Abstract
With the growing awareness and availability of proper screening methods, detection of breast lump is increasing globally and is now a very sensitive issue for females. The treatment of these lumps ranges from lumpectomy to wide local excision to mastectomy; hence, a proper diagnosis is very important to prevent under- or overtreatment in patients. Breast lesions are the heterogeneous diseases encompassing several distinct entities with remarkably different characteristics. While the more common forms of breast cancers are well recognized and understood better, there are many important unusual lesions and malignancies that are less known and less appreciated and can be challenging to diagnose. In such cases, due to rarity of the disease and lack of adequate treatment protocol, managing the patients can be a challenging task for surgeons and oncologist as well. In this article, we have shared our institutional experience in unusual breast lesions with emphasis on diagnostic as well as management challenges faced.
Keywords: Breast, Neoplasm, Unusual, Challenges
Introduction
Breast lesions have gained importance globally due to significant increase in mortality and morbidity caused by breast cancer, which is now one of the leading causes of death worldwide. As a result of increasing awareness and diagnosis, lump in the breast is now a very sensitive matter for females. Earlier, cervical cancer was most common cancer in Indian woman, but now, the incidence of breast cancer has surpassed cervical cancer in urban areas and has become the leading cause of cancer associated deaths in females nationwide [1, 2]. Timely and accurate diagnosis can be not only helpful in early management of this disease but also alleviates anxiety and psychological stress in these patents.
Breast neoplasm is heterogeneous in nature and benign breast lesions are more common than malignant tumors [3, 4]. Fine needle aspiration cytology and core needle biopsy are the primary diagnostic modalities based on which the management of the patient is planned. Most of the time there are no diagnostic issues; however, certain rarer neoplasm may place a diagnostic challenge thereby causing a dicey situation for the surgeons to plan the management of the patients.
The aim of our study is to present our institutional experience in breast pathology with special emphasis on certain less encountered/ rarer entities and the diagnostic challenges faced during their histopathological and cytological evaluation along with the challenges faced by surgeons and oncologist in their management.
Material and Methods
This is a retrospective descriptive study including all the patients who visited the Department of Endocrine and Breast Surgery (between June 2016 and December 2017) with a breast lump and were referred to our unit in Department of Pathology for fine needle aspiration cytology and/ or histopathological evaluation. Clinical data as well as pathological details were retrieved from the pathology registers and clinical files. Uncommon and interesting lesions identified were studied in detail and described in result section of this article.
As per our protocol, all patients with a breast lump first undergoes fine needle aspiration. If they are diagnosed as malignant or suspicious of malignancy, further core needle biopsy is performed, and later, lumpectomy/modified radical mastectomy is performed in resectable cases with adjuvant chemotherapy, whenever needed radiotherapy and hormonal therapy or without chemotherapy. All the cytological smears prepared were stained with May Grunwald Giemsa and all the cases were reviewed. They were reported, as per IAC Standardized Reporting of Breast Fine-Needle Aspiration Biopsy Cytology. The biopsies/resection specimens were processed and tissue was stained with hematoxylin and eosin stain for histopathological evaluation and was reported as per the College of American Pathologist (CAP) protocol for reporting breast lesions.
Results
Four hundred forty-two patients were evaluated histopathologically for various breast pathologies in the Department of Pathology, King George’s Medical University between June 2016 and September 2017. Age ranged from 14 to 83 years with mean age of 48.5 years with female predominance. Only 13 male patients were there and majority of them presented with gynecomastia. However, there were three male patients with breast cancer. Cytological correlation was available in 195 cases. There were 274 malignant lesions in which infiltrating duct carcinoma was the commonest diagnosis followed by phyllodes neoplasm. There were 168 benign lesions with fibroadenoma being the most common. The histopathological spectrum of breast lesions is shown in Table 1. There were six very unusual cases including one case each of desmoid tumor, vascular malformation, primary osteosarcoma, carcinoma en cuirasse, and two cases of diffuse large B cell lymphoma. These cases are described below.
Table 1.
Immunohistochemical characteristic of mimickers of fibromatosis
| IHC markers | Fibromatosis | Phyllodes | Metaplastic carcinoma (fibromatosis-like variant) | Nodular fasciitis | Solitary fibrous tumor |
|---|---|---|---|---|---|
| Vimentin | + | + | + | + | + |
| Cytokeratin | – | – | + | – | – |
| Smooth muscle actin | + | + | + | + | + |
| CD34 | – | – | – | – | + |
| CD68 | – | – | – | + | – |
| P63 | – | – | + | – | – |
Case 1
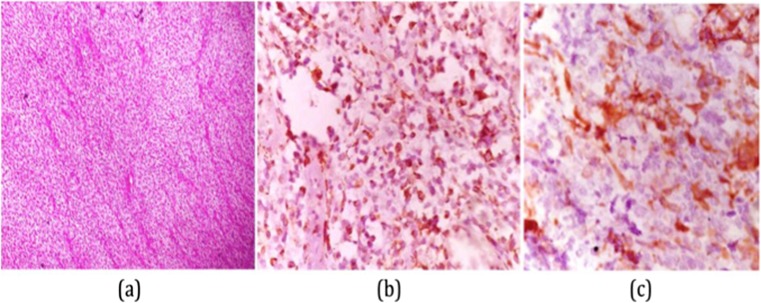
A 30-year-old female patient presented with a painless progressive lump in the right breast measuring 7 × 6 cm in size for 1 year with rapid increase in the last 2 months. There was a history of previous excision of this lump; however, the histopathological reports were not available. No axillary lymphadenopathy was present. USG breast using high-resolution linear probe showed a solid hypoechoic mass in the mammary layer in the right superior quadrant. Fine needle aspiration was performed and smears were paucicellular in spite of repeated aspiration showing occasional very small clusters of spindle-shaped cells with elongated nuclei. A possibility of benign phyllodes was considered. Later, a core needle biopsy was performed which also showed sheets of spindle-shaped cells. No mitosis, necrosis, or significant pleomorphism were identified. No epithelial component was identified in the material (Fig. 1a). A differential diagnosis of benign phyllodes neoplasm and fibromatosis/desmoid tumor was considered; hence, IHC was performed. On immunohistochemistry, the cells showed diffuse cytoplasm positivity for vimentin and smooth muscle actin (Fig. 1b, c) and were negative for cytokeratin, CD34, CD68, and p63. Ki 67 index was 1–2. Later, wide local excision was performed, and in spite of extensive grossing, no intrinsic epithelial component was identified. There was absence of significant stromal atypia, mitosis, necrosis, etc. Hence, with all these features in mind, a final diagnosis of fibromatosis/desmoid tumor breast was given. All the surgical resections’ margins were histopathologically negative. The patient was in follow-up for 15 months and recently has again presented with recurrence.
Fig. 1.
a Sheets of spindle-shaped cells with no mitosis, necrosis, or significant pleomorphism along with absence of epithelial component. b Tumor cells showing cytoplasmic positivity for vimentin. c Tumor cells showing cytoplasmic positivity for smooth muscle actin
Case 2
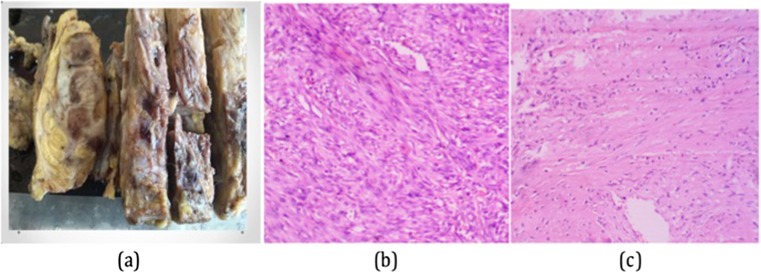
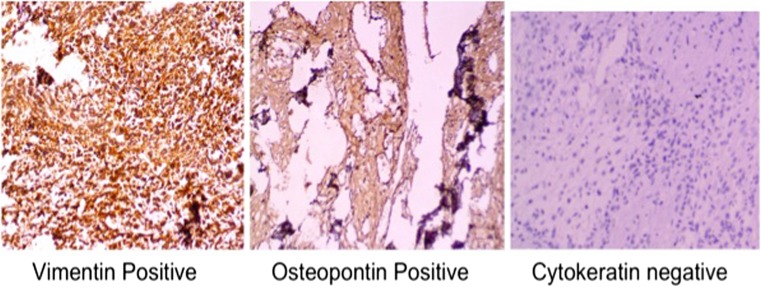
A 60-year-old lady presented with a rapidly increasing lump in the left breast for 6 months. On examination, the lump was hard with restricted mobility and fixed to overlying skin as well and was associated with axillary lymphadenopathy. The patient underwent left lumpectomy 3 months back in an outside hospital where it was reported as phyllodes neoplasm. Mammogram revealed an extremely dense (type D as per ACR classification for breast density) ill-defined mass with large areas of macro calcification abutting the underlying muscles. It was categorized as BIRADS 6. CT scan showed that the underlying bone was free and there was no continuation between them and the mass. Fine needle aspiration cytology from the lump was hemorrhagic with few clusters of spindly shaped atypical cells with pleomorphic nuclei. These smears were reported as positive for malignancy and differential diagnosis of phyllodes neoplasm possibly malignant, metaplastic carcinoma, and primary sarcoma was considered. In view of presence of infiltration and axillary lymphadenopathy, clinically, the infiltrating ductal carcinoma was considered and later left MRM was performed. Grossly, whole of the breast was replaced by a very hard and brittle mass which was difficult to cut (Fig. 2a). The sections showed a high-grade spindle-shaped osteoid-producing cells along with presence of variable amount of osteoid material (Fig. 2b, c). The case was diagnosed as primary osteosarcoma of breast which was infiltrating the pectoralis muscles. Ten axillary lymph nodes were dissected and all showed presence of reactive lymphoid hyperplasia with no evidence of metastatic tumor deposits. The tumor cells showed diffuse positivity for vimentin and osteopontin and were negative for cytokeratin (Fig. 3). The post op period was unremarkable. Due to the aggressive tumor biology, in spite of all multimodality treatment, she developed local recurrence in form of multiple skin nodules 1 year after the treatment, and few months later succumbed to the disease.
Fig. 2.
a Grossly, whole of the breast was replaced by a very hard and brittle mass which was difficult to cut. b A high-grade spindle-shaped cells (hematoxylin and eosin stain; × 400). c Tumor cells producing variable amount of osteoid material (hematoxylin and eosin stain; × 200)
Fig. 3.
Immunohistochemically, the tumor cells showed diffuse cytoplasmic positivity for vimentin as well as osteopontin and are negative for cytokeratin
Case 3
A 35-year-old female with a history of breast lump excision followed by three cycles of chemotherapy presented with a recurrent painless progressive lump in the right breast which was 7 × 5 cm. Being from a rural background, previous reports were not available. USG revealed a lobulated hypoechoic mass with heterogeneous texture and irregular outline along with presence of areas of necrosis. FNAC smears were positive for atypical cells. Core needle biopsy showed a malignant epithelial component along with sarcomatous component. Modified radical mastectomy was planned which histopathologically displayed a neoplasm with sarcomatous component and large areas of necrosis (Fig. 4). On immunohistochemistry, the sarcomatous component co expressed both cytokeratin and vimentin (Fig. 4). Tumor cells were negative for estrogen receptor, progesterone receptor as well as Her2 neu. Hence, the patient was finally diagnosed as a case of metaplastic carcinoma breast. There was absence of any nodal metastasis. The patient is presently in follow-up and free of any recurrences.
Fig. 4.
A neoplasm with a sarcomatous pattern and large areas of necrosis (hematoxylin and eosin stain; × 200) with tumor cells co expressing b vimentin and c cytokeratin
Case 4
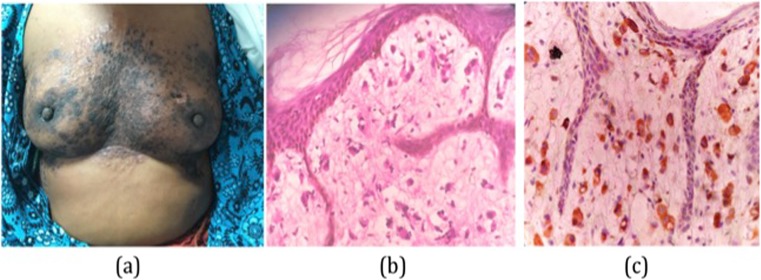
A 40-year-old female presented with papular, scaly, and confluent lesions over bilateral breast for 6 months which was also associated with swelling of right arm (Fig. 5). The skin over bilateral breast was thickened and breast volume was also decreased. Initially, the patient was evaluated for dermatological condition where excision skin biopsy was done from bilateral breast. No underlying breast lump was detected clinically or radiologically. The section showed infiltration of underlying dermis predominantly by singly scattered population of atypical cells. These atypical cells show high nucleocytoplasmic ratio, moderately pleomorphic, hyper chromatic nuclei, coarse chromatin, conspicuous nucleoli, and moderate amount of cytoplasm; the surrounding stroma showed myxoid changes. Overlying skin was free of any tumor infiltration (Fig. 5). These atypical cells were immunohistochemically negative for estrogen receptor and progesterone receptor, however showed membranous positivity for Her2 neu thereby confirming a mammary origin (Fig. 5). Leucocyte common antigen was also performed to rule out cutaneous lymphoma, which was not expressed on tumor cells. Hence, with all these features, the lesions were finally diagnosed as carcinoma en cuirasse. The patient was then referred to the Oncology Department for chemotherapy.
Fig. 5.
a Papular, scaly, and confluent lesions over bilateral breast. b Infiltration of underlying dermis predominantly by singly scattered population of atypical cells, with nucleocytoplasmic ratio, moderately pleomorphic, hyper chromatic nuclei, coarse chromatin, conspicuous nucleoli, and moderate amount of cytoplasm (hematoxylin and eosin stain; × 400). c Tumor cells showing diffuse immunohistochemical positivity for Her2 neu
Case 5
A 50-year-old female presented with a 5-month history of painless lump in the right breast. There was no skin edema, retraction, or nipple discharge. The lesion was associated with axillary lymphadenopathy. Mammography and ultrasonography were suggestive of breast cancer with nodal metastasis. Fine needle aspiration revealed a singly scattered population of medium to large size round cells with high n/c, dispersed chromatin, inconspicuous nucleoli, and scant cytoplasm (Fig. 6). Core needle biopsy was also performed which showed infiltration by similar types of atypical cells (Fig. 6). On immunohistochemical analysis, these cells showed diffuse cytoplasmic positivity for leucocyte common antigen, CD20, bcl-2, and CD79a (Fig. 6). The proliferation index was 75%. Hence, a final diagnosis of primary non-Hodgkin’s lymphoma (diffuse large B cell type) was made.
Fig. 6.
a Fine needle aspiration displaying singly scattered population of medium to large size round cells with high n/c, dispersed chromatin, conspicuous nucleoli, and scant cytoplasm (Giemsa stain; × 400). b Core needle biopsy displaying infiltration by sheets of similar types of atypical cells (hematoxylin and eosin stain; × 400). c On immunohistochemical analysis, these cells showed diffuse cytoplasmic positivity for CD20
Case 6
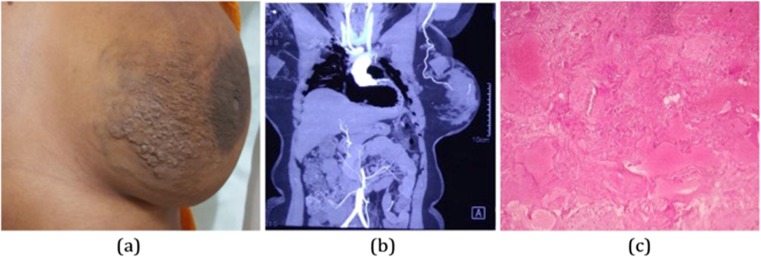
A 35-year-old lady presented with a left side breast skin irregularity, pain, and fullness of 1 year duration. On examination, there was tortuosity in subcutaneous venous channels along with absence of distinct lump or axillary lymphadenopathy (Fig. 7). Ultrasound showed multiple anechoic channels scattered diffusely throughout the breast with color flow noted and spectral pattern showed both arterial and venous pattern within channels. CT angiography was done which confirmed arteriovascular malformation (Fig. 7). Simple mastectomy was planned in view of large size of the lesion. Grossly, a large reddish brown area measuring 11 × 8.5 × 7 cm was identified at a distance of 2 cm from the overlying skin. Microscopy revealed arteries and veins of variable caliber (Fig. 7). Hence, the lesion was histologically confirmed as arteriovenous malformation breast. The patient is in follow-up for one and half year without any recurrence.
Fig. 7.
a Tortuosity in subcutaneous venous channels. b CT angiography displaying vascular malformation. c Microscopy revealing arteries and veins of variable caliber
Discussion
Breast lesions are the leading cause of morbidity and mortality among women worldwide with few cases being reported in males [5, 6]. Due to lack of breast screening practice in developing nations, patients present with palpable lumps. However, there are more benign breast lesions than the malignant breast lesions [3, 4, 6]. In the malignant category, there are more than dozen variants which are less common but still very well defined by the World Health Organization (WHO) classification [7]. They comprises less than 10% of the breast tumors. They have diverse biological behavior. So it is important to know their main characteristics in order to make the best treatment choice and foresee prognosis [5, 8]. The same is true for unusual lesion in benign and intermediate category. Hence, below, we describe the diagnostic entities encountered in our setting with special emphasis on various diagnostic and clinical challenges faced along with management issues.
Fibromatosis/desmoid tumor breast
Fibromatosis, also known as desmoid tumor of breast, is an extremely rare entity, constituting < 0.2% of breast tumor and is characterized by clonal proliferation of fibroblasts [9]. Some have suggested that this entity arises de novo from within the breast parenchyma itself while others have suggested that it arises de novo from the aponeurosis overlying the pectoralis major muscle [10]. It is not known to have a metastatic potential, but finite recognition of this entity is mandatory to avoid local recurrence due to inadequate excision [11]. Clinically, it may mimic malignancy due to presence of nipple retraction at times or due to rapid aggressive growth in an infiltrative manner. Radiological evaluation also raises a suspicion because fibromatosis is known to have irregular shape and speculated margins and is often radiologically categorized as BIRADS-4 [12]. Both on fine needle aspiration cytology as well as core needle biopsy possibility of phyllodes and metaplastic carcinoma may not be ruled out. Thus, a proper diagnosis is very important as fibromatosis is known to recur and hence require wide local excision/tamoxifen. It may histologically resemble certain other benign mesenchymal lesions like nodular fasciitis, myofibroblastic tumor, etc. and that is where the role of IHC comes [12]. Table 1 shows the characteristic markers to differentiate these conditions.
On IHC, both phyllodes and fibromatosis have a similar profile but phyllodes will also have interspersed benign ductal component which will be missing in fibromatosis. However, there may still be difficulty in arriving at a diagnosis in a core needle biopsy due to limited material. Fortunately, wide local excision is the treatment of choice of both these entities. However, differentiating it from malignancy is in fact very crucial, to prevent an overtreatment in the patients.
-
(b)
Primary osteosarcoma breast
Primary osteosarcoma of breast is an infrequent and highly aggressive neoplasm of breast and constitutes < 1% of primary breast malignancies such that only ~ 150 cases have been described in the last 60 decades [13, 14]. It arises either from totipotent mesenchymal cells of breast stroma or as a transformation from pre-existing fibroadenoma or phyllodes tumor [15]. The mean age of breast osteosarcoma is more than the conventional skeletal osteosarcomas [16, 17]. Proper clinical features and treatment protocol are not clear due to rarity of this entity. Usually, any sarcoma is treated with wide local excision with negative margins; however, for breast osteosarcoma, some authors have reported that the optimal treatment should be mastectomy as wide local excisions seem to be complicated by recurrences and documented positive margins after WLE [15, 17]. Breast osteosarcoma has a propensity for hematogenous spread instead of lymphatic spread. Axillary lymphadenopathy may be present but usually turns out to be reactive as seen in our case, and in such scenario, axillary lymph node dissection can be considered unnecessary [15]. However, when present, misleads the clinician/surgeons for the possibility of primary sarcoma breast, infiltrating duct carcinoma, or sarcomatoid carcinoma which often require lymph node dissection for adequate management. Osteosarcoma breast can arise from pre-existing benign lesions like fibroadenoma and benign phyllodes as seen in our case which developed in a background of phyllodes. Histomorphologically, osteoid metaplasia can be seen in metaplastic carcinoma, phyllodes, and fibroadenoma. However, proper history, histopathological evaluation, along with certain immunohistochemical markers can help in differentiating these entities as shown in Table 2. It is very important to differentiate these entities as the management is quite different. Phyllodes neoplasm is surgically managed by wide local excision where metaplastic carcinoma is treated as per the treatment protocol for infiltrating duct carcinoma which includes surgery with or without chemotherapy. On the other hand for osteosarcoma, mastectomy appears to be the most adapted primary treatment and the use of an adjuvant therapy still requires further investigations.
-
(c)
Metaplastic carcinoma breast
Table 2.
Differentiating features between phyllodes, metaplastic carcinoma, and primary osteosarcoma
| Phyllodes neoplasm | Metaplastic carcinoma | Primary osteosarcoma | |
|---|---|---|---|
| History | May be long or short | Short history | Short history with rapid increase in size. |
| Histopathology | - Benign epithelial component with hypercellularity in stromal compartment which is more around the ducts | Mesenchymal transformation of malignant epithelial component. | Malignant mesenchymal component with prominent osteoid formation around atypical cells |
| - Metaplastic changes may be present | |||
| Immunohistochemistry | P63 positive in myoepithelial cells | Both cytokeratin and vimentin positive | Vimentin positive |
| Cytokeratin negative | |||
| Osteopontin positive |
MCB represents 0.25–1% of all breast cancers diagnosed annually [18, 19]. The management as well as prognosis of this entity is largely unknown. As compared to IDC, they are larger, more aggressive, of higher grade with less hormone receptor positivity, less involvement of regional lymph node but higher potential of hematogenous spread as well as increased risk of tumor recurrence and hence worst overall prognosis [20, 21]. Mammographic and sonographic findings of MCB can be similar to IDC and benign lesions like fibromatosis. Histopathological mimickers of MCB include myoepithelial carcinoma breast, phyllodes, primary breast sarcoma, nodular fasciitis, and fibromatosis [22]. It is important to differentiate it from phyllodes, fibromatosis, and nodular fasciitis as the treatment in these entities is isomer conservative as compared to that of MCB which require mastectomy with lymph node dissection with or without chemo- or radiotherapy.
-
(d)
Carcinoma en cuirasse
Carcinoma en cuirasse is a form of metastatic cutaneous carcinoma characterized by diffuse sclerodermoid induration of the skin which usually appears in cases of local recurrence after mastectomy. This form of cutaneous metastasis is rarely found to be associated with adenocarcinomas of the lung, kidney, or gastrointestinal tract [23]. Its incidence varies from 0.7–9% and is characterized by dissemination of carcinoma cells along tissue spaces or through lymphatic [24]. It is extremely rare to see it as the first feature of carcinoma breast [23]. Our case is rare in the context that the first presenting feature of our patient was the cutaneous metastasis, which was initially diagnosed clinically as some dermatological condition. Skin metastasis when present as the initial symptoms may be confused as some dermatological conditions, as in our case, like erysipelas and herpes zoster eruption or a cutaneous lymphoma [23]. In breast carcinoma with skin metastasis presents as advanced tumor and shows very poor prognosis; hence, skin duration of long duration (months to years) is to be thoroughly investigated particularly in elderly patients. As a result of induration of the skin and subcutaneous tissue of the affected mammary gland reliable and thorough deep palpation may not be possible; hence, it should be accompanied by radiological examination for underlying breast lump. IHC phenotype specific to the tumors originating from the mammary tissue can be confirmed by using Her2 neu. Not much data are available on treating BC metastases to the skin; therefore, treatment options used in managing all types of BC metastases are preferred. Surgical methods are usually avoided as CMs are frequently a sign of an advanced process [23]. Miltefosine, a topical cytostatic, was employed in the treatment of patients with various forms of CMs with minor results [25]. No particular drug is advised for chemotherapy of CMs exclusively. Hence, clinicians should perform skin biopsy as it is an easily obtainable source of material for histologic verification and other assessments (e.g., IHC, PCR), thereby helpful in establishing the diagnosis and selecting proper treatment modality. Early and accurate recognition allows treating the spread of the cancer at the earliest possible stage to improve survival [23].
-
(e)
Primary breast lymphoma
PBL is defined as a malignant lymphoma primarily occurring in the breast in absence of previously detected lymphoma localization. It is a very rare disease and accounts for 0.04–0.5% of all breast malignancies [26]. Majority (80%) are B cells with most common histological diagnosis being diffuse large B cell lymphoma [27]. Fine needle aspiration, core biopsy, and excision biopsy are effective techniques in its diagnosis; however, sometimes, immunohistochemical and genetic studies may be necessary for establishing the diagnosis. Usually, cytomorphological features are clear cut but rarely may be confused with other small round cell tumor like PNET/Ewing’s breast and small cell carcinoma breast which again are extremely rare at this location. LCA, CD99, and synaptophysin can be effectively used to differentiate them. On cases of breast lymphoma, mastectomy is not recommended because it offers no benefit as regards to survival or recurrence. The most common therapy used is CHOP therapy as used in our case as well. It is important to differentiate between lymphoma and other SRCT that may occur at this location due to significant difference in their treatment plan as well as chemotherapeutic agents used.
To conclude, infiltrating duct carcinoma and fibroadenoma are the most common breast lesions presenting as lump, constituting more than two thirds of breast pathology reporting, but clinician, pathologists, and surgeons may encounter many unusual entities which should be diagnosed with caution since the management may vary, thereby effecting the overall survival and prognosis of the patients. Histopathology has been a gold standard for the diagnosis and predicting the prognosis. In this article, we described several rare and unusual lesion of breast which may have varied differentials and different prognosis. Using information gained from this study pathologist, clinician and surgeons will be able to diagnose and manage such patients in a better way.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Chanchal Rana, Phone: +91-9451250305, Email: Chanchal11aug@yahoo.com.
Pooja Ramakant, Email: poojaramakant@rediffmail.com.
References
- 1.National Cancer Registry Program. Ten year consolidated report of the hospital based cancer registries, 1984–1993, an assessment of the burden and care of cancer patients. New Delhi: Indian Council of Medical Research; 2001
- 2.Agarwal G, Ramakant P. Breast cancer care in India: the current scenario and the challenges for the future. Breast Care (Basel) 2008;3(1):21–27. doi: 10.1159/000115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai M. Role of obstetrician and gynecologist in management of breast lump. J Obstet Gynaecol India. 2003;53:389–391. [Google Scholar]
- 4.Sharkey FE, Craig Allred DC, Valente PT. Breast. In: Damjanov I, Linder J, (eds.) Anderson’s pathology. 10th
- 5.Kanthikar SN, Suryawanshi KH, Jagtap SV, Dravid NV, Gondane SR. Histopathological spectrum of unusual breast lesions: a seven year retrospective. Review. Indian J Pathol Oncol. 2016;3(3):456–462. doi: 10.5958/2394-6792.2016.00086.7. [DOI] [Google Scholar]
- 6.Tikku G, Umap P. Comparative study of CNB and FNAC in palpable breast lumps: scenario in developing nations. Turkish J Pathol. 2016;32(1):1–7. doi: 10.5146/tjpath.2015.01335. [DOI] [PubMed] [Google Scholar]
- 7.Yerushalmi R, Hayes MM, Gelmon KA. Breast carcinoma-rare types: review of the literature.2009: 20; 1763–1770 [DOI] [PubMed]
- 8.Teponavicience L, Gudavicience D, Meskauskas R. Rare types of breast carcinoma. ActaMedicaLituanica. 2012;19(2):81–91. [Google Scholar]
- 9.Erguvan-Dogan B, Dempsey PJ, Ayyar G, Gilcrease MZ. Primary desmoid tumor (extraabdominal fibromatosis) of the breast. AJR Am J Roentgenol2005; 185(2): 488–489 [DOI] [PubMed]
- 10.Ashoor A, Monti S, Pezzella M. Fibromatosis, a benign breast disease mimicking carcinoma. A case report. Int J Sur case report. 2017;41:392–397. doi: 10.1016/j.ijscr.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat D, Wear V, Weisenberg E, Alvarado R. Desmoid-type fibromatosis of the breast: a case report. Breast Disease. 2016;36:149–152. doi: 10.3233/BD-160227. [DOI] [PubMed] [Google Scholar]
- 12.Grimaldi MC, Trentin C, Gullo RL, Cassano E. Fibromatosis of the breast mimicking cancer: a case report. Radiol Case Rep. 2018;13:1–5. doi: 10.1016/j.radcr.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahrami A, Resetkova E, Ro JY, Ibanez JD, Ayala AG. Primary osteosarcoma of the breast: report of 2 cases. Arch Pathol Lab Med. 2007;131:7925. doi: 10.5858/2007-131-792-POOTBR. [DOI] [PubMed] [Google Scholar]
- 14.Silver SA, Tavassoli FA. Primary osteo genic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol. 1998;22:92533. doi: 10.1097/00000478-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Crevecoeur J, Jossa V, Gennigens C, Parmentier JC, Crevecoeu A. Primary osteosarcoma of the breast: a case report. Clinical Case Reports. 2016;4(1):62–66. doi: 10.1002/ccr3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argus A, Halton L. Case of the season: osteosarcoma of the breast. Semin Roentgenol. 2011;46:4–6. doi: 10.1053/j.ro.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Murakami S, Isozaki H, Shou T, Sakai K, Yamamoto Y, Oomori M, Toyoda H. Primary osteosarcoma of the breast. Pathol Int. 2009;59:111–115. doi: 10.1111/j.1440-1827.2008.02338.x. [DOI] [PubMed] [Google Scholar]
- 18.Leddy R, Irshad A, Rumboldt T, Cluver A, Campbell A, Ackerman S. Review of metaplastic carcinoma of the breast: imaging findings and pathological features. J Clin Imaging Sci. 2012;2(1):21. 6. doi: 10.4103/2156-7514.95435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberman HA. Metaplastic carcinoma of the breast: a clinicopathologic study of 29 patients. Am J Surg Pathol. 1987;11(12):918–929. doi: 10.1097/00000478-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Lai HW, Tseng LM, Chang TW, Kuo YL, Hsieh CM, Chen ST, Kuo SJ, Su CC, Chen DR. The prognostic significance of metaplastic carcinoma of the breast (MCB)—a case controlled comparison study with infiltrating ductal carcinoma. Breast. 2013;22(5):968–973. doi: 10.1016/j.breast.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Shah DR, Tseng WH, Martinez SR Treatment options for metaplastic breast cancer. ISRN Oncol 2012; 70:61–62 [DOI] [PMC free article] [PubMed]
- 22.McKinnon E, Xiao P. Metaplastic carcinoma of the breast. Arch Pathol Lab Med. 2015;139:819–822. doi: 10.5858/arpa.2013-0358-RS. [DOI] [PubMed] [Google Scholar]
- 23.Adam Reich A, Samotji D, Szczech J, Wozniak Z, Szepietowski J. Carcinoma en cuirasses’ an initial manifestation of in mammary breast cancer. Advances in Dermatology and Allergology 2, April / 20
- 24.Oliveira GM, Zachetti DBC, Barros HR, Tiengo A, Romiti A. Breast carcinoma en Cuirasse—case report. An Bras Dermatol. 2013;88(4):608–610. doi: 10.1590/abd1806-4841.20131926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clive S, Gardiner J, Leonard RC. Miltefosine as a topical treatment for cutaneous metastases in breast carcinoma. Cancer Chemother Pharmacol 1999; 44 Suppl.: S29–S30 [DOI] [PubMed]
- 26.Jinming X, Qi Z, Xiaoming Z, Jianming T. Primary non-Hodgkin’s lymphoma of the breast: mammography, ultrasound, MRI and pathologic findings. Future Oncol. 2012;8(1):105–109. doi: 10.2217/fon.11.132. [DOI] [PubMed] [Google Scholar]
- 27.Mouna B, Saber B, Tijani EH, Hind M, Amina T, Hassan E. Primary malignant non-Hodgkin’s lymphoma of the breast: a study of seven cases and literature review. World J Surg Oncol. 2012;10:151. doi: 10.1186/1477-7819-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]