Abstract
Breast cancer in India appears to be diagnosed more in the young women, but whether this is an actual higher incidence, and hence a matter of concern, needs more clarity, and the extent to which correctable measures can be taken to reduce or overcome this additional disease burden, if any, has to be better understood. The article analyzes these and more in a systematic manner and highlights the important issues in the very young women which makes the clinical management of breast cancer more complex.
Keywords: Breast cancer, Young women, India
Breast cancer in India appears to be diagnosed more in the young women, but whether this is an actual higher incidence, and hence a matter of concern, needs more clarity, and the extent to which correctable measures can be taken to reduce or overcome this additional disease burden, if any, has to be better understood. Cancer in the young women has major fallout as it afflicts them while they are still in the prime of their life and are caring for young families and some are also professionally engaged. The implications are huge and it affects not just the woman herself personally, financially, and socially but also the whole family and society at large
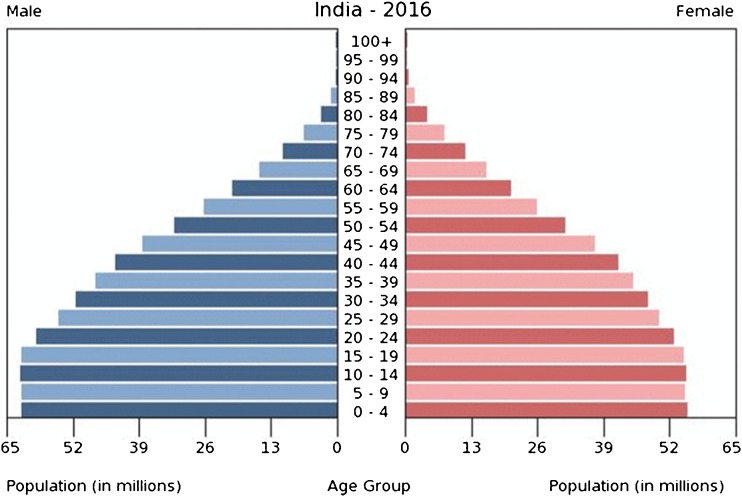
There is an apparent higher incidence of breast cancer noted in younger women in India as compared to the west. The median age of presentation at diagnosis is 49 years in India as against 62 in the western population. This is a relative figure as the population is made up of mostly younger women (nearly 75% below age 50, Fig. 1), and this unequal population age distribution with predominance of younger population in India as compared to the west (https://en.wikipedia.org/wiki/Demographics_of_India) tilts the balance to the left. Improvement in education and awareness towards cancer in this age group also is contributory. There is, however, no difference in age-specific incidence between India and the rest of the world.
Fig. 1.
Population pyramid illustrating the age and sex structure of India showing preponderance of younger women. Source: https://www.indexmundi.com/india/age_structure.html
As far as stage of breast cancer at presentation, nearly half the cancers across India are later stages or locally advanced at time of diagnosis. As cancer is not a national health problem (as against communicable diseases), there is no cancer screening program in India and awareness and health education plays an important role in early detection of breast cancer. As far as the total cancer burden in India is concerned, there is a prediction by the International Agency for Research in Cancer (IARC) in Lyon (GLOBOCAN project) [1], France, stating that in India, there is an expected increase of breast cancer incidence from the one million cases in 2012 to nearly 1.7 million in the next 15 years. Also, the deaths due to any cancer will nearly double from 7 million estimated in 2012 to 12 million in 2035 with the current cancer care scenario indicating a need for improving awareness and encouraging early detection, and priority need to improve breast cancer care across the country.
Standard screening modality of mammography has failed as a tool for early detection of breast cancer in young women below age of 50 and resulted in more harms than the small benefit observed in lowering mortality in these young women. Hence, the US preventive task force does not recommend routine screening for breast cancer in women aged 40–49 years [2] unless qualifying as a high risk, where additional MRI may be required to improve detection. Breast self-examination randomized trials [3] did not succeed in lowering mortality due to breast cancer due to its various limitations and poor compliance. The randomized trial of clinical breast examination [4] has been able to detect more cancers in the screened arm but yet to show a benefit of reducing mortality from breast cancer. The fact however remains that early detection of cancer in lower stages results in better survival and improving awareness helps in earlier diagnosis and treatment, and helps in achieving this objective.
The population-based registry in Mumbai (Mumbai Cancer Registry) reported time-trends in breast cancer from 1976 to 2005. Classifying the women as “younger” if 25–49 and “older” if 50–74, and using models to predict age-adjusted rates and number of breast cancer cases by 2025, the estimated annual percent change (EAPC) in incidence for older women over the next 3 decades was higher at 1.6% (95% CI 1.1–2.0) while it was lesser but still significant at 1% (95% CI 0.2–1.8) in younger women [5].
The changing landscapes over the regional variations in breast cancer risk in India do seem to indicate at how things may evolve in years to come. The vast difference in the incidence and risk of developing breast cancer between rural and urban areas itself is a warning that changing lifestyles and reproductive patterns in the urbanized society is increasing the risks for development of breast cancer [6]. The life time risk of breast cancer in rural registries is 1 in 60, while in the urban areas such as Mumbai, the life time risk for developing breast cancer is 1 in 28. Unless awareness on the risk factors is improved, the incidence will continue to rise and we could reach abreast to the risks seen in developed countries. This is still far lower than the risk for developing breast cancer seen in women living in the US and UK where the life time risk is 1 in 8 women.
Cancers in young women tend to be more aggressive, of higher grade, with rapid proliferation, and larger tumors, with a predominant triple-negative biology [7]. All these indicate a poorer prognosis. Young age has been reported in India as well to correlate with relatively poorer outcome disease [8–11]. Surprisingly, age had no impact on outcome in our patients with early cancer in a recent retrospective multivariate analysis of hospital-based data. It appears that outcome is inferior with later stage at presentation, higher grade, positive lymph node status and adverse biology of tumors, and features commonly seen in the younger women (unpublished data, under consideration for publication by the Indian Journal of Cancer).
Chemotherapy is also, thus, more frequently required in younger women, and due to its suppressive effect on ovarian function, it also has major implications on fertility preservation in this age group. With increasing age at marriage and delayed childbearing, many premenopausal women may not have had started family when they are diagnosed with breast cancer. Delayed childbirth itself is also a risk factor for onset of breast cancer. Detailed evaluation of fertility status, and having a fertility expert as part of the multidisciplinary disease management group aids in proper assessment, counseling, and appropriate fertility preservation advice for individual patient.
Standard methods of fertility preservation, like oocyte preservation or embryo preservation, are being offered before commencing chemotherapy, with reasonably encouraging results in those who subsequently avail of the conception procedure at the end of their cancer treatment [12], with cost being the highest limiting factor. It is important to understand the costs involved and actual success or failure of such procedures before offering them as standard routine. An interesting recent cross-sectional survey [13] in women and men (above 18 years of age) who had preserved gonadal tissue at start of their cancer treatment showed that there is a 58% chance of natural conception, very few women (13%) and men (22%) actually availed of the preserved tissue for attempting conception, and the procedure was more successful in men than in women (80 vs 18%). However, this was a survey wherein the respondents were only 302 of 870 who had availed of the facility of gonadal preservation. This indicates a need for a more focused prospective study to understand the success of such procedures keeping in mind the associated high costs and social implications.
Chemotherapy-induced amenorrhea is known to occur in premenopausal women on treatment. Conservation of fertility during chemotherapy is therefore very important in young women who have been advised neo-adjuvant or adjuvant chemotherapy, and have previously had either no children or are wishing to conceive again after completion of breast cancer treatment. This was reported in a randomized trial in 257 premenopausal eligible women, aptly called Preservation of Ovarian Function study (POEMS study) [14], wherein concurrent use of gonadotropin-releasing hormone analogs (GnRHa) along with chemotherapy versus chemotherapy alone, in hormone receptor-negative women, showed a benefit with increased resumption of ovarian function, better pregnancy rates, along with a significant DFS (p = 0.04) and OS (p = 0.05) benefit. Among 135 women with primary end-point data, there was a lower ovarian failure rate with goserelin plus chemotherapy use than chemotherapy alone, 8% vs. 22%, OR 0.30; 95% CI, 0.09–0.97, (2-sided p = 0.04). Pregnancy occurred significantly more in women in the goserelin group than in chemotherapy-alone group (21% vs. 11%, p = 0.03).
A recently completed pooled analysis of five randomized trials [15] investigating temporary ovarian suppression with GnRHa during chemotherapy as a strategy to preserve ovarian function and fertility in 873 premenopausal early breast cancer patients (in both HR negative and some HR positive) showed a significant reduction in risk of chemotherapy-induced premature ovarian insufficiency rate (14.1% vs 30.9%, OR 0.38, 95% CI 0.26–0.57, p < 0.001), with a greater number of women in GnRHa group reporting successful conception (10.3% vs 5.5%, IRR 1.83, 95% CI 1.06–3.15, p = 0.03). Thus, there appears to be a strong case for use of GnRHa concurrent with chemotherapy in preservation of ovarian function in young premenopausal women wishing for fertility, irrespective of hormone receptor status. The use, however, did not impact on DFS or OS in these women in the meta-analysis.
If a diagnosis of triple-negative breast cancer (TNBC) is established, a waiting period of 2 years (which is the duration of high early relapse risk) is advised post completion of adjuvant therapy before contemplating conception. In the subset of women who have hormone receptor (HR)-positive disease, the story is very different. Although they are considered to carry a lower relapse risk and relatively better outcome as compared to HR-negative breast cancer, pregnancy is either not feasible in presence of ongoing hormonal treatment such as tamoxifen plus ovarian suppression for 5 years (based on the combined results of SOFT plus TEXT trials of IBCSG) [16] or is contraindicated (when treated with tamoxifen alone) whereby the duration of treatment with tamoxifen in ER-positive breast cancer in premenopausal women may be continued for a total of 10 years based on the results of ATLAS trial [17].
The effect of tamoxifen on fertility preservation is also unclear and needs to be explored more in details. Proposed POSITIVE study [18] with BIG group will answer the question whether an intentional break in hormonal therapy with pregnancy being permitted after a drug-free washout period of 3 months, and delayed restarting hormones after successful completion of pregnancy has any detrimental effect on the cancer outcomes. Hormone receptor-expressing cancers are relatively better outcome disease and thus any harm by discontinuation of hormonal therapy will not be acceptable.
To sum up, breast cancer in young women has significant implications on quality of life, poses major challenges in detection, treatment, and is associated with relatively poorer outcome. Addressing all these issues is a priority to ensure overall better survival with a satisfactory quality of life.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 11. Lyon: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Siu AL, U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94:1445–1457. doi: 10.1093/jnci/94.19.1445. [DOI] [PubMed] [Google Scholar]
- 4.Mittra I, Mishra GA, Singh S, Aranke S, Notani P, Badwe R, Miller AB, Daniel EE, Gupta S, Uplap P, Thakur MH, Ramani S, Kerkar R, Ganesh B, Shastri SS. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. Int J Cancer. 2010;126(4):976–984. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon PK, Yeole BB, Dikshit R, Kurkure AP, Bray F. Trends in breast, ovarian and cervical cancer incidence in Mumbai, India over a 30-year period, 1976–2005: an age–period–cohort Analysis. British Journal of Cancer. 2011;105(5):723–730. doi: 10.1038/bjc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohandas K Mallath, David G Taylor, Rajendra A Badwe, et al.. Cancer burden and health systems in India 1 - the growing burden of cancer in India: epidemiology and social context. Published online April 11, 2014 10.1016/S1470-2045(14)70115-9www.thelancet.com/oncology
- 7.Lee H-B, Han W. Unique features of young age breast cancer and its management. J Breast Cancer. 2014;17(4):301–307. doi: 10.4048/jbc.2014.17.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma D, Singh G. Breast cancer in young women: a retrospective study from tertiary care center of north India. South Asian J Cancer. 2017;6(2):51–53. doi: 10.4103/2278-330X.208859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gogia A, Raina V, Deo SV, Shukla NK, Mohanti BK. Young breast cancer: a single center experience. Indian J Cancer. 2014;51(4):604–608. doi: 10.4103/0019-509X.175332. [DOI] [PubMed] [Google Scholar]
- 10.Mathew A, Pandey M, Rajan B. Do younger women with non-metastatic and non-inflammatory breast carcinoma have poor prognosis? World J Surg Oncol. 2004;2:2. doi: 10.1186/1477-7819-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinshaw KA, Sarin R, Budrukkar AN, Shrivastava SK, Deshpande DD, Chinoy RF, Badwe R, Hawaldar R. Safety and feasibility of breast conserving therapy in Indian women: two decades of experience at Tata Memorial Hospital. J Surg Oncol. 2006;94:105–113. doi: 10.1002/jso.20497. [DOI] [PubMed] [Google Scholar]
- 12.Martinez M, Rabadan S, Domingo J, Cobo A, Pellicer A, Garcia-Velasco JA. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod BioMed Online. 2014;29(6):722–728. doi: 10.1016/j.rbmo.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Hammarberg K, Kirkman M, Stern C, McLachlan RI, Clarke G, Agresta F, Gook D, Rombauts L, Vollenhoven B, Fisher JRW. Survey of reproductive experiences and outcomes of cancer survivors who stored reproductive material before treatment. Hum Reprod. 2017;32(12):2423–2430. doi: 10.1093/humrep/dex314. [DOI] [PubMed] [Google Scholar]
- 14.Moore HCF, Unger JM, Phillips K-A, for the POEMS/S0230 Investigators et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923–932. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matteo Lambertini, Halle C.F. Moore, Robert C.F. Leonard, et al. Pooled analysis of five randomized trials investigating temporary ovarian suppression with gonadotrophin-releasing hormone analogs during chemotherapy as a strategy to preserve ovarian function and fertility in premenopausal early breast cancer patients. San Antonio Breast Cancer Symposium, 2017
- 16.Saha P, Regan MM, Pagani O, Francis PA, Walley BA, Ribi K, Bernhard J, Luo W, Gómez HL, Burstein HJ, Parmar V, Torres R, Stewart J, Bellet M, Perelló A, Dane F, Moreira A, Vorobiof D, Nottage M, Price KN, Coates AS, Goldhirsch A, Gelber RD, Colleoni M, Fleming GF; SOFT; TEXT Investigators; International Breast Cancer Study Group. Treatment efficacy, adherence, and quality of life among women younger than 35 years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J Clin Oncol. 2017:JCO2016720946. doi: 10.1200/JCO.2016.72.0946. [DOI] [PMC free article] [PubMed]
- 17.Davies C, Pan H, Godwin J, Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):804. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagani O, Ruggeri M, Manunta S, Saunders C, Peccatori F, Cardoso F, Kaufman B, Paluch-Shimon S, Gewefel H, Gallerani E, Abulkhair OM, Pistilli B, Warner E, Saloustros E, Perey L, Zaman K, Rabaglio M, Gelber S, Gelber RD, Goldhirsch A, Korde L, Azim HA, Jr, Partridge AH. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast. 2015;24(3):201–207. doi: 10.1016/j.breast.2015.01.005. [DOI] [PubMed] [Google Scholar]