Abstract
As a treatment for solid tumors, dendritic cell (DC)-based immunotherapy has not been as effective as expected. Here, we review the reasons underlying the limitations of DC-based immunotherapy for solid tumors and ask what can be done to improve immune cell-based cancer therapies. Several reports show that, rather than a lack of immune induction, the limited efficacy of DC-based immunotherapy in cases of renal cell carcinoma (RCC) likely results from inhibition of immune responses by tumor-secreted TGF-β and an increase in the number of regulatory T (Treg) cells in and around the solid tumor. Indeed, unlike DC therapy for solid tumors, cytotoxic T lymphocyte (CTL) responses induced by DC therapy inhibit tumor recurrence after surgery; CTL responses also limit tumor metastasis induced by additional tumor-challenge in RCC tumor-bearing mice. Here, we discuss the mechanisms underlying the poor efficacy of DC-based therapy for solid tumors and stress the need for new and improved DC immunotherapies and/or combination therapies with killer cells to treat resistant solid tumors.
DC Immunotherapy Effectively Inhibits Tumor Metastasis and/or Recurrence, But Does Not Eradicate Established Solid Tumors
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that drive T cell-mediated immune responses. Vaccination with DCs pulsed with tumor lysates increases therapeutic antitumor immune responses both in vitro and in vivo [1], [2], [3]. DCs capture and process antigens, migrate into lymphoid organs, express lymphocyte costimulatory molecules, and secrete cytokines that initiate immune responses. They also stimulate immunological effector cells (T cells) that express receptors specific for tumor-associated antigens and reduce the number of immune repressors such as CD4+CD25+Foxp3+ regulatory T (Treg) cells [4], [5], [6].
Several reports show that a number of obstacles must be overcome before DC-based immunotherapy can be used as an effective therapy for solid tumors [1], [7]. One of the major difficulties with respect to treatment of advanced tumors seems to be that tumor cells suppress the patient's antitumor immune response. Recent reports show that Treg cells, which produce TGF-β, IL-10, and IL-4, play a crucial role in regulating the immune response to self- and/or non-self-antigens [8], [9], [10], [11]. Several Treg subsets, such as CD4+CD25+Foxp3+TGF-β+ cells differentiated by tolerogenic (t)DCs, inhibit autoimmune (e.g., rheumatoid arthritis and myocarditis) and inflammatory (e.g., myocardial infarction) [12], [13], [14], [15] responses, thereby maintaining immune tolerance in tumor-bearing hosts. In humans with solid cancers, high tumor infiltration by Treg cells and, more importantly, a low effector T (Teff) cell/Treg cell ratio, are associated with a poor outcome [16], [17]. Conversely, a high Teff/Treg cell ratio is associated with favorable responses to immunotherapy in both humans and mice [18], [19]. To date, most studies support the notion that targeting Treg cells, either by depletion or functional modulation, may offer significant therapeutic benefit, particularly in combination with other immune modulatory interventions such as vaccination and checkpoint blockade [20], [21], [22].
This review focuses on the mechanisms by which solid tumors escape immune responses and discusses immune cell-based therapy (particularly DC-based immunotherapy) for the treatment of solid tumors.
Recently, a DC vaccination strategy for renal cell carcinoma (RCC), which is based on a tumor cell lysate-DC hybrid, showed therapeutic potential in preclinical and clinical trials [1], [23]. Although such trials are promising, several hurdles must be overcome if we are to generate a more effective antitumor response. In a previous study, we developed a murine model of RCC by inoculating mice with mouse renal adenocarcinoma Renca cells. We then examined the efficacy of DC vaccination as a treatment for RCC by testing whether Renca cells induce any of the immunosuppressive effects generally reported in humans with RCC and other solid tumors; such effects might attenuate T cell-mediated antitumor immunity [1]. A DC vaccine would be the best method of inhibiting tumor growth in tumor-bearing mice and would be applicable to humans (cytokine therapies based on IL-12 and IL-2 are not applicable to humans due to toxicity and poor efficacy). Several studies show that, although vaccination with DCs induces a systemic response and can prevent tumor metastasis and recurrence, the response does not affect the solid tumor itself due to its secretion of protective TGF-β. Tumor cells secrete various immunosuppressive factors, including TGF-β [1], [24], VEGF [25], and IL-10 [25]. Among these, overexpression of TGF-β is closely associated with a poor prognosis in patients with malignant tumors [26], [27]. TGF-β suppresses differentiation of bone marrow (BM) DCs, as well as their capacity to secrete IL-12 [28], present antigens, stimulate tumor-sensitized T lymphocytes, and migrate into tumor-draining lymph nodes [29]. Indeed, we showed previously that high doses of TGF-β inhibit DC migration in vitro [1]. However, tumor lysate-pulsed DCs effectively migrated into regional lymph nodes and induced sufficient numbers of functional cytotoxic T lymphocytes (CTLs) in both sc tumor-bearing mice and metastatic tumor-bearing mice. Consequently, we believe that immunity induced by tumor lysate-pulsed DCs may not be restricted to peri-tumor tissue in vivo. By contrast, immunohistochemical analyses of sc-implanted tumor masses indicate that the number of Teff cells within the tumor-infiltrating lymphocyte (TIL) population is very low, possibly due to inhibition by tumor-derived TGF-β. These data can be explained by the fact that naïve T cells in sc-implanted tumor masses may be primed to differentiate into Treg (CD4+CD25+Foxp3+) cells by tumor-derived TGF-β [30], leading to subsequent inactivation of TILs. Therefore, sc-implanted tumors in mice are resistant to DC vaccination-induced antitumor immune responses. During tumor progression in humans, Treg cells accumulate in tumors and secondary lymphoid organs. Also, chemokines produced by tumor cells or tumor-infiltrating macrophages recruit Treg cells to the tumor bed [30], [31].
Although DC vaccination lacks efficacy against sc tumors in mice, it does inhibit further spread of metastatic tumors or tumor recurrence in mice after surgery, indicating that DC vaccination is effective at inducing long-lasting systemic antitumor immunity after surgery [1]. We expect that these results will form an important basis for clinical trials of DC-based immunotherapy under these conditions.
Improved DC Immunotherapy Is Highly Effective at Inhibiting Established Solid Tumors
An immune response is triggered by danger signals, which include microbial products (termed pathogen-associated molecular patterns) and fragments of dying cells; these signals are recognized by the cells that provide innate immunity [32], [33]. Of these, DCs are the major link between the innate and adaptive immune responses. Recent reports show that DCs pulsed with tumor lysates in vitro and in vivo drive increased therapeutic antitumor immune responses after vaccination [1], [3], [34]. However, several reports show that a number of obstacles must be overcome before DC-based immunotherapy can be used widely to treat tumors [7], [35].
In an attempt to overcome such problems, several studies focused on antigen cross-priming using heat shock proteins (HSPs), which are highly conserved and abundantly expressed proteins that have diverse functions [36], [37]. Recent studies show that these molecular chaperones interact with APCs; thus their ability to induce antigen-specific CTL and Th1 responses has attracted much attention [38]. In the context of the immune system, HSPs transfer antigenic peptides to CD8+ T cells [38]. During this process, HSP70- or gp96-peptide complexes are internalized by APCs, including DCs, through receptor-mediated endocytosis via CD40, TLR2/4, or scavenger receptor A [39].
Photodynamic therapy (PDT) is an established cancer treatment that uses a combination of light and photosensitizing drugs to damage tumor tissues [40], [41]. One of the most important factors induced by PDT is extracellular HSP70 [42], [43]; thus we think that in vitro exposure of DCs to tumor cell lysates treated with PDT may improve DC immunotherapy against tumors by enhancing their function. Several studies indicate that some HSPs might be suitable [44], [45]. Inducible HSPs (i.e., HSP60, HSP70, and HSP90) stimulate DC differentiation and induce expression of several cytokines, including IL-12 [45], thereby increasing their antigen-presenting capacity [46]. Various immune cells, including DCs, macrophages, natural killer (NK) cells, and B lymphocytes, express receptors specific for HSPs [37], including HSP70. Therefore, induction of HSP expression may constitute a ‘danger’ signal that triggers DC maturation.
It seems likely that PDT-generated tumor lysates contain all of the factors necessary to activate DCs; this may include loading them with antigen and inducing effective antitumor immune responses. In accordance with these findings, HSPs induced by PDT might improve the efficacy of DC vaccines by increasing cross-priming. However, more work needs to be done to fully examine and understand the interaction between tumor antigens and HSPs that is responsible for increasing antitumor responses following PDT-DC vaccination.
Taken together, the data suggest that DCs loaded with PDT tumor lysates are strongly immunogenic and can be used as effective antitumor vaccines [47]. Thus, we expect that PDT-DC vaccination may be developed as an effective immunotherapy for treatment of tumors.
DC Immunotherapy Combined with Cytokine-Induced Killer (CIK) Cells Effectively Suppresses Established Hepatocellular Carcinomas in Mice
Cytokine-induced killer (CIK) cells are a heterogeneous population of ex vivo-expanded T lymphocytes with different cellular phenotypes. CIK cells are generated from peripheral blood, BM, or cord blood mononuclear cells upon treatment with a cytokine cocktail (e.g., IFN-γ and IL-2) and an anti-CD3 monoclonal antibody. CIK cells express markers associated with both T cells and NK cells, including CD3+CD56+ (NK T), CD3+CD56− (typical T), and CD3−CD56+ (NK). The antitumor activity of CIK cells is mediated mainly by CD3+CD56+ (NK T) cells, which show NK-like, major histocompatibility complex (MHC)-unrestricted, tumor killing ability [48], [49]. Therefore, CIK-based adoptive immunotherapy represents a potential strategy for curing cancer. Indeed, CIK cells exhibit active proliferation and potent antitumor cytotoxicity in the presence of various tumor cells, both in vitro and in vivo [50].
Although the majority of clinical trials focusing on DC-based immunotherapy have succeeded in generating tumor-specific CTLs in cancer patients, the effects against most solid tumors have been rather disappointing [1], [35]. Several mechanisms may account for the limited effectiveness of DC vaccine-induced immune responses to solid tumors. One is that insufficient numbers of CD8+ CTLs are induced in response to DC vaccination alone [51], [52], [53]. Alternatively, CTLs generated in this manner may possess suboptimal antitumor efficacy in vivo, possibly due to weak activation or inadequate migration to tumor sites [53]. The susceptibility of such cells to host-derived regulatory mechanisms also appears to be a problem.
Preclinical and clinical models in which ex vivo-expanded CIK cells have been tested also demonstrate antitumor activity, but only modest therapeutic efficacy; this is due largely to the strategies used by tumors to evade the host immune system [52]. To overcome these problems, we wondered whether a combination of DC vaccination plus CIK cells would induce a stronger therapeutic antitumor effect than administration of DCs or CIK cells alone [54].
CIK cells inhibit proliferation of tumor cells and show tumor cell-specific cytotoxicity. CIK cell-based immunotherapy is associated with a significant increase in the survival rate of cancer patients. Recently, several clinical trials examined combining strategies based on CIK cells with immunization approaches; for example, the combined application of CIK cells and tumor lysate-pulsed DCs improved antitumor toxicity [55]. However, despite several decades of research on CIK cancer vaccines, the clinical effectiveness of immunotherapy remains disappointing.
The goal of cancer immunotherapy is to induce an effective immune response that specifically targets tumor cells. It is well established that activated CD4+ T and CD8+ CTLs are necessary to sustain an antitumor response. Ideally, vaccine-elicited CD8+ T cells should have high avidity and be able to recognize peptide–MHC class I complexes on tumor cells; they should express high levels of granzyme and perforin (molecules essential for cytotoxic activity against cancer cells); they should be able to enter the tumor microenvironment; and they should be able to circumvent immunomodulatory mechanisms in the tumor. At least four components of the immune response are required for this ideal response: the presence of fully-activated DCs; activation of induced IFN-γ-producing CD4+ T helper cells; elimination and/or non-activation of Treg cells; and breakdown of the immunosuppressive tumor microenvironment. DCs generated by ex vivo culture of hematopoietic progenitor cells or monocytes with combinations of cytokines have been tested as therapeutic vaccines in cancer patients for more than a decade [56], [57], [58], [59], [60].
Our previous report demonstrated that DC vaccination combined with adoptive transfer of CIK cells leads to significant suppression of hepatoma tumor cell growth and improved antitumor responses [54]. These results suggest that a combination of DCs plus CIK cells can increase antitumor activity, indicating a potential for clinical application to cancer patients in the future.
Conclusions
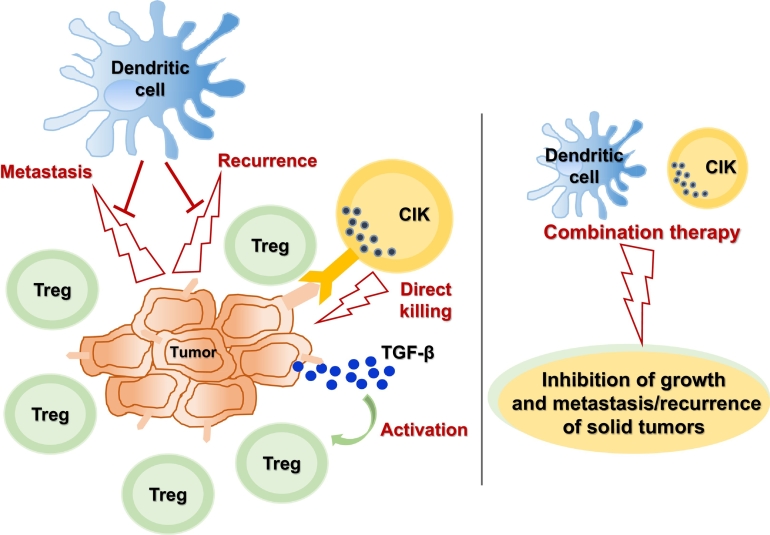
To overcome the above-mentioned limitations inherent in DC-mediated anticancer immune responses, we suggest the following: 1) depletion and/or inactivation (or non-activation) of Treg cells; 2) development of improved DC-based immunotherapies; and 3) combination therapy based on DCs plus other killer cells (CIK cells, T cells, or NK cells). In particular, we anticipate that DC immunotherapy will inhibit the metastasis/recurrence of solid cancers, and that killer cells such as CIK cells will kill tumor cells; such a combination may prove very effective against many difficult-to-treat solid tumors (Figure 1).
Figure 1.
Overview of immune cell-mediated anticancer immunity. DC immunotherapy inhibits the metastasis/recurrence of solid cancers, and killer cells such as CIK cells directly kill tumor cells; a combination of these cell-mediated therapeutics may prove very effective against many difficult-to-treat solid tumors. Treg, T regulatory cell; TGF-β, transforming growth factor-β; CIK, cytokine-induced killer cell.
Author contributions
N.-C.J. and D.-S.L. conceived of the presented idea and wrote the manuscript with support from J.-H.L., K.-H.C., and Y.S.K.. All authors discussed the idea and contributed to the final manuscript.
Footnotes
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation, funded by the Korean government (no. 2017M3A9C8062605).
The authors declare no conflict of interest.
References
- 1.Lim DS, Kim JH, Lee DS, Yoon CH, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother. 2007;56:1817–1829. doi: 10.1007/s00262-007-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 3.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt J, Eisold S, Buchler MW, Marten A. Dendritic cells reduce number and function of CD4+CD25+ cells in cytokine-induced killer cells derived from patients with pancreatic carcinoma. Cancer Immunol Immunother. 2004;53:1018–1026. doi: 10.1007/s00262-004-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Pereira LMS, Gomes STM, Ishak R, Vallinoto ACR. Regulatory T Cell and Forkhead Box Protein 3 as Modulators of Immune Homeostasis. Front Immunol. 2017;8:605. doi: 10.3389/fimmu.2017.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 12.Byun SH, Lee JH, Jung NC, Choi HJ, Song JY, Seo HG, Choi J, Jung SY, Kang S, Choi YS. Rosiglitazone-mediated dendritic cells ameliorate collagen-induced arthritis in mice. Biochem Pharmacol. 2016;115:85–93. doi: 10.1016/j.bcp.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Lim DS, Kang MS, Jeong JA, Bae YS. Semi-mature DC are immunogenic and not tolerogenic when inoculated at a high dose in collagen-induced arthritis mice. Eur J Immunol. 2009;39:1334–1343. doi: 10.1002/eji.200838987. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Kim TH, Park HE, Lee EG, Jung NC, Song JY, Seo HG, Seung KB, Chang K, Lim DS. Myosin-primed tolerogenic dendritic cells ameliorate experimental autoimmune myocarditis. Cardiovasc Res. 2014;101:203–210. doi: 10.1093/cvr/cvt246. [DOI] [PubMed] [Google Scholar]
- 15.Choo EH, Lee JH, Park EH, Park HE, Jung NC, Kim TH, Koh YS, Kim E, Seung KB, Park C. Infarcted Myocardium-Primed Dendritic Cells Improve Remodeling and Cardiac Function After Myocardial Infarction by Modulating the Regulatory T Cell and Macrophage Polarization. Circulation. 2017;135:1444–1457. doi: 10.1161/CIRCULATIONAHA.116.023106. [DOI] [PubMed] [Google Scholar]
- 16.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity. 2017;46:577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, Fulton A, Tamada K, Strome SE, Antony PA. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190:4899–4909. doi: 10.4049/jimmunol.1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höltl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin Jr JH, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–3376. [PubMed] [Google Scholar]
- 24.Conrad CT, Ernst NR, Dummer W, Brocker EB, Becker JC. Differential expression of transforming growth factor beta 1 and interleukin 10 in progressing and regressing areas of primary melanoma. J Exp Clin Cancer Res. 1999;18:225–232. [PubMed] [Google Scholar]
- 25.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91:964–971. [PubMed] [Google Scholar]
- 27.Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara H. An elevated serum level of transforming growth factor-beta 1 (TGF-beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res. 2000;20:4489–4493. [PubMed] [Google Scholar]
- 28.Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061–2070. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]
- 29.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL, Akporiaye ET. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 30.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker C, Fantini MC, Neurath MF. TGF-beta as a T cell regulator in colitis and colon cancer. Cytokine Growth Factor Rev. 2006;17:97–106. doi: 10.1016/j.cytogfr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Fonteneau JF, Kavanagh DG, Lirvall M, Sanders C, Cover TL, Bhardwaj N, Larsson M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood. 2003;102:4448–4455. doi: 10.1182/blood-2003-06-1801. [DOI] [PubMed] [Google Scholar]
- 33.Medzhitov R, Janeway C., Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 34.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 35.Chang DH, Dhodapkar MV. Dendritic cells and immunotherapy for cancer. Int J Hematol. 2003;77:439–443. doi: 10.1007/BF02986611. [DOI] [PubMed] [Google Scholar]
- 36.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 37.Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 39.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kataoka H, Nishie H, Hayashi N, Tanaka M, Nomoto A, Yano S, Joh T. New photodynamic therapy with next-generation photosensitizers. Ann Transl Med. 2017;5:183. doi: 10.21037/atm.2017.03.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim DS, Ko SH, Lee CH, Ahn WS, Lee WY. DH-I-180-3-mediated photodynamic therapy: biodistribution and tumor vascular damage. Photochem Photobiol. 2006;82:600–605. doi: 10.1562/2005-09-13-RA-683. [DOI] [PubMed] [Google Scholar]
- 42.Song S, Zhou F, Chen WR, Xing D. PDT-induced HSP70 externalization up-regulates NO production via TLR2 signal pathway in macrophages. FEBS Lett. 2013;587:128–135. doi: 10.1016/j.febslet.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 43.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 44.Vo MC, Nguyen-Pham TN, Lee HJ, Jung SH, Choi NR, Hoang MD, Kim HJ, Lee JJ. Chaetocin enhances dendritic cell function via the induction of heat shock protein and cancer testis antigens in myeloma cells. Oncotarget. 2017;8:46047–46056. doi: 10.18632/oncotarget.17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flohe SB, Bruggemann J, Lendemans S, Nikulina M, Meierhoff G, Flohe S, Kolb H. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J Immunol. 2003;170:2340–2348. doi: 10.4049/jimmunol.170.5.2340. [DOI] [PubMed] [Google Scholar]
- 46.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 47.Jung NC, Kim HJ, Kang MS, Lee JH, Song JY, Seo HG, Bae YS, Lim DS. Photodynamic therapy-mediated DC immunotherapy is highly effective for the inhibition of established solid tumors. Cancer Lett. 2012;324:58–65. doi: 10.1016/j.canlet.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 48.Eshima K, Chiba S, Suzuki H, Kokubo K, Kobayashi H, Iizuka M, Iwabuchi K, Shinohara N. Ectopic expression of a T-box transcription factor, eomesodermin, renders CD4(+) Th cells cytotoxic by activating both perforin- and FasL-pathways. Immunol Lett. 2012;144:7–15. doi: 10.1016/j.imlet.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 50.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou Y, Wang G, Lizee G, Kim GJ, Finkelstein SE, Feng C, Restifo NP, Hwu P. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen MH, Keikavoussi P, Brocker EB, Schuler-Thurner B, Jonassen M, Sondergaard I, Straten PT, Becker JC, Kampgen E. Induction of systemic CTL responses in melanoma patients by dendritic cell vaccination: cessation of CTL responses is associated with disease progression. Int J Cancer. 2001;94:820–824. doi: 10.1002/ijc.1536. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Diez A, Marincola FM. Immunotherapy against antigenic tumors: a game with a lot of players. Cell Mol Life Sci. 2002;59:230–240. doi: 10.1007/s00018-002-8419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung NC, Lee JH, Choi HJ, Hwang SU, Song JY, Seo HG, Choi J, Jung SY, Han SG, Lim DS. Dendritic cell immunotherapy combined with cytokine-induced killer cells effectively suppresses established hepatocellular carcinomas in mice. Immunol Invest. 2016;45:553–565. doi: 10.1080/08820139.2016.1183025. [DOI] [PubMed] [Google Scholar]
- 55.Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60:1497–1502. doi: 10.1007/s00262-011-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McIlroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother. 2003;52:583–591. doi: 10.1007/s00262-003-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massa C, Thomas C, Wang E, Marincola F, Seliger B. Different maturation cocktails provide dendritic cells with different chemoattractive properties. J Transl Med. 2015;13:175. doi: 10.1186/s12967-015-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castiello L, Sabatino M, Jin P, Clayberger C, Marincola FM, Krensky AM, Stroncek DF. Monocyte-derived DC maturation strategies and related pathways: a transcriptional view. Cancer Immunol Immunother. 2011;60:457–466. doi: 10.1007/s00262-010-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vopenkova K, Mollova K, Buresova I, Michalek J. Complex evaluation of human monocyte-derived dendritic cells for cancer immunotherapy. J Cell Mol Med. 2012;16:2827–2837. doi: 10.1111/j.1582-4934.2012.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han TH, Jin P, Ren J, Slezak S, Marincola FM, Stroncek DF. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J Immunother. 2009;32:399–407. doi: 10.1097/CJI.0b013e31819e1773. [DOI] [PMC free article] [PubMed] [Google Scholar]