of BCL2 leading to increased autophagy that supplemented energy in cisplatin-resistant cells, thus maintaining energy homeostasis (Figure 6G).
Discussion
The mechanisms for cisplatin resistance vary in different cancers. Intrinsic or acquired resistance of cervical cancer cells to cisplatin chemotherapy is the main reason for treatment failure of advanced or recurrent cervical cancer [4,5]. The findings of this preliminary study showed that the expression of microRNA-7-5p (mir-7-5p) was increased in cisplatin-resistant cervical cancer cells and that miR-7-5p maintained energy homeostasis via reducing energy consumption by targeting poly (ADP-ribose) polymerase 1 (PARP-1) and increasing energy generation by targeting the BCL2 gene in cisplatin-resistant cells.
The role of miR-7-5p in tumorigenesis of several types of cancer has been previously investigated and has been reported to have both a tumor-promoting and tumor-suppressive role, depending on the type of cancer. By targeting PI3K/Akt, miR-7-5p inhibited cell proliferation and metastasis in hepatocellular carcinoma and glioblastoma [7], and by regulating focal adhesion kinase (FAK) or Kruppel-like factor 4 (KLF4), miR-7-5p inhibited cell metastasis in breast cancer [6]. Although the functional target of miR-7-5p in renal cancer remains unclear, miR-7-5p has been shown to have a promoting role in renal cancer [9].
In a previously published study on cervical cancer tissue by Liu et al. [11], when compared with non-cancerous cervical tissue, the expression of miR-7 was decreased, and miR-7 exerted a tumor suppressive role by targeting X-linked inhibitor of apoptosis protein (XIAP) in cervical cancer. In the present study, the expression of miR-7-5p was decreased in cervical cancer when compared with non-cancerous tissue. However, for different types of cancer, the expression of miR-7-5p may vary depending on the individual patient response to cisplatin. In cisplatin-sensitive cancer tissues, the expression of miR-7-5p was decreased, indicating a tumor suppressive role of miR-7-5p. However, in cisplatin-resistant tissues, the expression of miR-7-5p was increased. Further studies are required to investigate the mechanisms of the oncogenic role of miR-7-5p in cisplatin-resistant cervical cancer. In this study, using chemotherapy-resistant and chemotherapy-sensitive cervical cancer cells in the in vitro model, in cisplatin-sensitive cells, the expression of miR-7-5p was decreased, and in cisplatin-resistant cells, the expression of miR-7-5p was increased.
The function of a given microRNA may vary in different circumstances, under different situations the functional target of microRNAs can change accompanied by a change in the role of the microRNA. The expression of microRNA is regulated at both the transcriptional and post-transcriptional level [12,13]. Currently, the mechanisms that explain the expression of miR-7-5p in cisplatin-resistant and cisplatin-sensitive cervical cancer cells remain unclear, and further studies are required.
The activation of the PARP-1 gene is correlated with a DNA damage response that is promoted by DNA repair and genomic integrity [14–16]. Under mild genotoxic stimulation, the activation of PARP-1 can facilitate DNA repair and maintain cell survival [17]. Excess DNA repair consumes most of the intracellular NAD+, causing ATP storage to provide the large cell energy demand, leading to cell dysfunction [18,19]. It is possible that cisplatin forms DNA-cisplatin adducts leading to DNA damage, and that excess DNA damage induces cellular apoptosis [20]. The findings of the present study showed that in cisplatin-resistant cervical cancer cells, miR-7-5p reduced levels of PARP-1 protein and PARP-1 gene expression levels, maintaining DNA repair activity and maintaining energy to avoid cell apoptosis.
Autophagy is a common stress response in eukaryotic cells that involves damage to cell organelles and autophagy of degraded proteins that could provide an alternative energy supply for the cell under stress condition [21]. In the present study, the level of autophagy was increased in cisplatin-resistant cervical cancer cells compared with cisplatin-sensitive cells, and that blocking autophagy with 3 3-methyladenine (3-MA) or chloroquine (CQ) could re-sensitize the cisplatin-resistant cell. This finding is supported by those of previous studies that have shown that autophagy promotes cisplatin resistance in several types of cancer [22,23].
The results of this study showed that the BCL2 gene was the functional target of miR-7-5p in the regulation of autophagy. Bcl-2 is a multifunction protein involving in the many aspects of cellular processes, including autophagy, and the expression of Bcl-2 inhibits autophagy via forming a complex with the BECN1 gene that encodes Beclin-1 [21,24,25]. miR-7-5p reduces cellular the expression levels of BCL2 in cisplatin-resistant cells, promoting autophagy, which explains its effects on cervical cancer cell survival during cisplatin treatment. Also, by promoting autophagy, miR-7-5p increased energy generation to maintain surviving of cisplatin-resistant cells under cisplatin exposure.
The results of this preliminary study have shown that in cisplatin-resistant cervical cancer cells, miR-7-5p maintained energy homeostasis of cisplatin-resistant cells via promoting autophagy and reducing DNA repair activity by targeting BCL2 and PARP-1 genes. These findings provide new insight on the role of miR-7-5p in cervical cancer and might stimulate further research leading to the prevention or reversal of cisplatin resistance in cervical cancer.
Conclusions
The results of this preliminary study have demonstrated a potential role for microRNA-7-5p (mir-7-5p) in cervical cancer and may shed light on the mechanism of cisplatin resistance of cervical cancer cells, by regulating the expression of PARP-1 and BCL2. Also, the study findings support that miR-7-5p might maintain energy homeostasis in cisplatin-resistant cervical cancer cells by reducing energy consumption via inhibiting DNA repair and increasing energy generation via activating autophagy.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Small W, Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer. 2017;123:2404–12. doi: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 2.Stehman FB, Ali S, Keys HM, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: Follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197:503.e1–6. doi: 10.1016/j.ajog.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturdza A, Potter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–33. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Eskander RN, Tewari KS. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr Opin Obstet Gynecol. 2014;26:314–21. doi: 10.1097/GCO.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Luo H, Zhang W, et al. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther. 2016;10:1885–95. doi: 10.2147/DDDT.S106412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Luo X, Li P, et al. miR-7-5p suppresses cell proliferation and induces apoptosis of breast cancer cells mainly by targeting REGgamma. Cancer Lett. 2015;358:27–36. doi: 10.1016/j.canlet.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Liu Y, Li L, et al. MiR-7-5p is frequently downregulated in glioblastoma microvasculature and inhibits vascular endothelial cell proliferation by targeting RAF1. Tumour Biol. 2014;35:10177–84. doi: 10.1007/s13277-014-2318-x. [DOI] [PubMed] [Google Scholar]
- 8.Giles KM, Brown RA, Epis MR, et al. miRNA-7-5p inhibits melanoma cell migration and invasion. Biochem Biophys Res Commun. 2013;430:706–10. doi: 10.1016/j.bbrc.2012.11.086. [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, Ni L, Chen D, et al. Identification of miR-7 as an oncogene in renal cell carcinoma. J Mol Histol. 2013;44:669–77. doi: 10.1007/s10735-013-9516-5. [DOI] [PubMed] [Google Scholar]
- 10.Xiang C, Zhang M, Zhao Q, et al. LncRNA-SLC6A9-5: 2: A potent sensitizer in 131I-resistant papillary thyroid carcinoma with PARP-1 induction. Oncotarget. 2017;8(14):22954–67. doi: 10.18632/oncotarget.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Zhang P, Chen Z, et al. MicroRNA-7 down-regulates XIAP expression to suppress cell growth and promote apoptosis in cervical cancer cells. FEBS Lett. 2013;587:2247–53. doi: 10.1016/j.febslet.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Morales S, Monzo M, Navarro A. Epigenetic regulation mechanisms of microRNA expression. Biomol Concepts. 2017;8:203–12. doi: 10.1515/bmc-2017-0024. [DOI] [PubMed] [Google Scholar]
- 13.Varghese VK, Shukla V, Kabekkodu SP, et al. DNA methylation regulated microRNAs in human cervical cancer. Mol Carcinog. 2018;57(3):370–82. doi: 10.1002/mc.22761. [DOI] [PubMed] [Google Scholar]
- 14.Mateu-Jimenez M, Cucarull-Martinez B, Yelamos J, Barreiro E. Reduced tumor burden through increased oxidative stress in lung adenocarcinoma cells of PARP-1 and PARP-2 knockout mice. Biochimie. 2016;121:278–86. doi: 10.1016/j.biochi.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Pascal JM, Ellenberger T. The rise and fall of poly(ADP-ribose): An enzymatic perspective. DNA Repair (Amst) 2015;32:10–16. doi: 10.1016/j.dnarep.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: An international molecule of mystery. DNA Repair (Amst) 2008;7:1077–86. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Virag L. Structure and function of poly(ADP-ribose) polymerase-1: Role in oxidative stress-related pathologies. Curr Vasc Pharmacol. 2005;3:209–14. doi: 10.2174/1570161054368625. [DOI] [PubMed] [Google Scholar]
- 18.Beneke S, Diefenbach J, Burkle A. Poly(ADP-ribosyl)ation inhibitors: Promising drug candidates for a wide variety of pathophysiologic conditions. Int J Cancer. 2004;111:813–18. doi: 10.1002/ijc.20342. [DOI] [PubMed] [Google Scholar]
- 19.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorusso D, Petrelli F, Coinu A, et al. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol Oncol. 2014;133:117–23. doi: 10.1016/j.ygyno.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Guo J, Yang Z, Yang X, et al. miR-346 functions as a pro-survival factor under ER stress by activating mitophagy. Cancer Lett. 2018;413:69–81. doi: 10.1016/j.canlet.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Cheng CY, Liu JC, Wang JJ, et al. Autophagy inhibition increased the anti-tumor effect of cisplatin on drug-resistant esophageal cancer cells. J Biol Regul Homeost Agents. 2017;31:645–52. [PubMed] [Google Scholar]
- 23.Liu J, Chen Z, Guo J, et al. Ambra1 induces autophagy and desensitizes human prostate cancer cells to cisplatin. Biosci Rep. 2017 doi: 10.1042/BSR20170770. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartolome A, Guillen C, Benito M. Autophagy plays a protective role in endoplasmic reticulum stress-mediated pancreatic beta cell death. Autophagy. 2012;8:1757–68. doi: 10.4161/auto.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Mi S, Li Z, et al. Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells. Autophagy. 2013;9:730–42. doi: 10.4161/auto.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]