Abstract
Quality Indicators for Sentinel Lymph Node Biopsy in Breast Cancer: Applicability and Clinical Relevance in a Non-screened Population: sentinel lymph node biopsy (SLNB) has replaced axillary lymph node dissection (ALND) as standard of care for management of early breast cancer. This study assessed our SLNB program against 11 published quality indicators (QIs). All breast cancer patients who underwent SLNB in our centre from June 2013–Dec 2015 were included. Clinical, pathological and follow-up data were extracted from the institutional REDCap data system. Analysis was done with SPSS 23. Following validation, 234 patients had SLNB, always performed along with primary surgery. Identification rate was 95.3% and > 1 SLN was identified in 72% of patients. SLNB positivity was 33%, of these, 100% underwent ALND. Overall 91% of QI eligible patients underwent SLNB. No ineligible patients (T4) underwent SLNB. For the patients who had radio colloid, injection criteria were met for 100%. Pathological evaluation and reporting criteria were met for 100% of patients. There were no axillary recurrences in a median follow-up of 2 years. 7.6% patients had SLN negative on frozen section but positive on final histology. 7.2% of patients with clinical negative nodes had pN2 disease in final histopathology report after surgery. Sixty percent of patients who had completion ALND had only positive SLN. This study supports the applicability of published QI of SLNB in a non-screened cohort of early breast cancer patients. Although QI were useful, modification based on patient characteristics and resource availability may be needed. These indicators can be used as audit tools to improve the overall accuracy of the procedure.
Keywords: Sentinel lymph node biopsy, Breast cancer, Quality indicators
Background
Sentinel lymph node biopsy (SLNB) is established as standard of care for axillary staging in women with clinically node-negative early-stage breast cancer [1–3]. The SLNB procedure requires multidisciplinary collaboration to ensure successful performance and accurate staging [4]. Many health systems require monitoring of SLNB procedures using established quality indicators (QIs) [5, 6], but there is no published data on the validity or use of such indicators in populations where patients present with symptomatic disease, with different disease characteristics and resource availability.
In early SLNB studies, technical accuracy was assessed by the number of SLNB performed under supervision, false-negative rate (FNR), and identification rate (IR) [7]. Guidelines for SLNB training programs recommend that surgeons must perform a minimum of 20 to 30 backup axillary lymph node dissections (ALND) after SLNB, and establish an IR > = 90% and an FNR < = 5% before performing SLNB alone [8–10]. A recent analysis of SLNB audits showed that IRs were consistent across centres, but half of the surgeons surveyed abandoned backup ALND prior to performing the recommended number [11]. As calculation of FNR requires ALND and it is impractical for surgeons to continuously quantify FNR after the initial validation or training period. As a consequence, novel SLNB QIs have been developed to monitor quality of the procedure.
A set of 11 QIs for SLNB in early-stage breast cancer was developed by Quan et al., using a modified Delphi process [12]. The QIs were retrospectively validated in different cohort [13, 14] of patients, and were found to be acceptable and clinically relevant. These QIs cover all aspects of SLNB, including patient selection criteria, methods and outcome, listed in Table 1. This study aimed to assess applicability of published QIs against our institutional SLNB program.
Table 1.
Sentinel Lymph Node Biopsy Quality Indicator Results
| Quality indicator | Description by Quan et al. | Proposed target rates | No. (%) of patients |
|---|---|---|---|
| Pathologic evaluation protocol | Proportion of patients in whom the SLN were examined using a recognised serial sectioning protocol | > 90% | 234 (100) |
| Pathologic reporting by AJCC guidelines | Proportion of SLNB final HPE reports that report the category of metastases identified and the patterns of tumour present according to (AJCC) criteria. | > 90% | 234 (100) |
| Protocol for injection of radio colloid | Proportion of patients having radio-colloid injected with defined nuclear medicine protocol for SLNB | > 90% | 136/136 (100) |
| Proper identification of SLN | Proportion of patients in whom sentinel lymph node(s) (SLNs) were identified as ‘hot’ and/or ‘blue’ and/or ‘clinically suspicious’ in the chart or operative note | > 90% | 223/223 (100) |
| SLNB performance in eligible patients | Proportion of patients undergoing SLNB in the setting of breast conserving surgery for T1 tumours | > 80% | T1 with BCS—30/33 (90.9) |
| Extended inclusion criteria | T1-3 with BCS/mastectomy—220/266 (82.7) | ||
| SLNB concurrent with lumpectomy/ mastectomy | Proportion of patients who underwent SLNB and lumpectomy concurrently | > 80% | 234 (100) |
| Completion ALND for positive SLNB | Proportion of patients with a positive SLNB (as defined by micrometastases greater than 0.2 mm) who received a completion axillary lymph node dissection (ALND) | > 75% | 74/74 (100) |
| SLNB performance in ineligible patients | Proportion of patients who undergo SLNB as a stand-alone axillary procedure who are ‘ineligible’ based on pre-operative disease characteristics | < 5% | 0 (0) |
| Axillary node positivity rate | Proportion of patients undergoing SLNB in whom SLNB was identified and found to be positive | 2–6%(T1a) | 0/3(0) |
| Extended inclusion criteria | Tis, Tmic, T1, T2, T3 74/223 (33) | ||
| Number of nodes removed (> 1) | Proportion of patient who underwent SLNB in whom the number of nodes removed > 1 | 60–70% | 163/223 (72.1) |
| Axillary recurrence rate at 5 years | Proportion of patients with a negative SLNB who develop an axillary recurrence | 3% at 5 years | aNo recurrence to date |
aMedian follow-up is 2-year only
Material and Methods
Methods
Study Design
The present study is a retrospective analysis of consecutive patients who underwent SLNB for breast carcinoma between June 2013 and Dec 2015. A waiver was obtained from the institutional review board. Patients diagnosed with clinically and radiologically node-negative operable breast cancer (T0/T1/T2/T3, N0) were offered SLNB, and have been included in the analysis. Patients with T4 tumours, N1-3 disease, or having neoadjuvant chemotherapy were not offered SLNB.
Sentinel Lymph Node Biopsy
SLNB was performed at the same time as breast surgery, with frozen section evaluation of SLNs. The preferred method for SLNB was a dual tracer technique. Methylene blue alone was used when isotope was not available. Isotope alone was used initially because blue dye was not available, and later for women with an atopic history. Radioactive colloid (micro filtered (0.22) micron 99 m Tc-S) was injected in divided doses peritumourally, at least 2 h before surgery. After induction of anaesthesia, 2 ml of methylene blue dye (1%) was injected subcutaneously superficial to the tumour, followed by 5 min gentle whole breast massage. A gamma probe (Europrobe3, EURORAD S.A2, Ettore Bugatti, 67201 ECKBOLSHEIM - FRANCE) was used to localise hot nodes. Hot, blue and clinically suspicious nodes were excised and sent for frozen section. Frozen section analysis was done by a trained breast pathologist, and reports followed institutional protocols based on the College of American Pathologists protocols (CAP) [15, 16]. Patients with negative frozen section did not undergo ALND. ALND was done if SLN was not identified; frozen section was positive for malignancy and if frozen section of SLN was negative but final histopathology was positive. Following our institutional protocol, all patients with positive SLNs, defined as isolated tumour cells (ITC), micro metastasis or macro metastasis had completion ALND.
Data Extraction
The clinical, radiological, pathological and follow-up data were extracted from the institutional REDcap data system [17]. Clinical data included age, body mass index, location, size of the primary tumour, axillary lymph node status assessed clinically and by ultrasonography, type of surgery, and the method used for SLNB. Pathological variables included the total number of SLNs removed, number of positive SLNs, size of SLN metastasis and for patients having completion ALND, the number of positive nodes in the rest of axillary tissue. The 11 QIs (Table 1) described by Quan et al. were considered as a standard reference and the data were analysed accordingly. Analysis of data was done with SPSS23.
Results
A total of 234 patients met inclusion criteria. The mean age of presentation was 54.8 (SD 11) years, and mean BMI was 26.99 (SD 4.9) kg/m2. For patients with a final diagnosis of DCIS, the mean tumour size was 4.6 cm, of whom 80% were clinically palpable. Mean pathological size for patients with invasive tumours was 2.77 (SD 1.23) cm, of which 94.5% were clinically palpable. Other patient characteristics are summarised in Table 2.
Table 2.
Clinical and Histopathological Characteristics
| Characteristic | No (%) of patients |
|---|---|
| Primary tumour stage | |
| Tis | 9 (3.8) |
| T1mic | 4 (1.7) |
| T1 | 44 (18.8) |
| T2 | 171 (73.1) |
| T3 | 5 (2.1) |
| Tx | 1 (0.4) |
| Histologic subtype | |
| DCIS | 9 (3.8) |
| DCIS + micro invasion | 4 (1.7) |
| Invasive ductal carcinoma | 195 (83.3)) |
| Invasive lobular carcinoma | 4 (3.4) |
| Others | 18 (7.7) |
| Case laterality | |
| Unilateral | 231 (98.7) |
| Bilateral | 3 (1.3) |
| Quadrant of tumour | |
| Lateral | 117 (50) |
| Central | 54 (23.1) |
| Medial | 56 (23.9) |
| Missing data | 7 (3) |
| Distribution of tumour | |
| Unifocal | 220 (94) |
| Multifocal | 14 (6) |
| Grade of invasive cancer (n = 225) | |
| G1 | 35 (15.5) |
| G2 | 89 (39.5) |
| G3 | 99 (44) |
| Missing data | 2 (1) |
| Oestrogen receptor status | |
| Positive | 184 (78.6) |
| Negative | 50 (21.4) |
| Progesterone receptor status | |
| Positive | 174 (74.4) |
| Negative | 60 (25.6) |
| HER 2 receptor status | |
| Positive | 44 (18.8) |
| Negative | 146 (62.4) |
| Equivocal | 33 (14.1) |
| Missing | 11 (4.7) |
Of the 234 patients, 19% had T1 disease, of whom 80% had breast conservation surgery (BCS), and 91% of T1 patients with BCS were offered SLNB. Including patients with T2 and T3 tumours and also those having mastectomy, 82% of eligible patients were offered SLNB, and in the 2-full calendar years of study, this figure was 75% in the first and 88% in the second year. No ineligible patients (T4) underwent SLNB. The dual method was used in 104 (44.4%), methylene blue in 98 (41.9%) and isotope in 32 (13.7%) patients. Institutional protocols were followed for all patients who had radio-colloid injection. Pathological reporting of both tumour pattern and metastases of SLN were in accordance with the criteria in all patients.
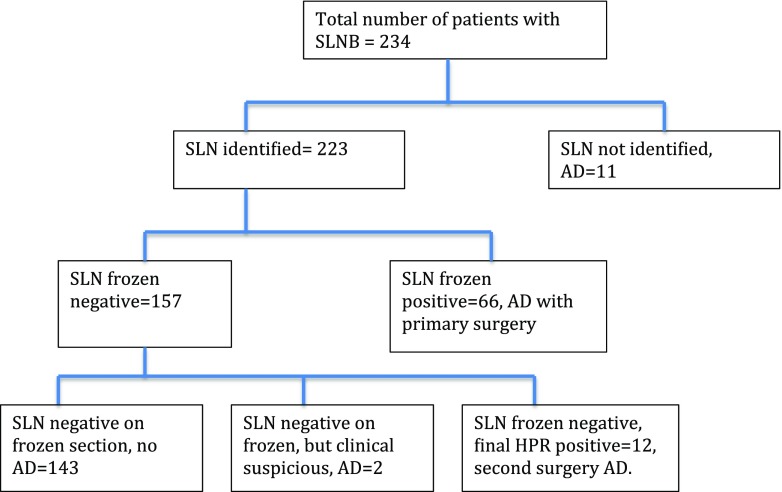
The IR (proportion of patients in whom a SLN was detected and excised) was 95.3%, and for all patients where the SLN was identified, the record clearly mentioned the number of nodes and whether they were hot, blue, hot and blue or clinically suspicious. In the 223 patients in whom SLNs were identified, 1 node was identified in 60 (27%), 1–4 in 156 (70%) and > 4 in 7 (3%). Frozen section of SLNs was positive in 66 (28%) patients, and immediate completion ANLD was done for all. In 12 patients (5.1%), the initial frozen report was negative but final histology was positive and completion ALND was done at a later date (Fig. 1). Two patients (1%) with clinically suspicious nodes intra-operatively had ALND in spite of a negative frozen section (both negative for malignancy on final histopathology, 1 had tubercular lymphadenitis). SLN histology showed ITC in 4 patients (5.1%), micro metastases in 8 (10.2%) and macro metastases in 66 (84.7%). Considering ITC (1.2%) as node negative, the SLN was positive in 74 patients (33%). For T1a patients, none of the 3 patients had a positive SLN. In the median follow-up of 24 months (IQR 18.8,29.4), there were no axillary recurrences. Results related to the published QI are summarized in Table 1.
Fig. 1.
Flow chart for sentinel lymph node biopsy
In addition to the predefined QIs, some other results were considered relevant in the context of quality. For 60% of patients having ALND after positive SLNB, the SLN was the only positive node. In patients with negative nodes on both clinical and radiological assessment, 7.2% had pN2 disease.
Discussion
SLNB is the recommended procedure for staging the axilla in early-stage breast cancer, and is part of all major treatment guidelines [1–3]. For reasons, which remain at least partially unexplained, analysis of breast cancer survival data has shown improved disease-free and overall survival for screen-detected breast cancer when compared with symptomatic cancers of the same stage [18, 19]. Prognostication tools such as PREDICT [20] include this information in their analysis. It is possible that the SLN positivity and other related information might be different where all patients have symptomatic disease, compared to studies where the majority of early cancer is screen detected. It is important that QIs should be relevant, allowing physicians in different circumstances to monitor outcomes and audit SLNB programs. However, there is no data on the use of published QI in non-screened cohort, where the majority of patients present with symptomatic disease. The aim of our study was to evaluate the applicability of published QI in non-screened breast cancer patients.
We found that the clinical information needed for these guidelines was simple. Once the entire team was acquainted with the data required, collecting data was not time consuming. All information can be collected during the course of routine work or retrieved from the hospital data system. Overall, our SLNB performance complied with almost all the indicators; although the 5-year axillary recurrence rate was not calculated as our median follow-up at the time of reporting was only 24 months.
The first three QIs address the structure of the SLNB program. We met 100% for the pathology reporting and radio-colloid injection QIs. We initially used radioactive colloid as our primary technique for SLN identification, and validated the process. However, our radioactive isotope is sourced internationally, and, as reported by other centres [21], for logistic reasons, there were significant periods of time when it was not available. As it was not possible to schedule surgery dependent on isotope availability, it became necessary to use blue dye alone to offer SLNB for maximum number of eligible patients. Additionally, the cost of the isotope-based technique in our hospital was over 100-fold that of the blue dye only method. Blue dye SLNB (variably using isosulphane blue, patent blue or methylene blue) is well supported by published evidence with acceptable IR and FNR [22–24], but this aspect is not addressed in published QIs. We were able to increase the proportion of patients having SLNB when this technique was offered and we propose that institutions should have a blue dye protocol, including the concentration of dye and the time difference between injection and removal of SLN.
The next set of QIs cover the process of SLNB. In the criteria under consideration here, only patients with T1 tumours having BCS should be included, and the proposed target rate was 80%. This study was conducted in a region without population-based screening, and apart from a very few with opportunistic identification of impalpable disease, most patients presented with palpable lumps [25, 26]. Only 19% of patients in this study presented with T1 disease, and 20% of them opted for mastectomy. Assessing our program against this QI would have meant that > 75% of our patients would have been excluded, although we offered SLNB to all patients with T1, T2 or T3 disease having either BCS or mastectomy [27].
SLNB was done concurrently with primary surgery in 100% of our patients, and all positive SLN were followed by ALND. Only 5% of patients with a negative frozen section but positive histology needed a second operation for completion ALND. SLNB alone, in advance of the breast procedure, would mean that a second operation was always needed, which may not be acceptable to patients, but is necessary in centres without frozen section facilities [21]. On the other hand, some studies have supported SLNB following diagnostic lumpectomy [27]. Over 7% of our patients finally had pN2 disease, which is lower than the 13% reported elsewhere [2] and this could be a QI for the diagnostic pathway.
Two QIs address surgical outcome measures. In our cohort of patients, there were no positive SLNs in patients with T1a tumours, but only 1% of tumours were T1a. The target positivity rate of 2–6% is based on data for T1a tumours only. Data from a SEER review for SLN positivity found the node-positive rate for T1a, b and c tumours was 2.8, 3.4 and 7–14%, indicating that a higher positivity rate is to be expected with increasing tumour size [28, 29]. Other published results with wider inclusion criteria show SLN positivity between 17 to 35% [30, 31]. Our data, where 73.1% of tumours were T2, with a median tumour size of 2.7 cm (SD 1.23), was towards the higher extreme for positivity. Definition of QIs for larger tumours would have been useful in these circumstances. In terms of the number of nodes removed, we met the QI with > 1 SLN removed in 72% of patients. In addition, > 4 nodes were removed in 3.1% of patients. Studies suggest that the FNR decreases when > 1 SLN is harvested, but removing more than 4 nodes provides no additional benefit and is associated with increased morbidity such as lymphoedema and paraesthesia, without increasing accuracy [32]. In parallel with the figure for the proportion of patients where a single node is identified, limits for the number of patients having > 4 SLN removed would also be a useful QI.
In the process of analysing this data, we found that we frequently needed to modify inclusion criteria for QIs to avoid leaving out a large proportion of patients. First among these was extension of size criteria for eligible patients to include T2, T3 disease and patients with mastectomy. On the other hand, 7% of our patients were pN2, and might be considered to have been ineligible, though it was not clear from this study how the pre-operative identification pathway could be improved. In addition, in spite of having nuclear medicine support, we found that availability of isotope was a significant problem, and concluded that quality metrics based on the easily available and economical blue dye method were also needed.
Accurate identification of the SLN at surgery is perhaps the most crucial part of the entire process of SLNB. Current evidence shows that micro metastasis [33] or up to two nodes with macro metastases [34] have such a low probability of additional positive non-SLNs, that ALND can be avoided without compromising outcomes in patients who receive appropriate adjuvant therapy. In all published studies, for > 50% of patients, SLNs are the only positive nodes [35]. In our series too, for 60% of patients, SLN was the only positive node. Clinical treatment algorithms in many parts of the world still advise ALND if the SLN is positive. However, based on ASOCOG Z-0011 study [34], recently published ASCO guidelines state that in certain circumstances SLNB alone is adequate in up to two positive SLN [36]. Until non-dissection becomes universally accepted, we suggest that the SLN being the only positive node is an indicator of accuracy, and a target rate should be defined as a QI.
Conclusion
This is the first study from India focusing on quality assurance for sentinel lymph node biopsy. In general, this study supports the applicability of published QI of SLNB in a non-screened cohort of early breast cancer patients. However, some criteria needed modification to make them relevant to the disease characteristics and resource availability in India. The indicators are useful as audit tools, and can be used at intervals to improve the overall accuracy of the procedure. They are easily monitored in tertiary care centres with access to good diagnostic facilities and well-maintained data systems. As SLNB becomes more common in private practice and de-centralised settings in India, guidance on maintenance of SLNB data records should be defined along with QIs.
Funding
None.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that that they have no conflict of interest.
References
- 1.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106(1):4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 2.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(30):7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Giammarile F, Alazraki N, Aarsvold JN, et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging. 2013;40(12):1932–1947. doi: 10.1007/s00259-013-2544-2. [DOI] [PubMed] [Google Scholar]
- 5.Rutgers EJ. Guidelines to assure quality in breast cancer surgery. Europ J Surg Oncol. 2005;31(6):568–576. doi: 10.1016/j.ejso.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Schachter HM, Mamaladze V, Lewin G, et al. Many quality measurements, but few quality measures assessing the quality of breast cancer care in women: a systematic review. BMC Cancer. 2006;6:291. doi: 10.1186/1471-2407-6-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal A, Newcombe RG, Chhabra A, Mansel RE. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer—results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99(2):203–208. doi: 10.1007/s10549-006-9192-1. [DOI] [PubMed] [Google Scholar]
- 8.Mansel RE, MacNeill F, Horgan K, et al. Results of a national training programme in sentinel lymph node biopsy for breast cancer. Br J Surg. 2013;100(5):654–661. doi: 10.1002/bjs.9058. [DOI] [PubMed] [Google Scholar]
- 9.McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg. 2001;234(3):292–299. doi: 10.1097/00000658-200109000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons RM. Review of sentinel lymph node credentialing: how many cases are enough? J Am Coll Surg. 2001;193(2):206–209. doi: 10.1016/S1072-7515(01)00998-X. [DOI] [PubMed] [Google Scholar]
- 11.Lucci A, Jr, Kelemen PR, Miller C, III, Chardkoff L, Wilson L. National practice patterns of sentinel lymph node dissection for breast carcinoma. J Am Coll Surg. 2001;192(4):453–458. doi: 10.1016/S1072-7515(01)00798-0. [DOI] [PubMed] [Google Scholar]
- 12.Quan ML, Wells BJ, McCready D, Wright FC, Fraser N, Gagliardi AR. Beyond the false negative rate: development of quality indicators for sentinel lymph node biopsy in breast cancer. Ann Surg Oncol. 2010;17(2):579–591. doi: 10.1245/s10434-009-0658-3. [DOI] [PubMed] [Google Scholar]
- 13.Wells B, Saskin R, Wright F, McCready D, Quan ML. Measuring the quality of sentinel lymph node biopsy (SLNB) for breast cancer: a population-based evaluation. Ann Surg Oncol. 2013;20(2):615–619. doi: 10.1245/s10434-012-2626-6. [DOI] [PubMed] [Google Scholar]
- 14.Acuna SA, Angarita FA, McCready DR, Escallon J. Quality indicators for sentinel lymph node biopsy: is there room for improvement? Can J Surg. 2013;56(2):82–88. doi: 10.1503/cjs.033011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgibbons PL, Connolly JL, Page DL. Cancer Committee College of American Pathologists. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Northfield: College of American Pathologists; 2005. [Google Scholar]
- 16.Lester SC, Bose S, Chen YY, et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med. 2009;133(10):1515–1538. doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wishart GC, Greenberg DC, Britton PD, et al. Screen-detected vs symptomatic breast cancer: is improved survival due to stage migration alone? Br J Cancer. 2008;98(11):1741–1744. doi: 10.1038/sj.bjc.6604368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mook S, Van’t Veer LJ, Rutgers EJ, et al. Independent prognostic value of screen detection in invasive breast cancer. J Natl Cancer Inst. 2011;103(7):585–597. doi: 10.1093/jnci/djr043. [DOI] [PubMed] [Google Scholar]
- 20.Wishart GC, Bajdik CD, Dicks E, et al. PREDICT Plus: development and validation of a prognostic model for early breast cancer that includes HER2. Br J Cancer. 2012;107(5):800–807. doi: 10.1038/bjc.2012.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.East JM, Valentine CSP, Kanchev E, Blake GO. Sentinel lymph node biopsy for breast cancer using methylene blue dye manifests a short learning curve among experienced surgeons: a prospective eoular cumulative sum (CUSUM) analysis. BMC Surg. 2009;9:2. doi: 10.1186/1471-2482-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golshan M, Nakhlis F. Can methylene blue only be used in sentinel lymph node biopsy for breast cancer? Breast J. 2006;12(5):428–430. doi: 10.1111/j.1075-122X.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 23.Nour A. Efficacy of methylene blue dye in localization of sentinel lymph node in breast cancer patients. Breast J. 2004;10(5):388–391. doi: 10.1111/j.1075-122X.2004.21360.x. [DOI] [PubMed] [Google Scholar]
- 24.Varghese P, Mostafa A, Abdel-Rahman AT, et al. Methylene blue dye versus combined dye-radioactive tracer technique for sentinel lymph node localisation in early breast cancer. Eur J Surg Oncol. 2007;33(2):147–152. doi: 10.1016/j.ejso.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Kamath R, Mahajan KS, Ashok L, Sanal TS. A study on risk factors of breast cancer among patients attending the tertiary care hospital, in Udupi District. Indian J Community Med. 2013;38(2):95–99. doi: 10.4103/0970-0218.112440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal G, Ramakant P. Breast cancer care in India: the current scenario and the challenges for the future. Breast Care. 2008;3(1):21–27. doi: 10.1159/000115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renaudeau C, Lefebvre-Lacoeuille C, Campion L, et al. Evaluation of sentinel lymph node biopsy after previous breast surgery for breast cancer: GATA study. Breast. 2016;28:54–59. doi: 10.1016/j.breast.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Helyer LK, Coburn NG, Law CH, McCready DR. Does the adoption of sentinel node biopsy account for the increase in node positivity in women with T1 tumors? Ann Surg Oncol. 2008;15(S2):75. [Google Scholar]
- 29.Helyer LK, Coburn NG, Law CH, McCready DR. Axillary staging is more accurate today than ever before: no increase in the false negative rate with wide-spread adoption of sentinel node technique. Breast Can Surg Treat. 2007;106(S1):s127–s128. [Google Scholar]
- 30.Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol. 2006;7(12):983–990. doi: 10.1016/S1470-2045(06)70947-0. [DOI] [PubMed] [Google Scholar]
- 31.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 32.Wong SL, Edwards MJ, Chao C, et al. Sentinel lymph node biopsy for breast cancer: impact of the number of sentinel nodes removed on the false-negative rate. J Am Coll Surg. 2001;192(6):684–689. doi: 10.1016/S1072-7515(01)00858-4. [DOI] [PubMed] [Google Scholar]
- 33.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–230. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pohlodek K, Bozikova S, Meciarova I, Mucha V, Bartova M, Ondrias F. Prediction of additional lymph node involvement in breast cancer patients with positive sentinel lymph nodes. Neoplasma. 2016;63(3):427–434. doi: 10.4149/312_150922N497. [DOI] [PubMed] [Google Scholar]
- 36.Gary HL, Mark RS, Linda DB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:561–564. doi: 10.1200/JCO.2016.71.0947. [DOI] [PubMed] [Google Scholar]