Abstract
Management of metastatic bone disease is still evolving and is dependent upon many factors including the primary tumour type, expected life expectancy, site and size of lesion, character of the lesion, lytic or blastic, and the number of lesions. Active orthopaedic surgical intervention is usually required at the time of pathological fracture either impending or actual. Management options are either in situ fixation or replacement/arthroplasty. The role of post-operative radiotherapy is still not clearly defined and its biological effect on healing of pathological fracture is unclear. In this report, we describe a case of breast carcinoma with a large metastatic lytic lesion in the proximal femur, which was treated by in situ fixation followed by hormonal therapy and external beam radiotherapy. In the post-operative period, rapid and dramatic ossification and reformation of the proximal femur was observed.
Keywords: Breast cancer, Femoral metastasis, Intramedullary nailing
Introduction
After the spine and pelvis, the femur is the most common site of bone metastasis. In females, the breasts and lungs are the most common sites of primary disease, with approximately 80% metastatic bone tumours arising in these locations [1]. In metastatic lesions of the proximal femur, surgical treatment is recommended for all impending or pathological fractures at this site, except in bedridden patients with a life expectancy of less than a few months. Mirels proposed a scoring system to help in decision-making regarding prophylactic fixation of bone metastases [2]. Fixation is recommended for scores above 8. The pertrochantric region being under enormous stress is especially prone to fracture and is attributed a score of 3. The goals of treatment are pain relief, restoration of mobility and facilitation of nursing care [3]. Locked intramedullary nailing is the preferred mode of treatment for pathological pertrochantric fractures. However, the management of pertrochanteric lesions with extensive osteolysis is controversial with proponents of both fixation and prosthetic replacement. We report a case of adenocarcinoma breast with solitary pertrochantric metastasis with extensive bone loss and pathological fracture in a 28-year-old female which was managed by locked cephalomedullary nail followed by radiotherapy and hormonal therapy. Remarkable bone formation with re-establishment of trochanters was seen on sequential X-rays over a span of 6 months.
Case Report
A 28-year-old female with a history of adenocarcinoma breast presented with progressively increasing pain in the right hip region. She was operated 2 years back for adenocarcinoma breast with MRM (modified radical mastectomy), followed by 8 cycles of chemotherapy and was on a maintenance dose of Tamoxifen. At presentation, the hip pain was moderate to severe in intensity, dull aching in nature, non-radiating and was aggravated by movements of the hip. Pain was also present at rest and weight bearing was not possible. On examination, the right lower limb was externally rotated with true shortening of approximately 2 cm localised to the supra-trochantric region. There was no distal neurovascular deficit. A firm swelling was present in the right trochanteric region which was tender, with normal overlying skin. X-rays of the site revealed a large osteolytic lesion in the pertrochantric region with pathological fracture (Fig. 1). FNAC of the lesion had been done earlier which confirmed adenocarcinoma breast. A whole-body PET CT was done and it revealed multiple FDG uptake avid lesions in the proximal femur right side, lungs and liver. The case was discussed in a multidisciplinary team meeting involving medical, radiation and surgical oncologist, pathologist and radiologist to formulate a treatment plan. Surgical options were en bloc resection followed by reconstruction using megaprosthesis or an intralesional curettage followed by in situ intramedullary nail followed by hormonal therapy and radiotherapy. The challenge in using intramedullary nail was to manage the large void due to extensive bone loss in the intertrochanteric region. There have been reports of using bone cement to fill such defects. However, considering the increased vascularity of bone metastatic lesions and the enormous amount of bone cement to be used for filling such a large defect, it was thought that there will be a high risk of increased monomer leakage into systemic circulation and resulting toxicity. Inability to achieve adequate margins during resection, increased morbidity after extensive resection, premature failure of megaprosthesis due to loss of abductors and high cost were the possible drawbacks of resection and reconstruction with megaprosthesis. All the treatment options were discussed and keeping in view the poor socioeconomic profile of the patient and guarded prognosis even after an extensive and expensive surgery with megaprosthesis reconstruction, it was decided to do an in situ fixation of the pathological fracture using a cephalomedullary A2FN nail (AO Synthes TM) (Fig. 2). Reaming tissue samples sent for HPE confirmed the metastatic nature of the lesion. Hormonal receptor analysis showed ER618/PR418/Her Neu2 positive. Post-operatively, palliative external beam radiotherapy (EBRT) (30 Gy in 10 fractions) was given. Anastrazole (aromatase inhibitor) was continued in the post-operative period and patient was started on Goserelin acetate (10.8 mg once in 3 months) which is a GnRH agonist. On follow-up of patient, both clinical and radiological improvements were seen (Fig. 3) which continued till the last follow-up without any new symptomatology. There was prolific new bone formation in the earlier lytic lesion eventually reconstituting the shape of a normal proximal femur (Fig. 4). Till the last follow-up at 2 years, patient was walking full weight bearing.
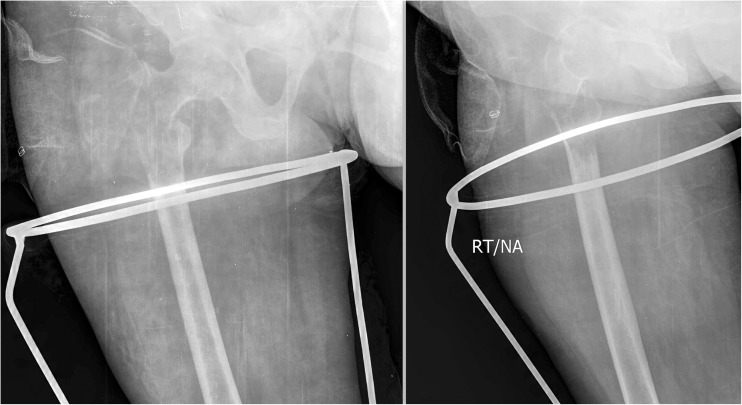
Fig. 1.
Pre-operative X-rays showing a large osteolytic lesion in the proximal femur and pathological fracture in the region
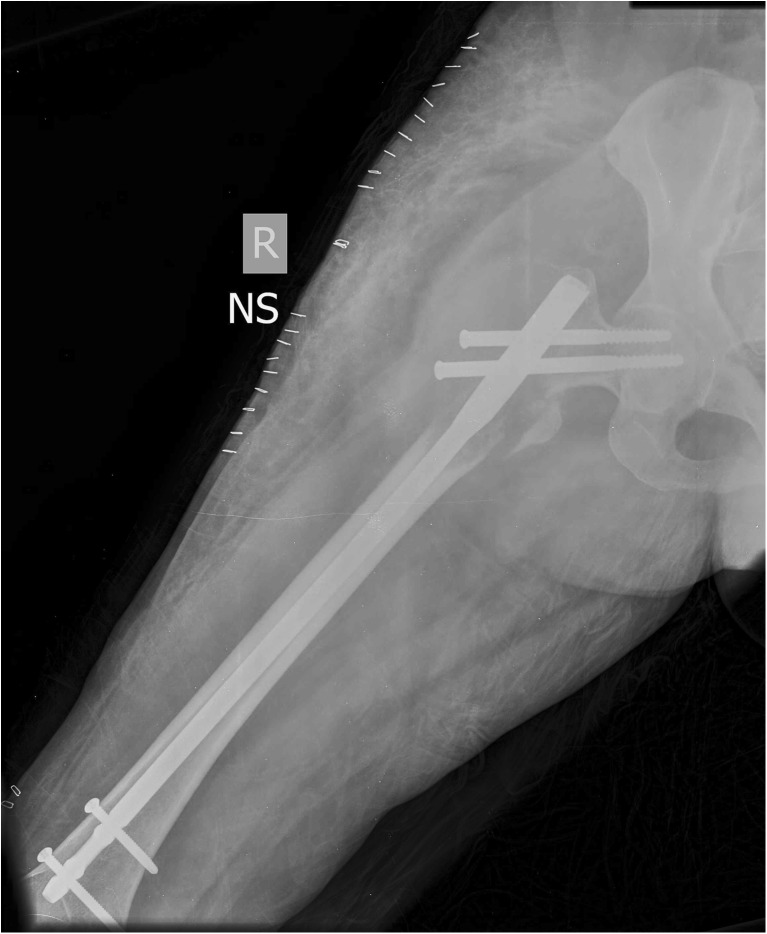
Fig. 2.
Showing the immediate post-operative X-ray after in situ nailing
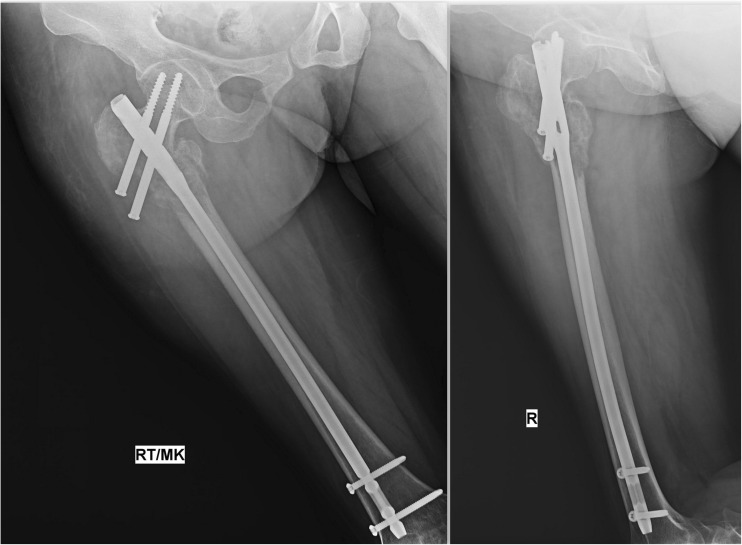
Fig. 3.
X-rays at 2 months post-operative showing re-ossification of the lytic lesion
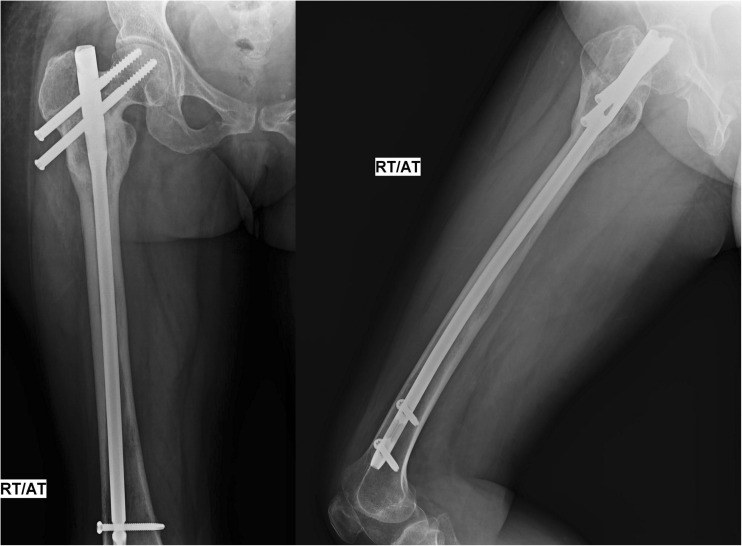
Fig. 4.
Showing extensive re-ossification and reformation of the proximal femur at the 5 months post-operative period
Discussion
The bone is one of the most favoured sites for metastatic disease next only to the lung and liver. Bone metastases can be classified as osteolytic with significant bone destruction, osteoblastic due to excess bone formation or a mixed phenotype can occur. It is estimated that bone metastases are present in approximately 70% of patients who die due to breast carcinoma. Metastases from breast carcinoma are typically lytic, whereas those from prostate are usually sclerotic.
The surgical management of metastatic deposits in the long bones is a source of debate. The surgical options available for pertrochantric lesions may be broadly classified into two categories: endoprosthetic replacement vs reconstruction nails or other osteosynthetic devices with or without the augmentation of cement. There are possible advantages and drawbacks of both the options and the treatment modality needs to be individualised. Complete resection followed by prosthetic reconstruction is traditionally favoured in young adults with good overall prognosis and low risk of recurrence. This procedure offers a chance for complete removal of metastatic tumour and in most cases allows immediate full weight bearing and mobilisation. Cemented components are usually preferred due to poor bone quality and limited potential for bone ingrowth over porous coated uncemented devices especially when post-operative radiation is being contemplated. Revision rates are reported to be quite low owing to limited life span and decreased ambulatory potential of patients. Patients with extensive lytic defects (as in the present case) are not amenable to routine endoprosthetic devices and require the use of megaprosthesis to reconstruct the defect. In addition to being an extensive and expensive surgery with increased blood loss and increased morbidity, megaprosthesis have their own set of complications including aseptic loosening, fatigue fractures of the stems, bushing failure, complications related to attachment of the soft tissues to the prosthesis, increased rate of dislocation, infection and wound necrosis [4].
Intralesional curettage followed by intramedullary nailing is preferred for solitary, less extensive lesions especially in inter-trochantric and subtrochantric region. Intramedullary nails stabilise the entire length of the femur, preserve the native hip joint, are affordable and are associated with fewer perioperative complications. Cement augmentation offers an attractive approach to deal with bone defects and also adds to the construct stability. However, there is an increased rate of implant failure reported with the use of intramedullary devices, which is understandable owing to the limited biological potential of fractures to heal. There are many adverse factors which impede fracture healing in patients with metastatic disease like malnutrition, chronic NSAIDS, chemotherapeutic drugs and steroid intake, pre- or post-operative radiotherapy and limited bone stock due to osteoporosis and tumour itself.
Thus, the exact choice (endoprosthesis vs replacement) depends on many factors: location, amount of bone loss, responsiveness of lesion to systemic therapy, expected life span and general health, solitary/oligometastases (2–4 lesions)/diffuse, and primary tumour type.
We in the present case performed intralesional curettage and intramedullary nailing for a large solitary pertrochantric metastatic lesion from primary adenocarcinoma breast in a young female. She was given adjuvant radiotherapy followed by hormonal therapy. The remarkable bone formation followed by reformation of the trochanters led us to investigate and publish this case. Radiotherapy (RT) has traditionally been known to retard bone healing, with numerous articles highlighting its negative impact on bone remodelling. Based on the same biological principle, radiotherapy is also prescribed for prevention of heterotrophic ossification. In metastatic lesions, radiotherapy may be either given with the intent of pain relief or re-ossification. Both have different protocols: for pain relief, 8 Gy single fraction is recommended, whereas for re-ossification, 30 Gy over 10 fractions is recommended [5].
However, its role on the healing of pathological fractures is still not clearly defined. RT has known to delay fracture healing in normal bone and the same effect has been observed in pathological fractures according to some reports [6]. However, when given in re-ossification dose, RT may enhance fracture healing, as observed in the present case with enormous re-ossification and reformation of trochanters. External beam irradiation produces ossification in 65 to 85% of lytic metastases in unfractured bone. Some of the ossification may occur by heterotopic ossification within the lesion; however, in most cases, there is formation of mature organised bone in the healed lesion, seemingly by direct osteogenesis [7]. Thus, the ultimate effect of RT on healing of pathological fractures is still controversial and unpredictable. The effect of RT on any malignant lesion is determined by a number of factors including the internal milieu/environment, rate of cell proliferation, primary cell type, oxygen concentration, inherent radiosenstivity, cellular metabolism of tumour, percentage of stem cells and presence or absence of tumour inhibitors. It is possible that RT may not have a consistent pattern of response in all pathological fractures and its ultimate result on healing of pathological fractures may be determined by interplay of numerous factors. One possible explanation of extensive reformation and re-ossification observed in the present case may be based on the logic of preferential kill of cells with high proliferation rate. An osteolytic bone metastasis lesion from primary breast malignancy would be having osteoclasts as the predominant cell type with higher mitotic rate. EBRT of such a lesion will thus result in preferential tumour kill of osteoclasts and relative sparing of osteoblasts, clinically resulting in reformation of bone by osteoblasts.
Thus, although there are clear guidelines for giving EBRT in cases of metastatic bone lesions, its role in pathological fractures after fracture fixation is still not defined. Even more debatable is the biological effect of radiation on healing of pathological fractures and the problem is compounded by a lack of data and conflicting reports. The remarkable and unexpected bone formation seen after EBRT in the present case might be an indication of the potential benefits that radiotherapy may offer such patients in the future.
References
- 1.Quattrocchi CC, Piciucchi S, Sammarra M, Santini D, Vincenzi B, Tonini G, Grasso RF, Zobel BB. Bone metastases in breast cancer: higher prevalence of osteosclerotic lesions. Radiol Med (Torino) 2007;112(7):1049–1059. doi: 10.1007/s11547-007-0205-x. [DOI] [PubMed] [Google Scholar]
- 2.Mirels H (1989) Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res (249):256–264 [PubMed]
- 3.Kunec JR, Lewis RJ. Closed intramedullary rodding of pathologic fractures with supplemental cement. Clin Orthop Relat Res. 1984;188:183–186. [PubMed] [Google Scholar]
- 4.Pala E, Trovarelli G, Calabrò T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Relat Res. 2015;473(3):891–899. doi: 10.1007/s11999-014-3699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz S, Lo S, Howell D et al (2011) American College of Radiology ACR Appropriateness Criteria® NON-SPINE BONE METASTASES expert panel on radiation oncology–bone metastases. J Palliat Med [DOI] [PubMed]
- 6.Bonarigo BC, Rubin P. Nonunion of pathologic fracture after radiation therapy. Radiology. 1967;88(5):889–898. doi: 10.1148/88.5.889. [DOI] [PubMed] [Google Scholar]
- 7.McBride WH, Withers HR. Biological basis of radiation therapy. In: Perez CA, Brady LW, Halperin EC, Schmidt-Ullrich RK, editors. Principles and practice of radiation oncology. 4. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 96–136. [Google Scholar]